Introduction
Pancreatic cancer, the fourth leading cause of
cancer-related mortality worldwide, constitutes one of the most
aggressive types of cancer (1).
There have been substantial advances in the therapeutic modalities
for advanced pancreatic cancer, including carbon beam ion
radiotherapy (2), systemic
chemotherapies using gemcitabine (GEM), tegaful-gimeracil-oteracil
potassium (S-1) (3) and
oxaliplatin, irinotecan, fluorouracil, leucovorin (Folfirinox)
(4), as well as an EGFR-inhibitor
erlotinib (5). However, despite
these advances, the median survival time (MST) of advanced
pancreatic cancer patients from the first or second line of
chemotherapy still remains approximately 7–11 (1–5) or 4–6
months (2,6), respectively. Therefore, the
development of novel therapeutic approaches including cancer
vaccines is needed.
We previously devised a new regimen of peptide-based
vaccination, named personalized peptide vaccination (PPV), in which
vaccine antigens were selected from 31 different pooled peptides,
and administered based on both HLA-class IA types and levels of
peptide-specific IgG responses before vaccination (7–10). In
our previous clinical trials, immune responses triggered by PPV
were well-associated with overall survival (OS) in advanced
pancreatic cancer patients under PPV in combination with GEM as the
first-line therapy (7,8). GEM did not inhibit immune responses
induced by PPV. Furthermore, the MST of advanced pancreatic cancer
patients with positive (n=10) or negative (n=8) immune responses
was 15.5 and 6 months, respectively, when non-resectable pancreatic
cancer patients were treated with PPV and GEM as the first-line
therapy. However, there is no trial of PPV for
chemotherapy-resistant advanced pancreatic cancer currently
available. Consequently, in the present study, a phase II study of
PPV in chemotherapy-resistant advanced pancreatic cancer patients
was performed.
Materials and methods
Patients
Patients pathologically and/or clinically diagnosed
with pancreatic cancer were eligible for inclusion in the present
study, when they had failed at least first-line chemotherapy and
showed positive IgG responses to at least 2 of the 31 different
vaccine candidate peptides as previously reported (10). Additional inclusion criteria were
the following: age between 20 and 80 years, Eastern Cooperative
Oncology Group (ECOG) performance status of 0 or 1, positive status
for the HLA-A2, -A24, -A3 supertype (A3, A11, A31 or A33) or -A26,
life expectancy of at least 12 weeks, and adequate hematologic,
hepatic and renal function. Exclusion criteria included pulmonary,
cardiac or other systemic diseases, acute infection, a history of
severe allergic reactions, pregnancy or nursing, and other
inappropriate conditions for enrollment as judged by clinicians.
The protocol was approved by the Kurume University Ethics
Committee, and was registered in the UMIN Clinical Trials Registry
(UMIN #08167). After a full explanation of the protocol, a written
informed consent was obtained from all the patients prior to
enrollment.
Clinical protocol
This was an open-label phase II study, in which the
main objectives were to evaluate safety and to address whether PPV
in combination with additional chemotherapeutic regimens for
chemotherapy-resistant pancreatic cancer patients prolongs MST.
Thirty-one peptides, the safety and immunological effects of which
were reported in previous clinical studies (8–11),
were employed for vaccination [12 peptides for HLA-A2, 14 for
HLA-A24, 9 for HLA-A3 supertype (A3, A11, A31 or A33) and 4 for
HLA-A26]. The peptides were prepared under the conditions of Good
Manufacturing Practice (GMP) by PolyPeptide Laboratories (San
Diego, CA, USA) and the American Peptide Company (Vista, CA,
USA).
The peptides for vaccination to individual patients
were selected in consideration of the pre-existing host immunity
before vaccination, by assessing the titers of IgG specific to each
of the 31 different vaccine candidates (10). A maximum of 4 peptides (3 mg/each
peptide), which were selected based on the results of HLA typing
and peptide-specific IgG titers, in complex with incomplete
Freund's adjuvant (Montanide ISA 51; Seppic, Paris, France) were
subcutaneously administered once a week for 6 consecutive
weeks.
After the first cycle of 6 vaccinations, up to 4
vaccine peptides were re-selected according to the titers of
peptide-specific IgG and administered every 2 weeks. Vaccine
peptides were re-selected at every cycle of 6 vaccinations until
the discontinuation of PPV. Adverse events were monitored according
to the National Cancer Institute Common Terminology Criteria for
Adverse Events (NCI-CTCAE) version 3.0. Complete blood counts and
serum biochemical tests were performed at every cycle of 6
vaccinations. The clinical responses were evaluated by the Response
Evaluation Criteria in Solid Tumors (RECIST) with radiological
findings of computed tomography (CT) scanning or magnetic resonance
imaging (MRI) before and after vaccinations.
Measurement of laboratory markers
Levels of C-reactive protein (CRP), serum amyloid A
(SAA) and IL-6 in plasma were examined by ELISA using kits from
R&D Systems (Minneapolis, MN, USA), Invitrogen (Carlsbad, CA,
USA) and eBioscience (San Diego, CA, USA), respectively. Bead-based
multiplex assays were used to measure cytokines, including IL-4,
IL-13, IL-21, IP-10, BAFF and TGF-β with the Luminex 200 system
(Luminex, Austin, TX, USA).
Measurement of immunoglobulins (Igs)
reactive to each of the 31 different peptides
The levels of Igs reactive to each of the 31
different peptides were measured using the Luminex 200 system as
previously reported (9–11). In brief, plasma was incubated with
100 μl of peptide-coupled color-coded beads for 1.5 h at 30°C,
followed by washing and incubation with 100 μl of biotinylated goat
anti-human IgG (Vector Laboratories, Burligame, CA, USA). After
washing, 100 μl of streptavidin-PE (Invitrogen) was added and
incubated for 30 min at 30°C. After washing, the fluorescence on
the beads was detected using the Luminex 200 system. The Igs levels
were expressed in fluorescence intensity units (FIU) as previously
reported (9–11). Peptide-specificity of IgG against
each of the 31 peptides was confirmed (unpublished data).
Statistical methods
The Wilcoxon signed-rank test and paired t-test were
used to compare differences between pre- and post-vaccination
measurements. OS was calculated from the first day of peptide
vaccination until the day of death or the last day when the patient
was known to be alive. Prognostic factors for OS were evaluated by
univariate and multivariate analyses with the Cox proportional
hazards regression model. Curves for OS were estimated using the
Kaplan-Meier method, and the log-rank test was conducted for the
comparison of survival curves. Two-sided P-values of <0.05 were
considered to indicate statistically significant differences. All
statistical analyses were conducted using the JMP version 10.0.1
software (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
Between November 2008 and March 2011, 41 advanced
pancreatic cancer patients who had failed at least first-line
chemotherapy were included in the present study. Patient
characteristics are listed in Table
I. There were 27 male and 14 female subjects with a median age
of 61 years (range, 44–78). All patients had advanced stages of
cancer (stage IVa, n=7; IVb, n=24; recurrent, n=10). Prior to
enrollment, the patients had failed 1 (n=11), 2 (n=24), 3 (n=5) or
4 (n=1) regimen(s) of chemotherapy. The median duration of
chemotherapy prior to PPV was 8 months with a range from 1 to 36
months. The performance status at the time of enrollment was grade
0 (n=37) or 1 (n=4). The numbers of vaccine peptides employed at
the first cycle of vaccinations were 4 peptides in 33 patients, 3
in 5 patients and 2 in 3 patients. The median number of
vaccinations was 10 with a range of 3 to 36. PPV was combined with
GEM (n=11), S-1 (n=6), GEM and S-1 (n=8) or other combinations of
chemotherapeutic agents including CDDP-based regimens (n=8). PPV
alone was administered to 8 patients, since chemotherapy could not
be tolerated (n=4) or due to patient refusal (n=4).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristic | Value |
|---|
| Age (years), median
(range) | 61 (44–78) |
| Gender, n |
| Male | 27 |
| Female | 14 |
| Disease location,
n |
| Head | 14 |
| Body | 15 |
| Limbs | 6 |
| Body and limbs | 6 |
| Performance status,
n |
| 0 | 37 |
| 1 | 4 |
| Stage, n |
| IVa | 7 |
| IVb | 24 |
| Recurrent | 10 |
| No. of previous
regimens, n |
| 1 | 11 |
| 2 | 24 |
| 3 | 5 |
| 4 | 1 |
| Duration of previous
treatment (months), median (range) | 8 (1–36) |
| No. of vaccinations,
median (range) | 10 (3–36) |
| Combined treatment,
n |
| (−) | 8 |
| GEM | 11 |
| S-1 | 6 |
| GEM and S-1 | 8 |
| Other regimens | 8 |
| Treatment response,
n |
| SD | 28 |
| PD | 13 |
| Overall survival time
(days), median (95% CI) | 238 (151–313) |
Toxicities
A grade 1 or 2 dermatological reaction at the
injection site was observed in 39 cases. Anemia (n=15),
lymphocytopenia (n=20), thrombocytopenia (n=11), leukocytopenia
(n=7), hypoalbuminemia (n=15) and hyperglycemia (n=8) were also
frequently observed. Grade 3 adverse events included anemia (n=1),
lymphocytopenia (n=1), hypertension (n=1), GGT increase (n=1) and
creatinine increase (n=1). According to assessment by the
Independent Safety Evaluation Committee in this trial, all the
grade 3 adverse events were concluded to be not directly associated
with PPV.
Humoral responses to peptides
IgG responses specific to the vaccine peptides in
pre- and post-vaccination plasma samples were analyzed.
Post-vaccination plasma samples were available from 36 and 17
patients after the 5th and 11th vaccination, respectively. When
peptide-specific IgG titers to at least one of the vaccine peptides
in the post-vaccination plasma were >2-fold higher compared to
those in the pre-vaccination plasma, antigen-specific humoral
responses were considered to be increased. The IgG responses
specific to at least one of the vaccine peptides were augmented in
14 of 36 patients (39%) and in 18 of 19 patients (95%) after the
5th and 11th vaccination, respectively (Table II).
 | Table IIIgG responses to the vaccinated
peptides. |
Table II
IgG responses to the vaccinated
peptides.
| | IgG response |
|---|
| |
|
|---|
| Patient no. | Peptide | Pre | 5th | 11th |
|---|
| 1 | ppMAPkkk-432 | 43 | 40 | na |
| WHSC2-103 | 68 | 69 | na |
| HNRPL-501 | 191 | 638 | na |
| HNRPL-140 | 209 | 189 | na |
| 2 | SART3-109 | 226 | 1,896 | na |
| Lck-422 | 44 | 66 | na |
| CypB-129 | 23 | 45 | na |
| WHSC2-103 | 322 | 401 | na |
| 3 | PSA-248 | 28 | 4,999 | 28,025 |
| MRP3-1293 | 75 | 70 | 3,259 |
| SART2-161 | 37 | 38 | 7,860 |
| Lck-486 | 38 | 31 | 23,697 |
| 4 | MRP3-503 | 57 | 56 | na |
| MRP3-1293 | 79 | 69 | na |
| SART2-161 | 51 | 53 | na |
| Lck-486 | 53 | ND | na |
| 5 | CypB-129 | 161 | 120 | 12,717 |
| ppMAPkkk-432 | 368 | ND | ND |
| UBE2V-43 | 396 | 399 | 60,508 |
| SART3-302 | 272 | 235 | 11,267 |
| HNRPL-501 | 150 | 343 | ND |
| 6 | HNRPL-140 | 13 | ND | na |
| SART3-302 | 40 | ND | na |
| 7 | SART3-109 | 42 | 52 | na |
| SART3-511 | 27 | ND | na |
| Lck-90 | 13 | ND | na |
| Lck-449 | 45 | ND | na |
| 8 | SART2-93 | 32 | 18 | na |
| PAP-213 | 1,249 | 1,573 | na |
| EGF-R-800 | 40 | ND | na |
| MRP3-503 | 98 | 38 | na |
| SART3-109 | 23 | 11 | na |
| 9 | Lck-246 | 376 | 623 | 3,264 |
| UBE2V-43 | 188 | ND | 16,549 |
| UBE2V-85 | 294 | 314 | 2,053 |
| SART3-302 | 207 | 330 | 1,929 |
| HNRPL-140 | ND | 494 | 2,780 |
| 10 | HNRPL-501 | 578 | ND | ND |
| UBE2V-85 | 70 | ND | 14 |
| SART3-302 | 36 | ND | ND |
| SART3-309 | 18 | ND | ND |
| 11 | SART3-109 | 21 | ND | 653 |
| MRP3-503 | 69 | ND | 14,787 |
| PTHrP-102 | 14 | ND | ND |
| 12 | SART2-93 | 164 | ND | na |
| Lck-208 | 206 | 13 | na |
| Lck-486 | 245 | 298 | na |
| EZH2-735388 | 503 | na | |
| Lck-422 | 783 | 532 | na |
| HNRPL-140 | 456 | 380 | na |
| 13 | SART3-109 | 1,475 | 1,279 | na |
| Lck-486 | 1,644 | 1,833 | na |
| 14 | SART3-109 | 2,309 | 2,136 | 6,782 |
| MRP3-1293 | 43 | 40 | 23,180 |
| SART2-161 | 32 | 27 | ND |
| Lck-486 | 1,515 | 1,234 | 267,768 |
| 15 | SART3-109 | 1,500 | 5,872 | 180,917 |
| SART2-161 | 31 | 22 | 3,278 |
| Lck-486 | 650 | 224 | 58,780 |
| Lck-488 | 54 | 37 | 21,889 |
| SART3-511 | 99 | 57 | ND |
| 16 | SART3-511 | 1,699 | 1,503 | 1,522 |
| PAP-248 | 70 | 69 | ND |
| Lck-422 | 180 | ND | 16 |
| WHSC2-103 | 188 | ND | 2,629 |
| Lck-90 | 35 | 45 | 63 |
| CypB-129 | 16 | 23 | 20 |
| 17 | ppMAPkkk-432 | 83 | 88 | ND |
| SART3-109 | 62 | 49 | ND |
| Lck-486 | 2,176 | 2,191 |
3,523,034 |
| PTHrP-102 | 129 | 162 | 135 |
| SART2-93 | 47 | 100 | 59 |
| 18 | MRP3-1293 | 103 | ND | na |
| Lck-486 | 5,731 | 10,510 | na |
| PSMA-624 | 99 | ND | na |
| ppMAPkkk-432 | 126 | 115 | na |
| SART3-109 | 55 | 50 | na |
| Lck-488 | 38 | 35 | na |
| 19 | CypB-129 | 57 | 53 | na |
| ppMAPkkk-432 | 106 | 90 | na |
| HNRPL-501 | 974 | 934 | na |
| SART3-302 | 473 | 2,233 | na |
| Lck-246 | 17 | 61 | na |
| 20 | Lck-246 | 409 | 441 | 2,349 |
| EGF-R-800 | 83 | 134 | 183 |
| Lck-486 | 95 | 72 | 37,353 |
| EZH2-735 | 117 | ND | 10,454 |
| CypB-129 | 183 | 192 | 190 |
| ppMAPkkk-432 | 120 | 185 | 233 |
| 21 | PAP-213 | 48 | 98 | na |
| Lck-486 | 20 | 22 | na |
| 22 | CypB-129 | 109 | 112 | 393 |
| Lck-246 | 22 | 13 | 56 |
| WHSC2-141 | 22 | ND | 15 |
| SART3-302 | 631 | 1,459 | 5,168 |
| Lck-422 | 14 | 12 | 78 |
| 23 | PAP-213 | 13 | 123 | 4,179 |
| Lck-486 | 25 | 580 | 2,552 |
| Lck-449 | 37 | 37 | 43 |
| WHSC2-103 | 40 | 14 | 165 |
| SART3-511 | ND | 289 | 173 |
| PAP-248 | ND | 1,200 | 63 |
| 24 | PAP-213 | 122 | 122 | na |
| Lck-449 | 129 | 102 | na |
| CypB-129 | 186 | 183 | na |
| WHSC2-103 | 69 | ND | na |
| 25 | PAP-213 | 16 | 2,772 | na |
| PSA-248 | 64 | 1,372 | na |
| Lck-486 | 17 | 105 | na |
| 26 | CypB-129 | 90 | 81 | 105 |
| Lck-246 | 20 | 12 | 39 |
| SART3-309 | 12 | 374 | 4,738 |
| PAP-248 | 21 | ND | ND |
| 27 | SART2-93 | 11 | ND | 55 |
| SART3-109 | 156 | 222 | 1,871 |
| Lck-486 | 185 | 313 | 12,511 |
| Lck-488 | 15 | 12 | 3,980 |
| PAP-213 | ND | 14 | ND |
| 28 | PAP-213 | 31 | 44 | 657 |
| PSA-248 | 45 | 446 | 15,954 |
| EGF-R-800 | 30 | 33 | 2,926 |
| Lck-486 | 22 | 23 | 11,356 |
| 29 | SART2-93 | 11 | 11 | na |
| Lck-486 | 25 | ND | na |
| Lck-488 | 14 | 16 | na |
| 30 | CypB-129 | 246 | 232 | na |
| WHSC2-141 | 317 | 21 | na |
| SART3-302 | 86 | 865 | na |
| Lck-208 | 11 | 2,016 | na |
| 31 | SART2-93 | 40 | 37 | 478 |
| Lck-486 | 23 | 32 | 2,567 |
| Lck-488 | 31 | 47 | 20,641 |
| PTHrP-102 | 40 | 46 | 523 |
| 32 | WHSC2-141 | 433 | 398 | 20,518 |
| PSA-248 | 29 | 2,109 | 13,221 |
| MRP3-1293 | 149
121 | 4,155 | 11,903 |
| Lck-486 | 121 | 18,577 | |
| 33 | SART2-93 | 22 | 51 | 60 |
| SART3-109 | 14 | ND | 16 |
| Lck-486 | 39 | ND | 2,479 |
| SART2-161 | ND | 76 | 59 |
| 34 | CypB-129 | 263 | 239 | na |
| WHSC2-103 | 43 | ND | na |
| WHSC2-141 | 231 | 125 | na |
| SART3-734 | 32 | ND | na |
| 35 | MRP3-1293 | 62 | ND | na |
| Lck-486 | 85 | ND | na |
| SART3-734 | 123 | ND | na |
| CypB-129 | 149 | 93 | na |
| 36 | SART2-93 | 13 | 11 | 12 |
| SART3-109 | 11,200 | 10,657 | 10,093 |
| Lck-488 | 16 | 13 | 2,017 |
| EGF-R-800 | ND | 11 | ND |
Laboratory markers
Two inflammation markers, CRP and SAA, and 7
cytokines including IL-4, IL-6, IL-13, IL-21, IP-10, BAFF and
TGF-β, were examined in plasma before and after the 5th
vaccination. Since 5 of 41 patients did not complete the first
cycle of 6 vaccinations due to rapid disease progression, they were
excluded from the marker analysis. However, no significant
differences before and after vaccinations were observed in the
markers tested (data not shown).
Clinical outcome
No complete response (CR) or partial response (PR)
was observed during PPV. Optimum clinical responses after the 6th
vaccination or at discontinuation of PPV were observed in 28 cases
of stable disease (SD) and 13 cases of progressive disease (PD)
(Table I). MST from the first
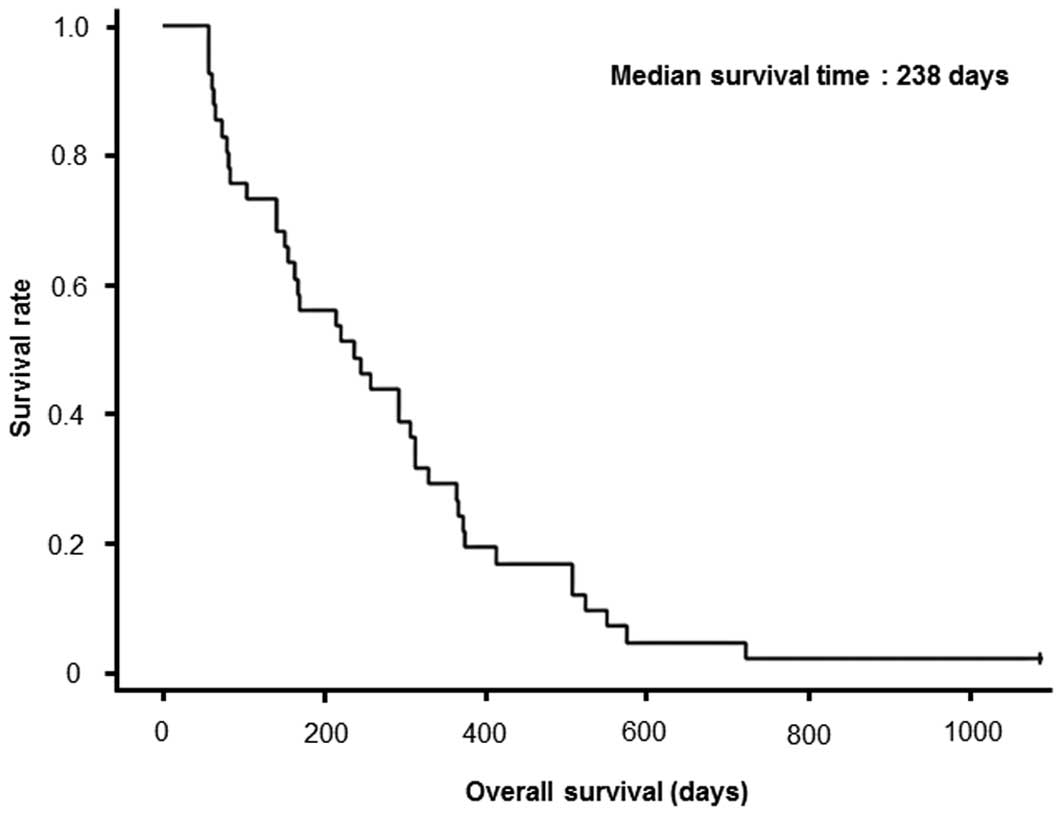
vaccination was 7.9 months (238 days) with a 1-year survival rate
of 26.8% (Table I). All the 41
patients, except for 1 patient, had succumbed to the disease at the
time of examination. Survival curve is shown in Fig. 1. MST in patients treated with PPV in
combination with (n=33) or without (n=8) chemotherapies was 9.6 or
3.1 months, respectively (P=0.0013) (data not shown). When
calculated from the initiation of the first-line chemotherapy, MST
of all 41 cases was 19.0 months [95% confidence interval (CI),
15.0–25.0 months].
Prognostic factors for OS
Pre-vaccination prognostic biomarkers for OS were
investigated in 36 patients who completed at least the first cycle
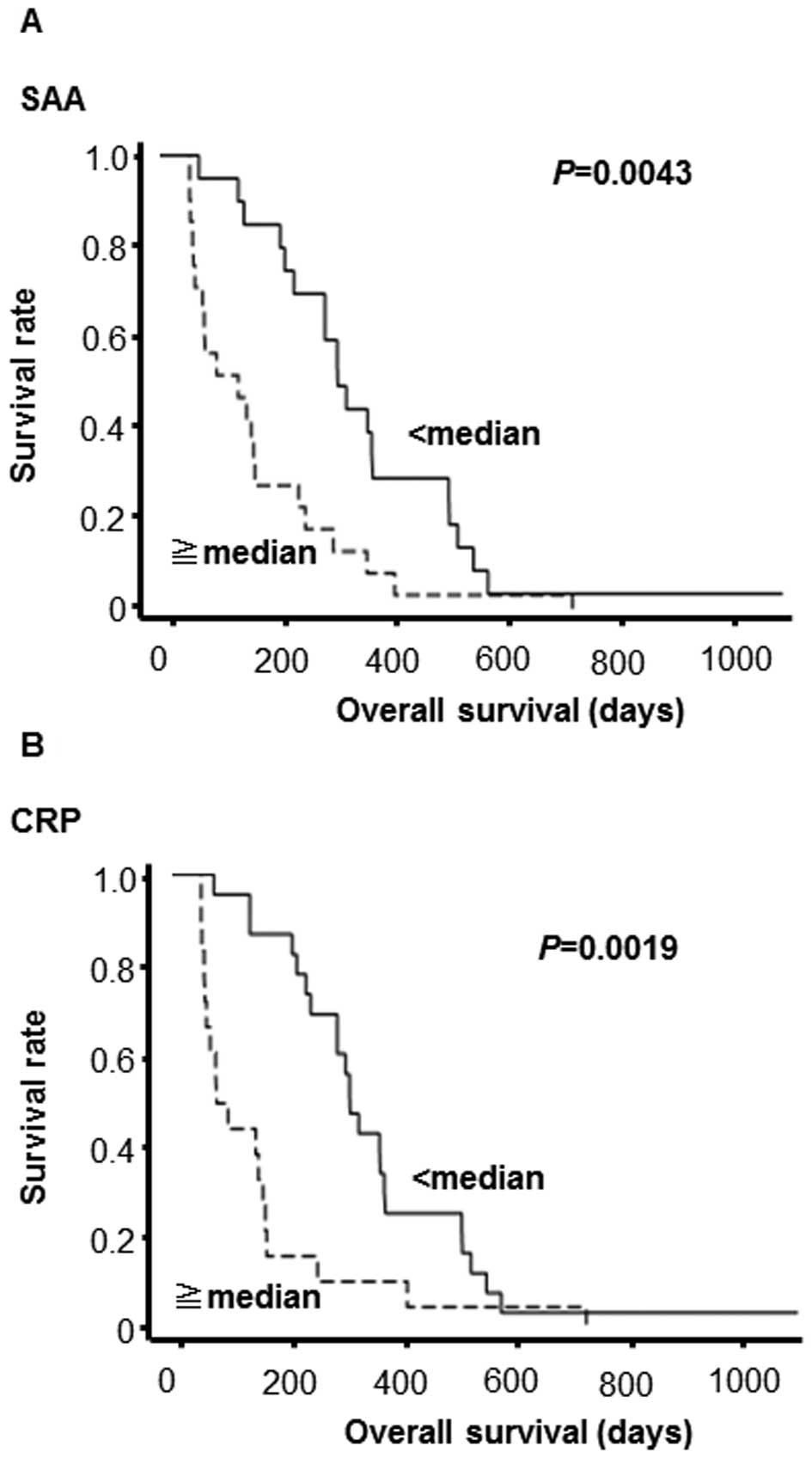
of 6 vaccinations. SAA levels in pre-vaccination samples were found
to be inversely associated with OS using the univariate Cox
proportional hazards model [hazard ratio (HR) per 1 mg/dl increment
= 1.10, 95% CI=1.03–1.15, P=0.004] (Table III). CRP levels also showed a
significant association (HR per 1 mg/dl increment = 1.68, 95%
CI=1.03–2.58, P=0.039). Similar results were obtained using the
multivariate Cox proportional hazards model. The patients were
allocated into two subgroups according to the median value of SAA
or CRP. The survival curves were estimated by the Kaplan-Meier
method and differences in survival rates were compared using the
log-rank test. The patients with higher SAA (P=0.0043) or CRP
levels (P=0.0019) in the pre-vaccination samples exhibited worse
prognosis (Fig. 2). In addition,
concerning post-vaccination samples, the patients with boosted IgG
responses (n=19) [in response to the vaccinated (n=14) or
unvaccinated peptides selected for the 2nd cycle of PPV (n=5)]
exhibited better prognosis compared to those with no IgG boosting
(n=17) (P=0.0485) (data not shown).
 | Table IIIUnivariate and multivariate analyses
with pre-vaccination clinical findings and laboratory data. |
Table III
Univariate and multivariate analyses
with pre-vaccination clinical findings and laboratory data.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| Factor | Hazard ratio (95%
Cl) | P-valuea | Hazad ratio (95%
Cl) | P-valuea |
|---|
| Age (years) | 1.58
(0.40–6.44) | 0.52 | | |
| Gender
(female<male) | 0.98
(0.52–1.95) | 0.96 | | |
| Clinical stage
(IVa<recurrent<IVb) | 1.18
(0.78–1.80) | 0.43 | | |
| Duration of
previous chemotherapy (months) | 0.98
(0.94–1.02) | 0.27 | | |
| Regimen no. of
previous chemotherapy | 0.93
(0.59–1.44) | 0.75 | | |
| Lymphocyte count
(x102/mm3) | 1.00
(1.00–1.00) | 0.39 | | |
| Hemoglobin
(g/dl) | 0.93
(0.75–1.16) | 0.53 | | |
| Albumin (g/dl) | 0.58
(0.32–1.10) | 0.09 | | |
| Creatinine
(mg/dl) | 1.88
(0.51–5.23) | 0.31 | | |
| SAA (mg/dl) | 1.09
(1.03–1.15) | 0.004b | 1.08
(0.99–1.18) | 0.09 |
| CRP (mg/dl) | 1.68
(1.03–2.58) | 0.039b | 0.95
(0.41–2.06) | 0.91 |
Discussion
The MST of 41 chemotherapy-resistant advanced
pancreatic cancer patients under PPV was 7.9 months with a 1-year
survival rate of 26.8%. Among them, the MST in patients treated
with PPV combined with (n=33) or without (n=8) chemotherapies was
9.6 or 3.1 months, respectively (P=0.0013). OS of the patients
treated with PPV not combined with chemotherapies was significantly
short, suggesting that PPV alone did not provide survival benefits
to advanced pancreatic cancer patients. This failure was expected
based on the results from our previous study (13). These results suggest that PPV has
the potential to improve OS in chemotherapy-resistant advanced
pancreatic cancer patients when administered in combination with
chemotherapeutic agents.
With regard to post-vaccination biomarkers, several
factors, including CTL responses, Th1 responses, delayed-type
hypersensitivity (DTH) and autoimmunity, have been reported to be
associated with clinical responses in some clinical trials
(14,15). We have also shown that an increase
in peptide-specific IgG and/or CTL responses after PPV is
significantly associated with longer OS (11,12).
In contrast to such post-vaccination biomarkers, there are
currently no validated pre-vaccination prognostic biomarkers widely
used. Therefore, this issue was addressed in the present study. As
a result, plasma SAA and CRP levels were inversely correlated with
OS. These results were expected based on our previous study on PPV
(10). These biomarkers are
suggested to be important not only in cancer vaccines, but also in
other treatment modalities for advanced pancreatic cancers.
Collectively, due to the safety profile and the
potential clinical efficacy of PPV, further clinical trials to
determine a protocol suitable for PPV-based therapy in
chemotherapy-resistant advanced pancreatic cancer patients are
warranted.
Acknowledgements
This study was supported in part by grants from the
Regional Innovation Cluster Program, a research program of the
Project for Development of Innovative Research on Cancer
Therapeutics (P-Direct), the Ministry of Education, Culture,
Sports, Science and Technology of Japan, and the Sendai Kousei
Hospital, Japan.
References
|
1
|
Kindler HL, Ioka T, Richel DJ, et al:
Axitinib plus gemcitabine versus placebo plus gemcitabine in
patients with advanced pancreatic adenocarcinoma: a double-blind
randomised phase 3 study. Lancet Oncol. 12:256–262. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Okada T, Kamada T, Tsuji H, et al: Carbon
ion radiotherapy: clinical experiences at National Institute of
Radiological Science (NIRS). J Radiat Res. 51:355–364. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakamura K, Yamaguchi T, Ishihara T, Sudo
K, Kato H and Saisho H: Phase II trial of oral S-1 combined with
gemcitabine in metastatic pancreatic cancer. Br J Cancer.
94:1575–1579. 2006.PubMed/NCBI
|
|
4
|
Conroy T, Desseigne F, Ychou M, et al;
Groupe Tumeurs Digestives of Unicancer and PRODIGE Intergroup.
FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N
Engl J Med. 364:1817–1825. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moore MJ, Goldstein D, Hamm J, et al:
Erlotinib plus gemcitabine compared with gemcitabine alone in
patients with advanced pancreatic cancer: a phase III trial of the
National Cancer Institute of Canada Clinical Trials Group. J Clin
Oncol. 25:1960–1966. 2007. View Article : Google Scholar
|
|
6
|
Pelzer U, Schwaner I, Stieler J, et al:
Best supportive care (BSC) versus oxaliplatin, folinic acid and
5-fluorouracil (OFF) plus BSC in patients for second-line advanced
pancreatic cancer: a phase III study from the German CONKO-study
group. Eur J Cancer. 47:1676–1681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yanagimoto H, Mine T, Yamamoto K, et al:
Immunological evaluation of personalized peptide vaccination with
gemcitabine for pancreatic cancer. Cancer Sci. 98:605–611. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yanagimoto H, Shiomi H, Satoi S, et al: A
phase II study of personalized peptide vaccination combined with
gemcitabine for non-resectable pancreatic cancer patients. Oncol
Rep. 24:795–801. 2010.PubMed/NCBI
|
|
9
|
Terasaki M, Shibui S, Narita Y, et al:
Phase I trial of a personalized peptide vaccine for patients
positive for human leukocyte antigen-A24 with recurrent or
progressive glioblastoma multiforme. J Clin Oncol. 29:337–344.
2011. View Article : Google Scholar
|
|
10
|
Terazaki Y, Yoshiyama K, Matsueda S, et
al: Immunological evaluation of personalized peptide vaccination in
refractory small cell lung cancer. Cancer Sci. 103:638–644. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Noguchi M, Mine T, Komatsu N, et al:
Assessment of immunological biomarkers in patients with advanced
cancer treated by personalized peptide vaccination. Cancer Biol
Ther. 10:1266–1279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mine T, Sato Y, Noguchi M, et al: Humoral
responses to peptides correlate with overall survival in advanced
cancer patients vaccinated with peptides based on pre-existing,
peptide-specific cellular responses. Clin Cancer Res. 10:929–937.
2004. View Article : Google Scholar
|
|
13
|
Yamamoto K, Mine T, Katagiri K, et al:
Immunological evaluation of personalized peptide vaccination for
patients with pancreatic cancer. Oncol Rep. 13:874–883.
2005.PubMed/NCBI
|
|
14
|
Lopez MN, Pereda C, Segal G, et al:
Prolonged survival of dendritic cell-vaccinated melanoma patients
correlates with tumor-specific delayed type IV hypersensitivity
response and reduction of tumor growth factor beta-expressing T
cells. J Clin Oncol. 27:945–952. 2009. View Article : Google Scholar
|
|
15
|
Firpo MA, Gay DZ, Granger SR, et al:
Improved diagnosis of pancreatic adenocarcinoma using haptoglobin
and serum amyloid A in a panel screen. World J Surg. 33:716–722.
2009. View Article : Google Scholar : PubMed/NCBI
|