Introduction
Lung cancer is one of the major causes of
cancer-related mortality worldwide (1). Lung cancer is generally classified
into two major types, small cell lung cancer (SCLC) and non-small
cell lung cancer (NSCLC). The latter is the most common type
accounting for ~80% of lung cancer cases (2). The early diagnosis of lung metastasis
can contribute to the decrease of mortality and morbidity, since
the migration and invasion of cancer cells from the primary tumor
sites into the surrounding tissues promote the progression of
metastasis.
Of note, epithelial to mesenchymal transition (EMT)
was known to promote the tumor cell infiltration into interstitial
stroma by extending microtubule-based protrusions and inhibiting
cell proliferation (3). There is
accumulating evidence that EMT plays an important role in cancer
progression, wound healing, invasion and tissue fibrosis as well as
in embryogenesis (4–6). The EMT process results in the loss of
epithelial cell phenotype and exhibits mesenchymal cell
characteristics. Therefore, epithelial cell type genes such as
E-cadherin, claudins and occludin are downregulated, while
mesenchymal cell type genes including N-cadherin, vimentin and
fibronectin related to the migration, invasion and proliferation
are upregulated during the EMT process (7,8).
However, although EMT transcriptional factors such as Twist, Snail,
Slug and Zinc finger E-box binding homeobox 1 (ZEB1) are aberrantly
overexpressed in a patient with NSCLC as a highly fibrotic
malignancy (9,10), their upstream factors remain
unclear.
ZNF746, which contains a Kruppel-associated box at
its N terminus and a C2HC/C2H2 type zinc finger at its C terminus,
interacts with Parkin as a ubiquitin E3 ligase (11,12) or
as a suppressor of peroxisome proliferator-activated receptor γ
coactivator-1 (PGC-1) in neurodegeneration in Parkinson’s disease
(12). Nevertheless, the role of
ZNF746 has yet to be elucidated in tumorigenesis. Thus, in the
present study, we investigated for the first time whether or not
the inhibition of ZNF746 suppresses the invasion and EMT process in
H460 NSCLC cells.
Materials and methods
Cell culture
H460 NSCLC cells (HTB-177™) were obtained from the
American Type Culture Collection (ATCC). H460 NSCLC cells were
maintained in Roswell Park Memorial Institute (RPMI)-1640 media
(WelGENE, Inc., Daegu, Korea) supplemented with 10% fetal bovine
serum (FBS; Gibco, Carlsbad, CA, USA) and 1% antibiotics at 37°C in
5% CO2.
Invasion assay
To investigate the migratory properties of H460
NSCLC cells, after transfecting with control or small interfering
RNA (siRNA) ZNF746 (Bioneer, Daejun, Korea) (80 nM) using
INTERFERin® reagent (Polyplus, Illkirch, France),
invasion assay was carried out using modified 48-well
microchemotaxis chambers (Neuro Probe Inc., Gaithersburg, MD, USA).
Polyvinylpyrrolidone-free polycarbonate filters (8 mm pore size)
(Neuro Probe, Inc.) were coated with Matrigel, a solubilized
basement membrane matrix. The lower chamber was filled with media
containing 10% FBS as chemoattractant agents. The coated filter and
upper chamber were laid over the lower chamber. The control siRNA
and ZNF746 siRNA (Bioneer, Daejun, Korea)-transfected H460 NSCLC
cells (1×104 cells/25 μl) were seeded onto the upper
chamber wells. After 24 h incubation at 37°C, the filter was fixed
and stained with Diff-Quick (Sysmex, Kobe, Japan) and non-migrated
cells on the upper surface of the filter were wiped off with a
swab. Then, randomly chosen fields were photographed under a
microscope (DFC420C; Leica, Germany) and the number of cells that
migrated to the lower surface was counted.
Real-time quantitative RT-PCR
(RT-qPCR)
Total RNA was isolated from H460 NSCLC cells with
QIAzol (Invitrogen, Carlsbad, CA, USA). A reverse transcription kit
(Promega, Madison, WI, USA) was used to construct the template
cDNA. RT-qPCR was performed with the LightCycler™ instrument (Roche
Applied Sciences, Indianapolis, IN, USA) according to the
manufacturer’s protocol. The mRNA level of GAPDH was used to
normalize the expression of genes of interest. The primers used
were: E-cadherin forward, 5′-CAAG CTATCCTTGCACCTCAG-3′ and reverse,
5′-GCATCAAGA GAACTCCTATCTTG-3′; matrix metalloproteinase (MMP)1
forward, 5′-CTGGCCACAACTGCCAAATG-3′ and reverse,
5′-CTGTCCCTGAACAGCCCAGTACTTA-3′; MMP2 forward,
5′-TCTCCTGACATTGACCTTGGC-3′ and reverse,
5′-CAAGGTGCTGGCTGAGTAGATC-3′; MMP7 forward,
5′-TGAGCTACAGTGGGAACAGG-3′ and reverse, 5′-TCAT
CGAAGTGAGCATCTCC-3′; MMP9 forward, 5′-TTGACA GCGACAAGAAGTGG-3′ and
reverse, 5′-GCCATTCACGT CGTCCTTAT-3′; Slug forward,
5′-GCGATGCCCAGTCTA GAAAA-3′ and reverse, 5′-GCAGTGAGGGCAAGAAA
AAG-3′; Nanog forward, 5′-CAGCTGTGTGTACTCAATG ATAGATTT-3′ and
reverse, 5′-ACACCATTGCATTTCTTCG GCCAGTTG-3′; octamer-binding
transcription factor 4 (OCT4) forward, 5′-GACAACAATGAAAATCTTCAGG
AG-3′ and reverse, 5′-CTGGCGCCGGTTACAGAACCA-3′; SMAD2 forward,
5′-GTTCCTGCCTTTGCTGAGAC-3′ and reverse, 5′-TCTCTTTGCCAGGAATGCTT-3′;
vimentin forward, 5′-CAACCTGGCCGAGGACAT-3′ and reverse,
5′-ACGCATTGTCAACATCCTGTCT-3′; ZNF746 forward,
5′-GCTGCACACCGGCGAGCGGCCC-3′ and reverse, 5′-CT
TGCGGAGGTGGTCCTTGCG-3′; GAPDH forward, 5′-CCA CTCCTCCACCTTTGAC-3′
and reverse, 5′-ACCCTGTTGC TGTAGCCA-3′.
Immunofluorescence assay
H460 NSCLC cells transfected with control or ZNF746
siRNA (Bioneer) plasmids were fixed with 4% paraformaldehyde and
permeabilized in 0.1% Triton X-100. The fixed H460 NSCLC cells were
then washed with 1X PBS and blocked with 10% normal goat serum
blocking solution (Zymed Laboratories, Carlsbad, CA, USA) for 30
min at room temperature. Fixed cells were incubated with the
specific primary antibodies against ZNF746 (Sigma-Aldrich, St.
Louis, MO, USA), E-cadherin, β-catenin (Cell Signaling Technology,
Inc., Danvers, MA, USA) overnight at 4°C. After washing, the cells
were incubated with Alexa Fluor 594 goat anti-rabbit IgG
(Invitrogen) for 30 min at room temperature. After washing, the
cells were mounted with Vectashield/DAPI (Vector Laboratories,
Inc., Burlingame, CA, USA) and visualized by a Carl Zeiss LSM5
confocal microscope.
Western blotting
Whole cell lysates from H460 NSCLC cells transfected
with control or ZNF746 siRNA plasmids were prepared using lysis
buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.1%
SDS, 1 mM EDTA, 1 mM Na3VO4, 1 mM NaF and
protease inhibitor cocktail). The concentration of protein was
measured by using a Bio-Rad DC protein assay kit II (Bio-Rad,
Hercules, CA, USA). The proteins were separated on 8 or 12%
Bis-Tris Gels (Invitrogen). Hybond ECL transfer membrane (GE
Healthcare Bio-Sciences, Piscataway, NJ, USA) was used to transfer
the gel. The membranes were blocked with 5% nonfat dry milk.
Primary antibodies were used for detecting ZNF746 (Sigma-Aldrich),
E-cadherin, β-catenin (Cell Signaling Technology, Inc.), Twist
(Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), and β-actin
(Sigma-Aldrich). Secondary antibody was used with horseradish
peroxidase (HRP)-conjugated secondary antibodies. Enhanced
chemiluminescence (ECL) system (Amersham Pharmacia Biotech, Inc.,
Piscataway, NJ, USA) was used.
Results
Silencing of ZNF746 suppresses the
invasive property in H460 NSCLC cells
To investigate the role of ZNF746 in tumorigenesis,
the effect of the silencing of ZNF746 was evaluated on the invasion
of H460 NSCLC cells transfected with ZNF746 siRNA plasmid. Boyden
chamber invasion assay was performed to examine the effect on the
invasive activity of ZNF746. Knockdown of ZNF746 significantly
inhibited the invasive property compared to untreated control in
H460 NSCLC cells (Fig. 1A and B).
Western blot assay showed that ZNF746 was efficiently inhibited by
siRNA transfection (Fig. 1C).
Silencing of ZNF746 attenuates the
expression of MMP1, MMP2 and MMP9, but not MMP7, in H460 NSCLC
cells by RT-qPCR
MMPs promote tumor survival, invasion as well as
metastasis (13). In this regard,
we evaluated whether ZNF746 depletion regulates the expression of
MMPs (MMP1, MMP2, MMP7 and MMP9) in H460 NSCLC cells by RT-qPCR.
The depletion of ZNF746 attenuated the expression of MMP1, MMP2 and
MMP9, but not MMP7 (Fig. 2).
Silencing of ZNF746 upregulates
E-cadherin and β-catenin in H460 NSCLC cells by western blotting
and immunohistochemistry
EMT is a key phenotype by which cancer cells acquire
motility and invasion (4,8). In this regard, to explore whether or
not ZNF746 is associated with EMT in H460 NSCLC cells, we examined
the biomarkers of epithelial and mesenchymal phenotypes after
ZNF746 siRNA knockdown in H460 NSCLC cells by western blotting.
ZNF746 knockdown enhanced the expression of E-cadherin and
β-catenin as epithelial markers, while the expression of Slug was
suppressed as a mesenchymal marker (Fig. 3A). Consistently,
immunohistochemistry revealed that the silencing of ZNF746 reduced
the red color expression for β-catenin and E-cadherin in H460 NSCLC
cells (Fig. 3B).
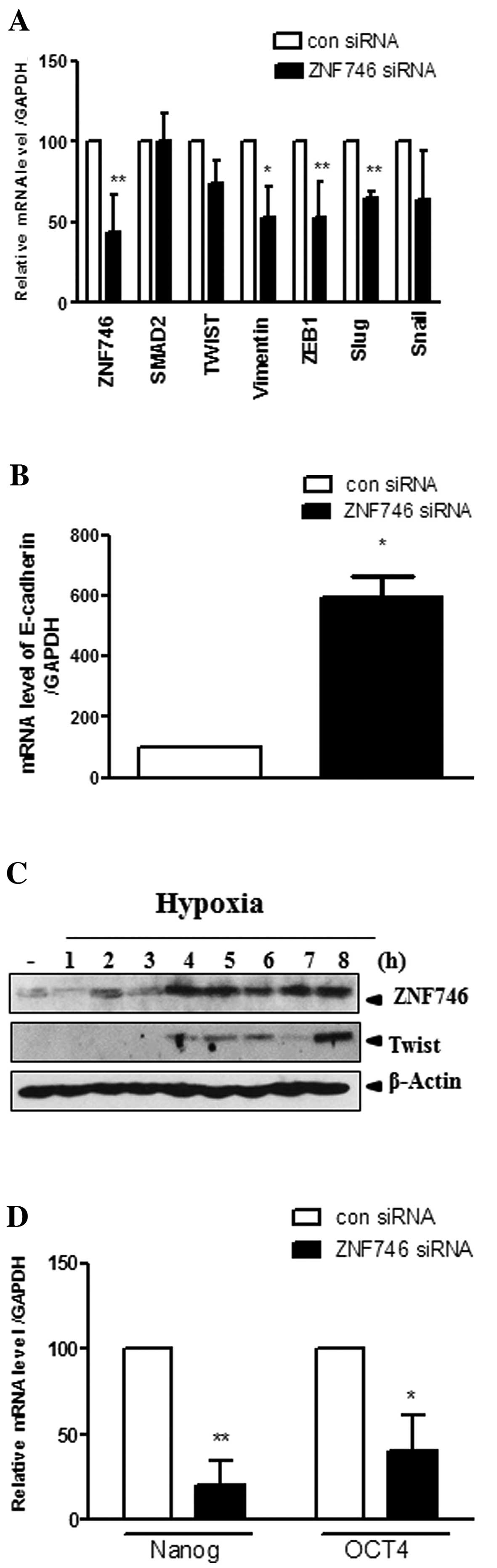
Silencing of ZNF746 upregulates EMT
molecules at the mRNA level in H460 NSCLC cells and hypoxia
enhances the protein expression of ZNF746 in parallel with
Twist
To verify that ZNF746 regulates EMT molecules at the
mRNA level, RT-qPCR analysis was performed. Expression of
epithelial marker (E-cadherin), mesenchymal marker (vimentin) or
transcriptional factors such as ZEB1, Twist, Snail and Slug, was
evaluated in ZNF746 siRNA-transfected H460 NSCLC cells. ZNF746
knockdown downregulated the expression of Twist, vimentin, ZEB1,
and Slug, but not SMAD2, and Snail, and also upregulated the
expression of E-cadherin in H460 NSCLC cells (Fig. 4A and B).
Hypoxia accelerates the cell invasion (14) and plays an important role in EMT
(15). To confirm that ZNF746 or
Twist can be induced under hypoxia, western blotting was carried
out in H460 NSCLC cells. The expression of ZNF746 was induced from
2 to 8 h in hypoxic H460 NSCLC cells (Fig. 4C). Of note, Twist, one of the EMT
molecules, was also induced from 4 to 8 h in hypoxic H460 NSCLC
cells almost in parallel with ZNF746.
Silencing of ZNF746 significantly
suppresses the expression of Nanog and OCT4 at the mRNA level in
H460 NSCLC cells
Although Nanog and OCT4 are transcriptional factors
in embryonic stem cells (16), they
have been studied in cell metastasis through the EMT process
(17,18). In this regard, we determined the
mRNA expression of OCT4 and Nanog by RT-qPCR following ZNF746 siRNA
transfection. OCT4 and Nanog were significantly suppressed at the
transcriptional level in ZNF746 siRNA-treated H460 NSCLC cells
(Fig. 4D).
Discussion
Lung cancer is generally classified into small cell
lung carcinoma (SCLC) and non-small cell lung carcinoma (NSCLC).
The lung is a common metastatic site from other parts of the body
and primary lung cancer usually metastasizes to the brain, bones,
liver and adrenal glands (19).
Metastases represent the end products of a multistep
cell-biological process known as the invasion-metastasis cascade in
several types of cancer (20). In
the present study, the role of ZNF746 was examined in the invasion
and EMT in H460 NSCLC cells.
In the present study, invasion assay using Boyden
chamber revealed that ZNF746 knockdown significantly reduced the
number of the invaded H460 NSCLC cells. It is well documented that
several MMP family members such as MMP1 (21), MMP2 (22), MMP7 (23) and MMP9 (24) are involved in invasion and
metastasis. RT-qPCR analysis showed that the silencing of ZNF746
attenuated the expression of MMP1, MMP2 and MMP 9, but not MMP7, in
H460 NSCLC cells, indicating that the ZNF746 knockdown can suppress
the invasion of H460 NSCLC cells, since ZNF746 may mediate
invasion-metastasis cascades.
Accumulating evidence suggests that EMT is a
critical event in tumor invasion and metastasis (3,4,25).
Thus, activation of the EMT is known to endow the invasive and
metastatic properties upon cancer cells for the successful
colonization of distal target organs (26). Previous studies also revealed that
EMT is crucial in drug resistance to EGFR inhibitor in NSCLC
(27) and promotes the cell
invasion and metastatic property. NSCLC cells expressing epithelial
phenotype are more sensitive to EGFR inhibition than NSCLC lines
expressing mesenchymal phenotype such as vimentin (28–30).
Here, western blotting showed that E-cadherin and β-catenin as
epithelial phenotypes were upregulated, while Slug as a mesenchymal
phenotype was downregulated in ZNF746 siRNA vector-transfected H460
NSCLC cells compared to untreated control, indicating that the
silencing of ZNF746 inhibits invasion-metastasis cascades via
regulation of EMT molecules. Accordingly, mRNA expression of
E-cadherin was upregulated, while that of vimentin or Slug, Twist,
ZEB was also attenuated at the mRNA level in ZNF746
siRNA-transfected H460 NSCLC cells, strongly demonstrating that the
silencing of ZNF746 favorably regulates EMT molecules at the mRNA
and protein levels in H460 NSCLC cells.
Hypoxia plays an important role in the progression
and metastasis of cancer (31).
Also, hypoxia inducible factor (HIF) α regulates transcriptional
regulators of EMT such as Twist (32)and ZEB1 (33–35).
In several cancer cell lines, N-cadherin, vimentin and fibronectin
are upregulated in hypoxia exposure (32). Similarly, ZNF746 was induced by
hypoxia in parallel with Twist expression in H460 NSCLC cells. In
addition, several studies showed that transcriptional marker Nanog
and OCT4 promoted malignancy and metastasis in lung adenocarcinoma
(18), melanoma (16) and colorectal (17) cancer. Here, ZNF746 siRNA-transfected
H460 NSLC cells suppressed the expression of Nanog and OCT4 in H460
NSCLC cells, indicating that ZNF746 knockdown may possibly regulate
lung cancer progression and metastasis mediated by Nanog and
OCT4.
In summary, the silencing of ZNF746 suppressed the
invasion by inhibition of MMP1, MMP2 and MMP9, upregulated
E-cadherin and β-catenin as well as downregulated Slug at the
protein level, consistently enhanced E-cadherin and attenuated the
expression of EMT key transcriptional factors such as vimentin,
Slug, Twist and ZEB, suppressed the expression of Nanog and OCT4 in
H460 NSCLC cells. Also, ZNF746 was induced by hypoxia in parallel
with Twist expression in H460 NSCLC cells. Overall, our findings
suggest that ZNF746 may be a critical target molecule in the
invasion and EMT process in H460 NSCLC cells. Nevertheless, animal
studies should be performed in the future to further validate our
in vitro evidence.
Acknowledgements
This study was supported by the National Research
Foundation of Korea (NRF) grant funded by the Korean government
(MEST) (no. 2012-0005755).
References
|
1
|
Lee W, Jiang Z, Liu J, Haverty PM, Guan Y,
Stinson J, Yue P, Zhang Y, Pant KP, Bhatt D, Ha C, Johnson S,
Kennemer MI, Mohan S, Nazarenko I, Watanabe C, Sparks AB, Shames
DS, Gentleman R, de Sauvage FJ, Stern H, Pandita A, Ballinger DG,
Drmanac R, Modrusan Z, Seshagiri S and Zhang Z: The mutation
spectrum revealed by paired genome sequences from a lung cancer
patient. Nature. 465:473–477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Walker S: Updates in non-small cell lung
cancer. Clin J Oncol Nurs. 12:587–596. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oyanagi J, Ogawa T, Sato H, Higashi S and
Miyazaki K: Epithelial-mesenchymal transition stimulates human
cancer cells to extend microtubule-based invasive protrusions and
suppresses cell growth in collagen gel. PLoS One. 7:e532092012.
View Article : Google Scholar
|
|
4
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tse JC and Kalluri R: Mechanisms of
metastasis: epithelial-to-mesenchymal transition and contribution
of tumor microenvironment. J Cell Biochem. 101:816–829. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Patel SA, Dave MA, Murthy RG, Helmy KY and
Rameshwar P: Metastatic breast cancer cells in the bone marrow
microenvironment: novel insights into oncoprotection. Oncol Rev.
5:93–102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kokkinos MI, Wafai R, Wong MK, Newgreen
DF, Thompson EW and Waltham M: Vimentin and epithelial-mesenchymal
transition in human breast cancer-observations in vitro and in
vivo. Cells Tissues Organs. 185:191–203. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thomson S, Petti F, Sujka-Kwok I, Mercado
P, Bean J, Monaghan M, Seymour SL, Argast GM, Epstein DM and Haley
JD: A systems view of epithelial-mesenchymal transition signaling
states. Clin Exp Metastasis. 28:137–155. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peinado H, Portillo F and Cano A:
Transcriptional regulation of cadherins during development and
carcinogenesis. Int J Dev Biol. 48:365–375. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang SP, Wang WL, Chang YL, Wu CT, Chao
YC, Kao SH, Yuan A, Lin CW, Yang SC, Chan WK, Li KC, Hong TM and
Yang PC: p53 controls cancer cell invasion by inducing the
MDM2-mediated degradation of Slug. Nat Cell Biol. 11:694–704. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khoo TK: Parkin inactivation via PARIS
(ZNF746) may lead to neurodegeneration in Parkinson’s disease. Mov
Disord. 26:7722011.PubMed/NCBI
|
|
12
|
Shin JH, Ko HS, Kang H, Lee Y, Lee YI,
Pletinkova O, Troconso JC, Dawson VL and Dawson TM: PARIS (ZNF746)
repression of PGC-1α contributes to neurodegeneration in
Parkinson’s disease. Cell. 144:689–702. 2011.PubMed/NCBI
|
|
13
|
Zheng H, Takahashi H, Murai Y, Cui Z,
Nomoto K, Niwa H, Tsuneyama K and Takano Y: Expressions of MMP-2,
MMP-9 and VEGF are closely linked to growth, invasion, metastasis
and angiogenesis of gastric carcinoma. Anticancer Res.
26:3579–3583. 2006.PubMed/NCBI
|
|
14
|
Miyoshi A, Kitajima Y, Ide T, Ohtaka K,
Nagasawa H, Uto Y, Hori H and Miyazaki K: Hypoxia accelerates
cancer invasion of hepatoma cells by upregulating MMP expression in
an HIF-1α-independent manner. Int J Oncol. 29:1533–1539.
2006.PubMed/NCBI
|
|
15
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Borrull A, Ghislin S, Deshayes F, Lauriol
J, Alcaide-Loridan C and Middendorp S: Nanog and Oct4
overexpression increases motility and transmigration of melanoma
cells. J Cancer Res Clin Oncol. 138:1145–1154. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dai X, Ge J, Wang X, Qian X, Zhang C and
Li X: OCT4 regulates epithelial-mesenchymal transition and its
knockdown inhibits colorectal cancer cell migration and invasion.
Oncol Rep. 29:155–160. 2013.PubMed/NCBI
|
|
18
|
Chiou SH, Wang ML, Chou YT, Chen CJ, Hong
CF, Hsieh WJ, Chang HT, Chen YS, Lin TW, Hsu HS and Wu CW:
Coexpression of Oct4 and Nanog enhances malignancy in lung
adenocarcinoma by inducing cancer stem cell-like properties and
epithelial-mesenchymal transdifferentiation. Cancer Res.
70:10433–10444. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li M, Zhou M, Gong M, Ma J, Pei F, Beamer
WG, Shultz LD, Hock JM and Yu X: A novel animal model for bone
metastasis in human lung cancer. Oncol Lett. 3:802–806.
2012.PubMed/NCBI
|
|
20
|
Valastyan S and Weinberg RA: Tumor
metastasis: molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu H, Kato Y, Erzinger SA, Kiriakova GM,
Qian Y, Palmieri D, Steeg PS and Price JE: The role of MMP-1 in
breast cancer growth and metastasis to the brain in a xenograft
model. BMC Cancer. 12:5832012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rojiani MV, Alidina J, Esposito N and
Rojiani AM: Expression of MMP-2 correlates with increased
angiogenesis in CNS metastasis of lung carcinoma. Int J Clin Exp
Pathol. 3:775–781. 2010.PubMed/NCBI
|
|
23
|
Okayama H, Kumamoto K, Saitou K, Hayase S,
Kofunato Y, Sato Y, Miyamoto K, Nakamura I, Ohki S, Sekikawa K and
Takenoshita S: CD44v6, MMP-7 and nuclear Cdx2 are significant
biomarkers for prediction of lymph node metastasis in primary
gastric cancer. Oncol Rep. 22:745–755. 2009.PubMed/NCBI
|
|
24
|
Li Y, Liu W, Fang L, Nan J, Zhang Z and
Zhou Q: Chemokine receptor 7 induces metastasis of NSCLC via
upregulating MMP-9 expression. Zhongguo Fei Ai Za Zhi.
13:1016–1020. 2010.(In Chinese).
|
|
25
|
Hugo H, Ackland ML, Blick T, Lawrence MG,
Clements JA, Williams ED and Thompson EW: Epithelial - mesenchymal
and mesenchymal - epithelial transitions in carcinoma progression.
J Cell Physiol. 213:374–383. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao D, Vahdat LT, Wong S, Chang JC and
Mittal V: Microenvironmental regulation of epithelial-mesenchymal
transitions in cancer. Cancer Res. 72:4883–4889. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sequist LV, Waltman BA, Dias-Santagata D,
Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger
S, Cosper AK, Akhavanfard S, Heist RS, Temel J, Christensen JG,
Wain JC, Lynch TJ, Vernovsky K, Mark EJ, Lanuti M, Iafrate AJ,
Mino-Kenudson M and Engelman JA: Genotypic and histological
evolution of lung cancers acquiring resistance to EGFR inhibitors.
Sci Transl Med. 3:75ra262011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thomson S, Buck E, Petti F, Griffin G,
Brown E, Ramnarine N, Iwata KK, Gibson N and Haley JD: Epithelial
to mesenchymal transition is a determinant of sensitivity of
non-small-cell lung carcinoma cell lines and xenografts to
epidermal growth factor receptor inhibition. Cancer Res.
65:9455–9462. 2005. View Article : Google Scholar
|
|
29
|
Rho JK, Choi YJ, Lee JK, Ryoo BY, Na II,
Yang SH, Kim CH and Lee JC: Epithelial to mesenchymal transition
derived from repeated exposure to gefitinib determines the
sensitivity to EGFR inhibitors in A549, a non-small cell lung
cancer cell line. Lung Cancer. 63:219–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yauch RL, Januario T, Eberhard DA, Cavet
G, Zhu W, Fu L, Pham TQ, Soriano R, Stinson J, Seshagiri S,
Modrusan Z, Lin CY, O’Neill V and Amler LC: Epithelial vs.
mesenchymal phenotype determines in vitro sensitivity and predicts
clinical activity of erlotinib in lung cancer patients. Clin Cancer
Res. 11:8686–8698. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moen I, Oyan AM, Kalland KH, Tronstad KJ,
Akslen LA, Chekenya M, Sakariassen PO, Reed RK and Stuhr LE:
Hyperoxic treatment induces mesenchymal-to-epithelial transition in
a rat adenocarcinoma model. PLoS One. 4:e63812009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim HM, Haraguchi N, Ishii H, Ohkuma M,
Okano M, Mimori K, Eguchi H, Yamamoto H, Nagano H, Sekimoto M, Doki
Y and Mori M: Increased CD13 expression reduces reactive oxygen
species, promoting survival of liver cancer stem cells via an
epithelial-mesenchymal transition-like phenomenon. Ann Surg Oncol.
19(Suppl 3): S539–S548. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yoo YG, Christensen J, Gu J and Huang LE:
HIF-1α mediates tumor hypoxia to confer a perpetual mesenchymal
phenotype for malignant progression. Sci Signal. 4(pt4)2011.
|
|
34
|
Salnikov AV, Liu L, Platen M, Gladkich J,
Salnikova O, Ryschich E, Mattern J, Moldenhauer G, Werner J,
Schemmer P, Buchler MW and Herr I: Hypoxia induces EMT in low and
highly aggressive pancreatic tumor cells but only cells with cancer
stem cell characteristics acquire pronounced migratory potential.
PLoS One. 7:e463912012. View Article : Google Scholar
|
|
35
|
Sun S, Ning X, Zhang Y, Lu Y, Nie Y, Han
S, Liu L, Du R, Xia L, He L and Fan D: Hypoxia-inducible
factor-1alpha induces Twist expression in tubular epithelial cells
subjected to hypoxia, leading to epithelial-to-mesenchymal
transition. Kidney Int. 75:1278–1287. 2009. View Article : Google Scholar : PubMed/NCBI
|