Introduction
Prostate cancer (PCa) is a common malignancy
associated with high morbidity and mortality. Radical prostatectomy
combined with pharmacological androgen ablation is effective during
the initial stages of the disease, yet the cancer often quickly
progresses to form distant metastases for which no known effective
therapy is available. The etiology of metastatic PCa remains poorly
understood.
The process of epithelial-mesenchymal transition
(EMT) is an important pathological process in tumor progression.
When cancer cells interact with the surrounding mesenchymal
environment, they lose epithelial cadherins, and aberrantly express
certain mesenchymal proteins, causing them to transform into
spindle-shape cells with stronger migrating capability (1). Previous studies have verified that EMT
is one of the crucial processes through which cancer cells migrate
from the primary microenvironment to distant sites (2,3). It
may also explain the process by which tumors evade host immune
surveillance (3,4) and acquire resistance to chemotherapy
(5–7).
It is generally accepted that tumors are composed of
a heterogeneous population of cells, among which only a small
proportion, behaving as stem cells, have infinite self-renewal
capacity, together with unlimited proliferation and differentiation
potential. This subset of cells is highly efficient at propagating
tumor progression, and they act functionally as cancer stem cells
(CSCs) (8). CSCs have been shown to
be involved in tumor progression, disease recurrence and
therapeutic resistance (9).
EMT and CSCs act together to promote tumor
progression. Several studies have established a crucial link
between occurrence of tumor EMT and acquisition of stem cell-like
properties (10,11). However, the underlying correlation
between these factors remains to be elucidated. In a previous
study, we showed that hypoxia-inducible factor-1α (HIF-1α)
triggered EMT in human PCa cells (12). Here, we further compared the EMT
activity of prostate CSCs with that of bulk population cells
subjected to HIF-1α transfection. Based on these findings, we
attempted to define relationships between EMT and tumor
heterogeneity, by identifying the possible ‘cell carriers’ involved
in EMT, and thereby develop a potential cell target to suppress EMT
progression.
Materials and methods
Cell culture
Human PCa cell lines (LNCaP, PC-3, Du145, TSU-PrL
and IA8) were maintained in Dulbecco’s modified Eagle’s medium
(DMEM) containing 1 mM sodium pyruvate, 2.5 mM glutamine, 10% fetal
bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin.
Stem-like population cells were cultured in keratinocyte medium
(ScienCell, Carlsbad, CA, USA) supplemented with 20 ng/ml epidermal
growth factor (Sigma), 50 μg/ml bovine pituitary extract (Sigma), 2
ng/ml leukemia inhibitory factor (Sigma), 2 ng/ml stem cell factor
(Sigma) and 100 ng/ml cholera toxin (Sigma). All cells were
aseptically handled and grown at 37°C in a humidified incubator
containing 5% CO2. Cells transfected with HIF-1α were
grown in an atmosphere of 1% oxygen.
Fluorescence-activated cell sorting
(FACS)
Combinations of fluorochrome-conjugated monoclonal
antibodies against human CD133 and CD44 (Miltenyi, Bergisch
Gladbach, Germany) and the respective isotype controls were added
to the cell suspension and incubated at 4°C in the dark for 15–30
min. After washing and fixing in PBS, the labeled cells were
analyzed by flow cytometry. Gates were determined by analysis of
double staining. Cells were also harvested for Hoechst 33342
staining, and verapamil (Sigma), an inhibitor of the ABCG2
transporter, was used to identify specific cell populations. A
350-nm UV laser was used to excite Hoechst 33342.
Analysis was performed using dual-wavelength
analysis (blue, 424 nm; red, 675 nm). The side population was
identified by gating the characteristic fluorescence emission
profile. Equal numbers of side population (SP) and non-SP cells
(NSP) were collected for the following experiments.
Colony formation assay
Colony formation assays were performed to evaluate
the cell self-renewal potential in vitro. Briefly, 1,000
cells were suspended in DMEM containing 0.4% agarose, and overlaid
onto a 6-cm dish (Corning Incorporated Corning, NY, USA). Cells
were incubated for 1–3 weeks until colonies were visible. Only
colonies with >32 cells were scored. Colony formation efficiency
(CFE) was determined by the percentage of cells that initiated a
clone.
HIF-1α cDNA transfection
Recombinant plasmid pcDNA3.1(−)/HIF-1α was
transfected into stem-like SP cells and homologous non-SP cells
using the Lipofectamine 2000 system (Life Technologies, Carlsbad,
CA, USA). The cells were then cultured in medium containing 400
μg/ml G418. After 4 weeks of selection, all non-transfected cells
died and separate clones were visible in the transfected cells.
These clones were further expanded using 200 μg/ml G418.
Western blotting
Clarified protein lysates were electrophoretically
resolved on denaturing SDS-PAGE and electro-transferred onto
nitrocellulose membranes. The membranes were initially incubated
with 5% non-fat dry milk in TBS for 2 h, and then probed with the
primary antibodies (all from Santa Cruz Biotechnology, Santa Cruz,
CA, USA) against various proteins at 4°C overnight. The proteins
tested were ABCG2, integrin α2, OCT4, CD44, Nanog, nestin (as
stemness markers), HIF-1α (as an EMT inducer), Glut-1 and VEGF (as
HIF-1α target proteins), E-cadherin and CK18 (as epithelial
markers), N-cadherin, fibronectin, vimentin, cathepsin D, MMP-2 and
uPAR (as mesenchymal markers) and GAPDH (as an internal
control).
The membranes were hybridized with an appropriate
horseradish peroxidase (HRP)-conjugated secondary antibody (Boshide
Co., Ltd., Hebei, China) for 2 h at room temperature. An enhanced
chemiluminescence system (Amresco, Solon, OH, USA) was used to
detect the immunopositive protein bands.
In vitro invasion assay
Polycarbonate filters (8-μm) (Millipore, Billerica,
MA, USA) were coated with 50 μg/cm2 of reconstituted
Matrigel (Sigma). Fifty thousand cells in 300 μl of serum-free
growth medium were seeded into the upper chamber. Cells were
incubated in normoxic conditions and allowed to migrate toward the
complete growth medium for 24, 48 and 72 h. Non-invading cells were
removed mechanically using cotton swabs, and the migrated cells
located on the lower surface were fixed with methanol.
The number of migrating cells was determined by
counting 10 high-power fields of view on each membrane. Data are
presented as the mean number of cells per field. Each experiment
was repeated three times.
MTT assay
Cells (1,000/well) were placed into 96-well plates.
At various time-points, MTT solution was added and incubated for an
additional 4 h. Cell-associated MTT crystals were dissolved in
DMSO. The color intensity was measured at 570 nm using a Bio-Rad
Technologies microplate reader.
Xenograft experiments
Surgery was performed under sodium pentobarbital
anesthesia, and all efforts were made to ameliorate suffering.
Six-week-old male nude mice (Charles River Laboratories
International) were injected subcutaneously with 5×105
cells in the left lateral flank. Animals (15 per group) were
monitored daily, and tumor volumes were determined by the formula:
V = (LW2)/2, where L is the length and W is the width.
Eight weeks later, tumors and other organs were fixed overnight at
4°C in 4% paraformaldehyde and embedded in paraffin for
histological analysis. Tumor tissue cells were used for primary
culture in the subsequent experiments.
This part of the study was carried out in strict
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals in the Weatherall Report. In addition,
the protocol was approved by the Committee on the Ethics for Animal
Experiments of the Capital Medical University.
Immunohistochemical staining
Paraformaldehyde-fixed cells and paraffin-embedded
sections of xenograft cancer tissues were used for either H&E
or immunostaining. Primary antibodies against stem cell markers
(ABCG2, OCT4, integrin α2, Nanog, CD44 or nestin) and EMT markers
(E-cadherin, vimentin, MMP-2, α-SMA and cathepsin D) were used.
Immunohistochemical analysis was carried out using a commercially
available kit (Boshide). Positive and negative controls were used
throughout all immunostaining protocols.
Statistical analysis
Statistical analysis was carried out using the
Statistical Package for Social Sciences (SPSS), version 13.0, for
Windows. Data are presented as means ± standard deviations (SD).
Student’s t-tests were used to compare results between the
different experimental groups. A value of P<0.05 was considered
to indicate a statistically significant result.
Results
Evidence for stem-like cells in the LNCaP
cell line
According to a previous finding (13), cells with the phenotype of
CD133+α2β1highCD44+
represent typical CSCs in primary PCa tissues. However, we observed
that there were few CD133+CD44+ cells
detected in the 5 PCa cell lines examined (Fig. 1A). Therefore, we used SP sorting to
isolate stem-like cells (Fig. 1B),
and found that although almost no SP cells were detected in the
PC-3 cells, we were able to obtain SP cells from each of the other
4 cell lines. The different percentages of SP cells were 0.15±0.02%
in Du145, 0.6±0.07% in IA8, 0.8±0.1% in LNCaP and 2.0±0.4% in
TSU-PrL cells.
SP cells from the IA8, LNCaP and TSU-PrL cell lines
sustained holoclone growth and formed characteristic compact round
clones, whereas most NSP cells exhibited elongated or flattened
shapes and grew in the pattern of a scattered paraclone (Fig. 2A). SP cells in the Du145 cell line
also grew as a tight holoclone, but they were no more clonogenic
than their NSP counterparts.
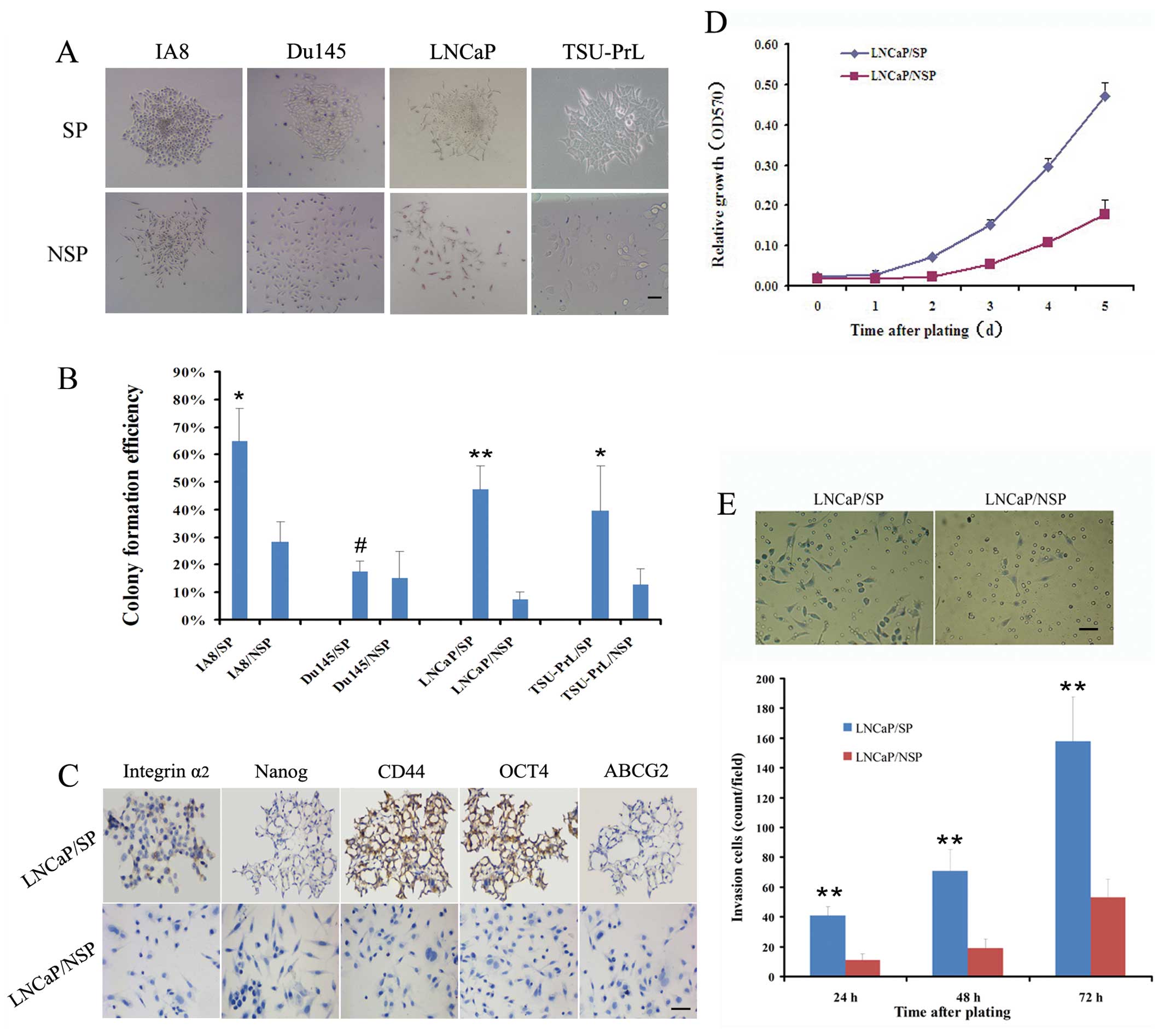 | Figure 2Identification of stem cell
properties in LNCaP/SP cells in vitro. (A) SP and NSP cells
from the IA8, LNCaP and TSU-PrL cell lines exhibited completely
different growth patterns after plating at low density and
continuous culture for 1–3 weeks. Most of the SP cells sustained a
holoclone growth and formed compact round colonies, whereas most
NSP cells grew in a scattered pattern as elongated or flattened
cells. (B) The colony formation efficiency of SP cells from the
IA8, LNCaP and TSU-PrL cell lines was significantly greater than
that of the homologous NSP cells. However, SP cells in the Du145
cell line were no more clonogenic than the NSP cells. (C)
Immunostaining analysis indicated that LNCaP/SP cells were
specifically positive for stem-cell property marker expression
(integrin α2, Nanog, CD44, OCT4 and ABCG2), when compared with the
LNCaP/NSP cells. In vitro, LNCaP/SP cells exhibited a
significantly higher rate of (D) proliferation and (E) invasion
than LNCaP/NSP cells. Scale bar, 50 μm. #P>0.05,
*P<0.05 and **P<0.01 for SP cells
compared with NSP cells. |
The colony formation efficiency (CFE) (Fig. 2B) of the Du145/SP cells was not
significantly greater than that of the Du145/NSP cells (17.4±10.1
vs. 15.1±11.7%; P>0.05). However, the CFE of IA8/SP cells was
2.3-fold greater than that of the IA8/NSP cells (64.6±12.2 vs.
28.4±19.3%; P<0.05), the CFE of the LNCaP/SP cells was 6.5-fold
greater than that of the LNCaP/NSP cells (47.2±8.6 vs. 7.3±5.9%;
P<0.01) and the CFE of the TSU-PrL/SP cells was 3.1-fold greater
than that of the TSU-PrL/NSP cells (39.4±16.3 vs. 12.7±6.1%;
P<0.05). These data highlight the stemness-associated clonogenic
ability of SP cells derived from the LNCaP, IA8 and TSU-PrL cell
lines.
In a previous study we confirmed that LNCaP is an
EMT-negative (epithelial-type) cell line; whereas IA8 and TSU-PrL
are EMT-positive (mesenchymal-type) cell lines (14). We further assessed the
stemness-associated molecular characteristics in the LNCaP/SP cells
in order to collect EMT-negative stem-like cells for EMT induction
trials. Compared with LNCaP/NSP, LNCaP/SP cells displayed specific
positive expression of stemness markers in immunostaining
detection, including integrin α2, Nanog, CD44 and OCT4 (Fig. 2C).
MTT trials showed that the doubling time of LNCaP/SP
cells was shorter than that of the LNCaP/NSP cells (2 vs. 3 days)
(Fig. 2D), which indicated that the
LNCaP/SP cells proliferated markedly faster. Additionally, the
number of cells that digested Matrigel and migrated through the
filter with 8-μm pores was significantly higher in the LNCaP/SP
group than the migratory cell number in the LNCaP/NSP group at 24,
48 and 72 h after plating (Fig.
2E). These results suggest that stem-like cells (LNCaP/SP) have
a much higher proliferative and invasive potential than their bulk
counterparts (LNCaP/NSP) in vitro.
The in vivo models (Fig. 3A) indicated that the tumor volume of
the LNCaP/SP models was 5.2-fold greater than the tumor volume in
the LNCaP/NSP models (526±53 vs. 100±29 mm3; P<0.01)
(Fig. 3B). LNCaP/SP cells also
showed a noticeably higher rate of tumor incidence (60 vs. 13.3%)
(Fig. 3C) and bone metastasis (26.7
vs. 0%; Fig. 3D) when compared with
the LNCaP/NSP cells. Thus, collectively, LNCaP/SP cells appeared to
represent a sub-population of stem-like cells in the LNCaP cell
line which display a stemness phenotype.
Comparison of EMT activity in LNCaP/SP
and LNCaP/NSP cells
To confirm that transfection of HIF-1α was
functional, we firstly analyzed the expression of HIF-1α and its
downstream proteins (Glut-1 and VEGF) in transfected cells. Western
blot analysis indicated that all three proteins were positively
expressed in the LNCaP/HIF-1α/SP and LNCaP/HIF-1α/NSP cells
(Fig. 4A). EMT-phenotype analysis
showed that although LNCaP/SP and LNCaP/NSP cells were both typical
epithelial-type cells, LNCaP/HIF-1α/SP cells acquired a relatively
complete mesenchymal-type with no evidence of expression of
epithelial markers, E-cadherin or CK18, nor aberrant expression of
mesenchymal proteins, vimentin, N-cadherin, fibronectin, cathepsin
D, MMP-2 and uPAR (Fig. 4B). By
contrast, LNCaP/HIF-1α/NSP cells displayed characteristics of a
partial mesenchymal-type with weak expression of vimentin and
cathepsin D and loss of CK18 (Fig.
4B).
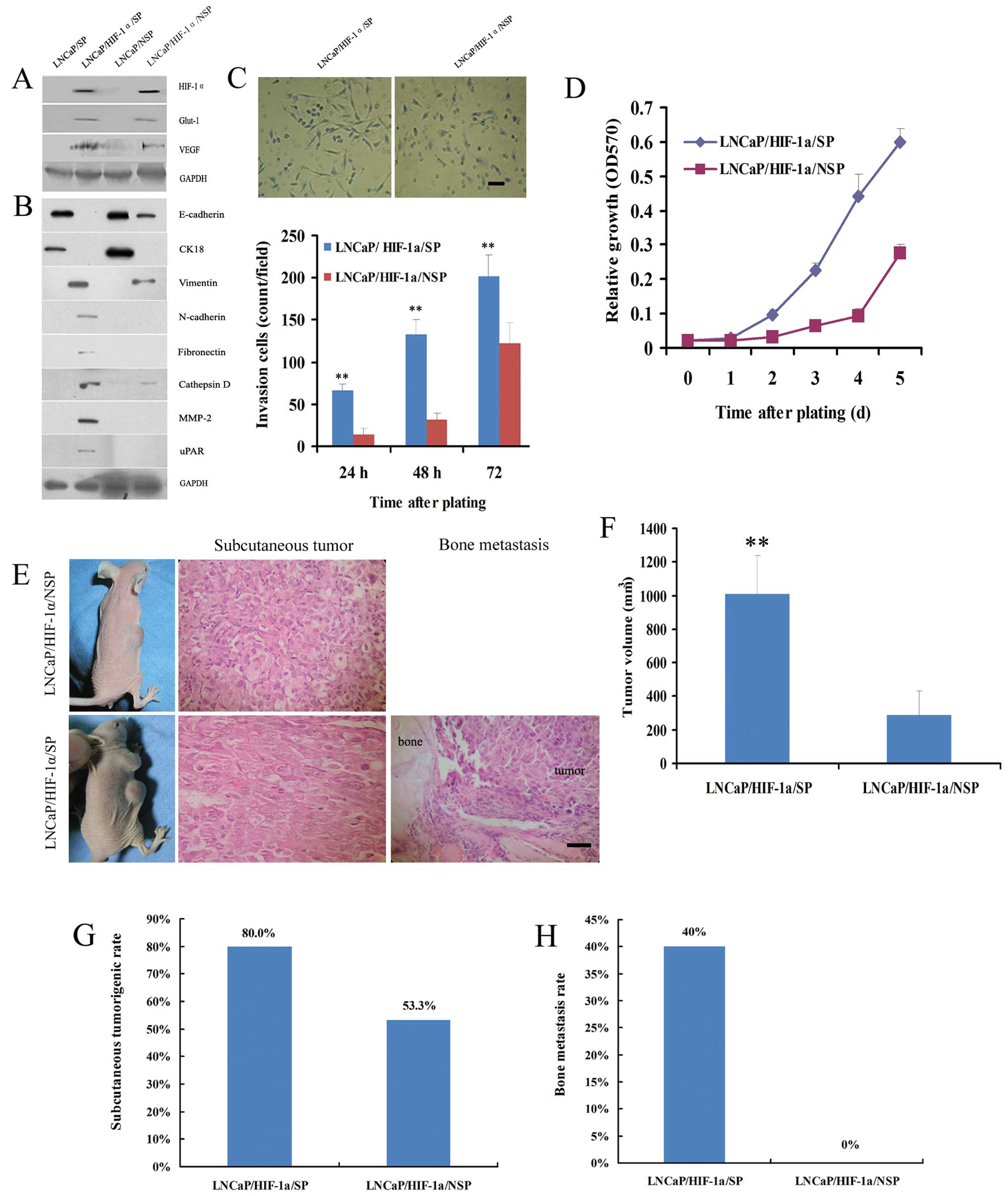 | Figure 4Comparison of EMT activity between
LNCaP/SP and LNCaP/NSP cells. (A) Western blot analysis confirmed
the definitive expression of HIF-1α and its two important
downstream proteins, Glut-1 and VEGF, in stably transfected
LNCaP/HIF-1α/SP and LNCaP/HIF-1α/NSP cells. (B) Although the
LNCaP/SP and LNCaP/NSP cell lines were both epithelial phenotypes,
LNCaP/HIF-1α/SP cells typically acquired a mesenchymal phenotype,
and LNCaP/HIF-1α/NSP cells displayed weak mesenchymal
characteristics. (C) Representative fields of cells that digested
Matrigel and migrated through the chemotaxis filter were
photographed after 48 h incubation in Transwells. The number of
LNCaP/HIF-1α/SP cells penetrating through the filter was markedly
higher than the number of LNCaP/HIF-1α/NSP cells after 24, 48 and
72 h of incubation. (D) LNCaP/HIF-1α/SP cells propagated much
faster than LNCaP/HIF-1α/NSP cells when plated at low density. (E)
After subcutaneously injection, LNCaP/HIF-1α/SP and
LNCaP/HIF-1α/NSP cells both formed neoplasias, while bone
metastasis was only observed in the LNCaP/HIF-1α/SP xenograft
models. Further analysis showed that injection of LNCaP/HIF-1α/SP
cells generated tumors of much greater volume (F), and at a much
higher rate of tumorigenesis (G), than the LNCaP/HIF-1α/NSP cells.
(H) The rate of bone metastasis in the LNCaP/HIF-1α/SP group was
also noticeably higher than that in the LNCaP/HIF-1α/NSP group,
which produced no visible bone metastases. Scale bar, 50 μm.
**P<0.01 for LNCaP/HIF-1α/SP models compared with
LNCaP/HIF-1α/NSP models. |
In the Transwell invasion assays, the number of
LNCaP/HIF-1α/SP cells penetrating through the Matrigel-coated
filter was noticeably higher than the number of LNCaP/HIF-1α/NSP
cells after plating for 24, 48 and 72 h (Fig. 4C). MTT experiments showed that the
doubling time of LNCaP/HIF-1α/SP cells was shorter than that of
LNCaP/HIF-1α/NSP cells (2 vs. 3 days) (Fig. 4D).
Transfected cells were used in xenograft experiments
to validate the influence of the EMT phenotype on tumor growth and
distant metastases. Pathological examination verified that both
LNCaP/HIF-1α/SP and LNCaP/HIF-1α/NSP cells generated macroscopic
tumors subcutaneously in nude mice, but only LNCaP/HIF-1α/SP cells
resulted in bone metastasis (Fig.
4E).
Further analysis demonstrated that the volume of
subcutaneous tumors produced from the LNCaP/HIF-1α/SP cells was
3.5-fold greater than that of tumors produced from the
LNCaP/HIF-1α/NSP cells (1,008±230 vs. 288±145 mm3;
P<0.01) (Fig. 4F). The rate of
tumorigenesis resulting from LNCaP/HIF-1α/SP cells was also much
higher than that noted with LNCaP/HIF-1α/NSP cells (80 vs. 53%)
(Fig. 4G).
We also demonstrated that LNCaP/HIF-1α/SP cells were
able to induce conspicuous bone metastasis, whereas
LNCaP/HIF-1α/NSP did not result in bone metastasis at the
experimental end point (40 vs. 0%) (Fig. 4H). These findings suggest that
LNCaP/SP cells are more likely to acquire mesenchymal and
aggressive phenotypes than LNCaP/NSP cells.
Identification of the EMT phenotype in
the PCa xenograft tumors
Immunohistochemical staining indicated that
xenografted tumors generated by LNCaP/SP and LNCaP/NSP cells both
displayed characteristics of an epithelial phenotype with
expression of E-cadherin and loss of mesenchymal markers (vimentin,
MMP-2, α-SMA and β-catenin) (Fig.
5). By contrast, tumors generated by LNCaP/HIF-1α/SP cells
displayed a strong mesenchymal phenotype characterized by loss of
E-cadherin and acquisition of mesenchymal markers. Xenografts from
the LNCaP/HIF-1α/NSP models also showed mesenchymal phenotype
because of their abundant expression of MMP-2 and weak expression
of β-catenin and vimentin, even though they showed low expression
of E-cadherin. These findings indicated that xenograft tumor
tissues produced by the injection LNCaP/SP cells were suitable for
isolating stem-like cells with an epithelial phenotype.
Evidence for stem-like cells in human PCa
xenograft tissues
Tumor cells in subcutaneous xenografts produced from
LNCaP/SP cells were cultured and used for secondary SP sorting. The
isolated SP cells, and their non-SP counterparts were referred to
as LNCaP/SP2 and LNCaP/NSP2, respectively.
FACS indicated that LNCaP/SP2 cells represented
~1.1±0.3% of the total cell population (Fig. 6A). These cells grew in a typical
holoclone pattern and produced much higher numbers in regards to
CFE than LNCaP/NSP2 cells (68.35±9.67 vs. 10.60±3.4%; P<0.01)
(Fig. 6B). Stemness markers,
including ABCG2, OCT4, integrin α2, Nanog and CD44, were positively
expressed in the LNCaP/SP2 cells and negatively expressed in the
LNCaP/NSP2 cells (Fig. 6C).
Additionally, LNCaP/SP2 cells exhibited a much
higher rate of subcutaneous tumorigenesis (100 vs. 20%) and bone
metastasis (73.3 vs. 0%) in the nude mouse xenograft models when
compared with the LNCaP/NSP2 cells (Fig. 6D). This was characterized by a much
greater tumor volume (1470±470 vs. 126±48 mm3;
P<0.01) (Fig. 6E). These
observations suggest that LNCaP/SP2 cells represent a stem-like
cell subpopulation of LNCaP/SP cells in the PCa xenograft
tissues.
Comparison of the EMT activity between
LNCαP/SP2 and LNCaP/NSP2 cells
Functional transfection of LNCaP/HIF-1α/SP2 cells
with HIF-1α resulted in loss of epithelial markers (E-cadherin and
CK18) and acquisition of mesenchymal markers (vimentin, N-cadherin,
fibronectin, cathepsin D, MMP-2 and uPAR) (Fig. 7A). LNCaP/HIF-1α/NSP2 cells showed a
partial mesenchymal phenotype, with loss of epithelial proteins,
E-cadherin and CK18, and sporadic expression of mesenchymal
proteins, cathepsin D and MMP-2 (Fig.
7B).
LNCaP/HIF-1α/SP2 cells displayed markedly higher
proliferative ability than LNCaP/HIF-1α/NSP2 cells. The doubling
time of LNCaP/HIF-1α/SP2 cells was shorter than that of the
LNCaP/HIF-1α/NSP2 cells (2 vs. 4 days) (Fig. 7C).
The number of LNCaP/HIF-1α/SP2 cells that penetrated
through the Matrigel filter after 24, 48 and 72 h was noticeably
higher than the number of LNCaP/HIF-1α/NSP2 cells, respectively
(Fig. 7D).
Following subcutaneous injection in nude mice,
LNCaP/HIF-1α/SP2 cells exhibited conspicuously higher tumorigenic
and metastatic ability than the LNCaP/HIF-1α/NSP2 cells (Fig. 7E and F). Taken together, these
findings imply that stem-like SP cells from PCa xenograft tissues
possess more active potential than their bulk counterparts to
undergo EMT. This result was consistent with the findings in the
stem-like SP cells from the PCa cell lines.
Discussion
SP cells are a small subset of cells characterized
by exclusion of Hoechst 33342 DNA binding dye. Breast
cancer-resistant protein-1 (BCRP1 or ABCG2) has been identified as
a crucial protein involved in maintaining the SP phenotype. A large
body of evidence indicates that CSCs are enriched in SP cells in
several types of solid carcinomas (15–17).
In the present study, we investigated the underlying relationship
between EMT phenomenon and prostate CSCs, replaced by SP cells.
Reports indicate that the acquisition of the EMT
phenotype and induction of the stemness phenotype are highly
interrelated during tumor progression (18). Many experiments support a ‘twinborn
relationship’ between EMT and CSCs based on common molecular
mechanisms shared by EMT development and CSC generation. For
example, two evolutionarily conserved families, miR-200 and let-7,
have been shown to play a dual role in controlling EMT processes by
direct targeting E-box binding protein ZEB1 and ZEB2 (19–21).
They have also been shown to affect the self-renewal capacity and
stem-like cell signatures of breast cancer cells by regulating the
expression of BMI1 (22,23). It has also been proposed that
several nuclear transcription factors, including β-catenin,
ESE3/EHF and FoxM1, may bidirectionally regulate the EMT process
and the stemness phenotype (24–26).
The signaling pathway mediated by IKKβ/IκBα/RelA, uPAR and Notch-1
have been found to be synchronously involved in the activation of
the EMT process and the acquisition of a stemness phenotype during
tumor progression (27–29).
Other studies describe a ‘parent-child relationship’
between EMT and CSCs based on the observation that EMT promotes the
generation of a stemness phenotype. In a mammary tumor progression
model, the acquisition of stemness characteristics in breast
epithelial cells was found to be driven by EMT induction following
the activation of the Ras-MAPK pathway (30). Additional research demonstrated that
breast cancer cells that have undergone EMT provide a rich source
of stem-like cancer cells. In these experiments, the induction of
EMT in differentiated immortalized human mammary epithelial cells
resulted in acquisition of the CD44+/CD24−
stem cell phenotype (31).
Additionally, our previous study also found that the EMT process
triggered by HIF-1α significantly promoted the stem-like SP cell
proportion in human thyroid cancer FTC133 cells (32).
In the present study, we propose a novel
‘parasite-host relationship’ between EMT and CSCs. This is based on
the findings of Biddle et al that a population of CSCs with
CD44highESAlow phenotype undergo EMT in
squamous cell carcinoma (33). We
further compared differences in EMT initiated by HIF-1α in cancer
stem-like cells and bulk population PCa cells, and confirmed that
stem-like cells are susceptible to EMT.
Previous published findings (31) indicate that putative
CD44+/CD24− CSCs isolated from neoplastic
human breast tissues express high levels of a specific mRNA that
encodes the EMT-associated markers Snail1, Snail2 and Twist. When
compared with parental cells (34),
stemness spheroid-derived head and neck squamous cell carcinoma
cell lines showed significantly increased mRNA levels of
EMT-related transcription genes (Snail1, Snail2 and Twist),
together with increased protein expression of α-SMA and vimentin,
and decreased protein expression of E-cadherin. Collectivelly,
these previously published findings indicate that genetic programs
relevant to EMT are significantly activated in stem-like cells.
They also indicate that CSCs not only represent the prerequisite
for the EMT process, but that they may also form a reservoir of
cells responsible for achieving EMT.
The interrelationship between CSCs and EMT
phenotypic cells represents a potentially promising new therapeutic
target. A growing body of evidence demonstrates a strong link
between the biology of EMT and CSCs in terms of the sequences and
compositions of miRNA molecules, suggesting that targeting these
miRNAs may provide a novel therapeutic strategy. Other researchers
(35) have demonstrated that
antagonism of miR-21 reverses the EMT and CSC phenotype by
inactivating the AKT/ERK1/2 pathways, and thereby relieves the
aggressiveness of breast cancer. Moreover, an in vivo study
(36) showed that miR145 reduces
xenograft tumor growth and metastasis, sensitizes tumors to
chemoradiotherapy, and prolongs patient survival, by inhibiting the
lung adenocarcinoma cell phenotype of EMT and stemness. This was
thought to be achieved by regulating Oct4/Sox2/Fascin1, Tcf4 and
Wnt5a.
It is believed that miR-34a plays a critical
regulatory role in pancreatic cancer progression by regulating CSC
characteristics. Experimental evidence indicates that re-expression
of miR-34α in human pancreatic CSCs not only eliminates CSC
characteristics, but also retards in vitro migration and
invasion of CSCs and enhances the CSC response to chemotherapy
(37). CDF is a novel analogue of
curcumin, which may also play a role as a therapeutic marker, as it
has been shown to attenuate CSC characteristics and suppress CSC
function by targeting an EZH2-miRNA regulatory circuit. These
characteristics resulted in inhibition of pancreatic tumor growth
and a reduction in aggressiveness in vivo (38,39).
In summary, our comparison of EMT activity in
stem-like cells and their counterparts in PCa support the notion
that prostate stem-like cells play a representative ‘cell carrier’
role in EMT progression. This finding may help in the development
of more effective strategies for targeting stemness and EMT, that
may in turn help to suppress local tumor relapse and minimize
distant metastatic spread.
Acknowledgements
This project was supported by the National Natural
Science Foundation of China (NSFC no. 30700968 and 30901725).
References
|
1
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang MH, Wu MZ, Chiou SH, Chen PM, Chang
SY, Liu CJ, Teng SC and Wu KJ: Direct regulation of TWIST by HIF-1α
promotes metastasis. Nat Cell Biol. 10:295–305. 2009.
|
|
3
|
Gjerdrum C, Tiron C, Hoiby T, Stefansson
I, Haugen H, Sandal T, Collett K, Li S, McCormack E, Gjertsen BT,
Micklem DR, Akslen LA, Glackin C and Lorens JB: Axl is an essential
epithelial-to-mesenchymal transition-induced regulator of breast
cancer metastasis and patient survival. Proc Natl Acad Sci USA.
107:1124–1129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kudo-Saito C, Shirako H, Takeuchi T and
Kawakami Y: Cancer metastasis is accelerated through
immunosuppression during Snail-induced EMT of cancer cells. Cancer
Cell. 15:195–206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gupta PB, Onder TT, Jiang G, Tao K,
Kuperwasser C, Weinberg RA and Lander ES: Identification of
selective inhibitors of cancer stem cells by high-throughput
screening. Cell. 138:645–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Skvortsova I, Skvortsov S, Raju U, Stasyk
T, Riesterer O, Schottdorf EM, Popper BA, Schiestl B, Eichberger P,
Debbage P, Neher A, Bonn GK, Huber LA, Milas L and Lukas P:
Epithelial-to-mesenchymal transition and c-myc expression are the
determinants of cetuximab-induced enhancement of squamous cell
carcinoma radioresponse. Radiother Oncol. 96:108–115. 2010.
View Article : Google Scholar
|
|
7
|
Bandyopadhyay A, Wang L, Agyin J, Tang Y,
Lin S, Yeh IT, De K and Sun LZ: Doxorubicin in combination with a
small TGFβ inhibitor: a potential novel therapy for metastatic
breast cancer in mouse models. PLoS One. 5:e103652010.
|
|
8
|
Lobo NA, Shimono Y, Qian D and Clarke MF:
The biology of cancer stem cells. Annu Rev Cell Dev Biol.
23:675–699. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu Z, Pestell TG, Lisanti MP and Pestell
RG: Cancer stem cells. Int J Biochem Cell Biol. 44:2144–2151. 2012.
View Article : Google Scholar
|
|
10
|
Kong D, Banerjee S, Ahmad A, Li Y, Wang Z,
Sethi S and Sarkar FH: Epithelial to mesenchymal transition is
mechanistically linked with stem cell signatures in prostate cancer
cells. PLoS One. 5:e124452010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Turner C and Kohandel M: Investigating the
link between epithelial-mesenchymal transition and the cancer stem
cell phenotype: a mathematical approach. J Theor Biol. 265:329–335.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luo Y, He DL, Ning L, Shen SL, Li L, Li X,
Zhau HE and Chung LW: Over-expression of hypoxia-inducible
factor-1α increases the invasive potency of LNCaP cells in
vitro. BJU Int. 98:1315–1139. 2006.
|
|
13
|
Collins AT, Berry PA, Hyde C, Stower MJ
and Maitland NJ: Prospective identification of tumorigenic prostate
cancer stem cells. Cancer Res. 65:10946–10951. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang YG, Luo Y, He DL, Li X, Zhang LL,
Peng T, Li MC and Lin YH: Role of Wnt/beta-catenin signaling
pathway in epithelial-mesenchymal transition of human prostate
cancer induced by hypoxia-inducible factor-1α. Int J Urol.
14:1034–1039. 2007.
|
|
15
|
Yu SC and Bian XW: Enrichment of cancer
stem cells based on heterogeneity of invasiveness. Stem Cell Rev.
5:66–71. 2007.PubMed/NCBI
|
|
16
|
Oates JE, Grey BR, Addla SK, Samuel JD,
Hart CA, Ramani VA, Brown MD and Clarke NW: Hoechst 33342 side
population identification is a conserved and unified mechanism in
urological cancers. Stem Cells Dev. 18:1515–1522. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou J, Wang H, Cannon V, Wolcott KM, Song
H and Yates C: Side population rather than CD133+ cells
distinguishes enriched tumorigenicity in hTERT-immortalized primary
prostate cancer cells. Mol Cancer. 10:1122011.PubMed/NCBI
|
|
18
|
May CD, Sphyris N, Evans KW, Werden SJ,
Guo W and Mani SA: Epithelial-mesenchymal transition and cancer
stem cells: a dangerously dynamic duo in breast cancer progression.
Breast Cancer Res. 13:2022011. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cano A and Nieto MA: Non-coding RNAs take
centre stage in epithelial-to-mesenchymal transition. Trends Cell
Biol. 18:357–359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gregory PA, Bert AG, Paterson EL, Barry
SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y and Goodall GJ:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Y, VandenBoom TG II, Kong D, Wang Z,
Ali S, Philip PA and Sarkar FH: Up-regulation of miR-200 and let-7
by natural agents leads to the reversal of
epithelial-to-mesenchymal transition in gemcitabine-resistant
pancreatic cancer cells. Cancer Res. 69:6704–6712. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wellner U, Schubert J, Burk UC,
Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D,
zur Hausen A, Brunton VG, Morton J, Sansom O, Schüler J, Stemmler
MP, Herzberger C, Hopt U, Keck T, Brabletz S and Brabletz T: The
EMT-activator ZEB1 promotes tumorigenicity by repressing
stemness-inhibiting microRNAs. Nat Cell Biol. 11:1487–1495. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong
C, Huang Y, Hu X, Su F, Lieberman J and Song E: let-7 regulates
self renewal and tumorigenicity of breast cancer cells. Cell.
131:1109–1123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brabletz T, Jung A, Spaderna S, Hlubek F
and Kirchner T: Opinion: migrating cancer stem cells - an
integrated concept of malignant tumour progression. Nat Rev Cancer.
5:744–749. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Albino D, Longoni N, Curti L, Mello-Grand
M, Pinton S, Civenni G, Thalmann G, D’Ambrosio G, Sarti M, Sessa F,
Chiorino G, Catapano CV and Carbone GM: ESE3/EHF controls
epithelial cell differentiation and its loss leads to prostate
tumors with mesenchymal and stem-like features. Cancer Res.
72:2889–2900. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bao B, Wang Z, Ali S, Kong D, Banerjee S,
Ahmad A, Li Y, Azmi AS, Miele L and Sarkar FH: Over-expression of
FoxM1 leads to epithelial-mesenchymal transition and cancer stem
cell phenotype in pancreatic cancer cells. J Cell Biochem.
112:2296–2306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang R, Li Y, Xu Y, Zhou Y, Pang Y, Shen
L, Zhao Y, Zhang J, Zhou J, Wang X and Liu Q: EMT and CSC-like
properties mediated by the IKKβ/IκBα/RelA signal pathway via the
transcriptional regulator, Snail, are involved in the
arsenite-induced neoplastic transformation of human keratinocytes.
Arch Toxicol. 87:991–1000. 2013.PubMed/NCBI
|
|
28
|
Jo M, Eastman BM, Webb DL, Stoletov K,
Klemke R and Gonias SL: Cell signaling by urokinase-type
plasminogen activator receptor induces stem cell-like properties in
breast cancer cells. Cancer Res. 70:8948–8958. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bao B, Wang Z, Ali S, Kong D, Li Y, Ahmad
A, Banerjee S, Azmi AS, Miele L and Sarkar FH: Notch-1 induces
epithelial-mesenchymal transition consistent with cancer stem cell
phenotype in pancreatic cancer cells. Cancer Lett. 307:26–36. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morel AP, Lièvre M, Thomas C, Hinkal G,
Ansieau S and Puisieux A: Generation of breast cancer stem cells
through epithelial-mesenchymal transition. PLoS One. 3:e28882008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou Y, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell
LL, Polyak K, Brisken C, Yang J and Weinberg RA: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lan L, Luo Y, Cui D, Shi BY, Deng W, Huo
LL, Chen HL, Zhang GY and Deng LL: Epithelial-mesenchymal
transition triggers cancer stem cell generation in human thyroid
cancer cells. Int J Oncol. 43:113–120. 2013.PubMed/NCBI
|
|
33
|
Biddle A, Liang X, Gammon L, Fazil B,
Harper LJ, Emich H, Costea DE and Mackenzie IC: Cancer stem cells
in squamous cell carcinoma switch between two distinct phenotypes
that are preferentially migratory or proliferative. Cancer Res.
71:5317–5326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen C, Wei Y, Hummel M, Hoffmann TK,
Gross M, Kaufmann AM and Albers AE: Evidence for
epithelial-mesenchymal transition in cancer stem cells of head and
neck squamous cell carcinoma. PLoS One. 6:e164662011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Han M, Liu M, Wang Y, Chen X, Xu J, Sun Y,
Zhao L, Qu H, Fan Y and Wu C: Antagonism of miR-21 reverses
epithelial-mesenchymal transition and cancer stem cell phenotype
through AKT/ERK1/2 inactivation by targeting PTEN. PLoS One.
7:e395202012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chiou GY, Cherng JY, Hsu HS, Wang ML, Tsai
CM, Lu KH, Chien Y, Hung SC, Chen YW, Wong CI, Tseng LM, Huang PI,
Yu CC, Hsu WH and Chiou SH: Cationic polyurethanes-short branch
PEI-mediated delivery of Mir145 inhibited epithelial-mesenchymal
transdifferentiation and cancer stem-like properties and in lung
adenocarcinoma. J Control Release. 159:240–250. 2012. View Article : Google Scholar
|
|
37
|
Nalls D, Tang SN, Rodova M, Srivastava RK
and Shankar S: Targeting epigenetic regulation of miR-34a for
treatment of pancreatic cancer by inhibition of pancreatic cancer
stem cells. PLoS One. 6:e240992011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bao B, Ali S, Kong D, Sarkar SH, Wang Z,
Banerjee S, Aboukameel A, Padhye S, Philip PA and Sarkar FH:
Anti-tumor activity of a novel compound-CDF is mediated by
regulating miR-21, miR-200, and PTEN in pancreatic cancer. PLoS
One. 6:e178502011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bao B, Ali S, Banerjee S, Wang Z, Logna F,
Azmi AS, Kong D, Ahmad A, Li Y, Padhye S and Sarkar FH: Curcumin
analogue CDF inhibits pancreatic tumor growth by switching on
suppressor microRNAs and attenuating EZH2 expression. Cancer Res.
72:335–345. 2012. View Article : Google Scholar : PubMed/NCBI
|





















