Introduction
Hepatocellular carcinoma (HCC) is one of the common
malignancies in the world, and it is especially common in east Asia
and sub-Saharan Africa (1–3). It is well known that the development
of most HCCs is a multistep process under the presence of various
chronic liver diseases (4,5). During such hepatocellular
carcinogenesis, changes in the blood supply have been reported,
based on pathological and radiological studies (6–9).
Briefly, a dysplastic nodule shows deterioration of arterial and
portal blood flow, and in well-differentiated HCC (wHCC) arterial
blood flow becomes dominant as a result of arterial
neo-vascularization. Subsequently, classical (moderately and poorly
differentiated) HCC shows abundant arterial blood flow and
decreased portal blood flow.
The detection of wHCC is one of the most important
issues in the management of patients with chronic liver disease.
Previous studies have reported the imaging features of wHCCs,
especially on computed tomography hepatic angiography (CTHA) and CT
during arterial portography (8,9). In
those studies, wHCCs showed varying attenuation levels
(hypoattenuation to hyperattenuation) on CTHA, suggesting that wHCC
is the transitional stage of the arterial blood supply from a
dysplastic nodule to classical HCC. However, to the best of our
knowledge, the upregulation mechanism of angiogenesis in wHCC has
not been fully elucidated.
Immunological studies have identified two distinct
states of macrophages (10,11). Classically activated (M1)
macrophages activate microbial killing and show antitumor immunity.
Tumor-associated macrophages (TAMs) are known to possess the
immunosuppressive M2 macrophage phenotype. M2 macrophages are
alternatively activated macrophages and contribute to tumor
progression, including angiogenesis (12,13). A
significant correlation between TAMs and tumor vascularity has been
reported in many tumors, including breast carcinoma (14), esophageal carcinoma (15), bladder (16) and prostate cancer (17).
However, it is known that macrophages in an HCC
decrease as the histological grade advances, as confirmed by
superparamagnetic iron oxide (SPIO)-enhanced magnetic resonance
(MR) imaging findings (18). In
fact, the macrophage count in wHCC is higher than that in classical
(moderately and poorly differentiated) HCC (18). Considering these reports, TAMs may
have a role in promoting angiogenesis of wHCCs.
In the present study, we attempted to clarify
whether TAMs are associated with the angiogenesis of HCC, with a
special focus on the early stage of HCC during the multistep
development.
Materials and methods
Patients
The present study conformed to the ethical
guidelines of the 1975 Declaration of Helsinki as revised in Tokyo
2004 and was performed with the approval of the Institutional
Ethics Committee of Kyushu University. We could not obtain written
informed consent from all the patients, therefore we removed
identifying information from all samples before analysis for strict
privacy protection. This procedure was in accordance with the
Ethical Guidelines for Human Genome/Gene Research enacted by the
Japanese Government.
The cases included 73 HCCs surgically resected at
our institution between March 1993 and September 2010. These
consisted of 43 wHCCs and 30 well- to moderately differentiated
HCCs (wmHCCs). Histological diagnosis was evaluated based on the
classification proposed by the World Health Organization (19) and the International Consensus Group
for Hepatocellular Neoplasia (20).
WmHCC was defined as a nodule-in-nodule lesion when an inner nodule
(moderately differentiated component) and an outer part
(well-differentiated component) could be clearly separated. Patient
details and tumor profiles are summarized in Table I. Twenty-six patients with wHCC and
all 30 patients with wmHCCs underwent CTHA before surgery. Any
cases with preceding therapy before surgery were excluded from the
present study.
 | Table IPatient details and tumor
profiles. |
Table I
Patient details and tumor
profiles.
| Characteristics | Data |
|---|
| wHCC |
| Gender
(male/female) | 33/10 |
| Age (years) mean
(range) | 63.2 (28–87) |
| Hepatitis viral
infection (B/C) | 8/31 |
| Liver cirrhosis | 21 |
| Tumor size (cm) mean
(range) | 1.6 (0.6–3.8) |
| wmHCC |
| Gender
(male/female) | 16/14 |
| Age (years) mean
(range) | 71.8 (55–87) |
| Hepatitis viral
infection (B/C) | 5/22 |
| Liver
cirrhosis | 14 |
| Tumor size (cm)
mean (range) | 2.1 (0.9–4.5) |
Immunohistochemistry
Ten-percent formalin-fixed, paraffin-embedded tissue
sections (4 μm) containing sufficient tumor tissue were used for
the study. Immunohistochemical staining was performed by the
streptavidin-biotin-peroxidase method (Histofine SAB-PO kit;
Nichirei, Tokyo, Japan). To retrieve antigens, the specimens were
preincubated in trypsin (Sigma-Aldrich, St. Louis, MO, USA) in
phosphate-buffered saline (PBS) for 30 min at 37°C for anti-CD34
antibody, while specimens were pretreated by heating in a microwave
oven for 20 min for anti-CD68 and anti-CD163 antibody. The
antibodies used in the present study were mouse monoclonal
antibodies, and their dilutions were 1:50 for CD34 (QBEnd/10;
Novocastra, Newcastle, UK), 1:300 for CD68 (KP-1; Dako, Glostrup,
Denmark) and 1:200 for CD163 (10D6; Novocastra).
Evaluation of immunostaining
Microvessel density (MVD) was quantified using
immunohistochemical staining for CD34 as a marker for endothelium
following the method of a previous report (21). Three of the most vascularized areas
were selected by scanning under a low-power view (×40), and the
number of CD34-positive vessels was counted at a magnification of
×100. The mean count of the three areas was defined as the MVD.
CD68-positive macrophages were also counted in both
the tumor and the non-tumorous tissue. Five areas with the greatest
number of macrophages were selected under a low-power view (×40),
and the number of CD68-positive macrophages was counted at ×200
magnification. The mean count of the five areas was defined as the
macrophage count (MC). Macrophages in the portal tracts of the
non-tumorous tissue were excluded from evaluation, as inflammatory
cell infiltration in this region depends on the presence or
condition of chronic hepatitis. To evaluate the MC in the tumor
compared with the non-tumorous tissue, we defined the MC ratio as
follows: MC ratio = MC in the tumor/MC in the non-tumorous tissue.
We also counted CD163-positive MC as a marker of M2 macrophages
(22–24) in the same way we counted MC.
All measurements were performed by two pathologists
(S.A. and Y.K.) without any knowledge of the clinicopathological
findings.
CT protocols
CTHA was performed using a 4-row multidetector CT
system (MDCT; Aquilion; Toshiba, Tokyo, Japan) or 16-row
multidetector CT system (Aquilion). The scanning parameters of the
4-MDCT were as follows: collimation 3 mm; pitch 5.5; reconstruction
5 mm. The parameters of the 16-MDCT were: collimation 1 mm; pitch
15; reconstruction 3 mm. The hepatic arterial catheter for CTHA was
placed in the proper, right or left hepatic arteries. Data
acquisition of CTHA began 15 sec (first phase) and 30 sec (second
phase) after the initiation of 15–40 ml iopamidol solution (150
mg/ml) (Iopamiron 150; Bayer Health Care) at a rate of 1–2.5 ml/sec
using an automated power injector. The appropriate injection rate
for CTHA was determined as the maximum injection rate that would
not cause a backward flow of contrast material on hepatic
arteriography. The duration of the arterial injection was 15–20
sec.
Imaging assessment
The CTHA images of HCCs were evaluated by two
abdominal radiologists (N.F. and A.N.) in a consensus fashion. The
observers were blinded to the pathological and clinical findings
for each case. We evaluated the tumor attenuation level on the
first phase of CTHA as we surmised that this phase represents tumor
vascularity. On the basis of the enhancement pattern in relation to
non-tumorous liver, we divided the wHCCs into two groups: a
hyperattenuation group and a hypo- or isoattenuation group. The
hyperattenuation was determined when the whole lesion showed
hyperattenuation or a hyperattenuation component was observed
within the lesion on CTHA. Although the CTHA images of the wmHCCs
were analyzed similarly, the two components were evaluated
independently. In particular, we focused on the difference in the
attenuation level on CTHA between the inner nodule (moderately
differentiated component) and the outer part (well-differentiated
component).
Statistical analysis
The relationships between the MVD and MC or MC
ratio, and between MC and CD163-positive MC in the wHCCs were
evaluated using the Pearson correlation test. Intraclass
correlation coefficient was used to evaluate the agreement between
MC and CD163-positive MC in the wHCCs. The correlation between the
attenuation level on CTHA and the MVD or MC in wHCCs was analyzed
by the Mann-Whitney U-test. In the wmHCCs, the Mann-Whitney U-test
was used for comparison of the MVD or MC between the well- and
moderately differentiated components. Continuous variables are
expressed as means ± SD. JMP 7.0.1 software (SAS Institute, Inc.,
Cary, NC, USA) and SPSS software version 13.0 (SPSS Inc., Chicago,
IL, USA) were used for the analysis. P-values <0.05 were
considered to indicate a statistically significant difference.
Results
Pathological and imaging analysis of the
wHCCs
The pathological analysis of the 43 wHCCs revealed
an MVD of 116.8±67.6 and an MC of 93.5±38.2. The MC ratio was
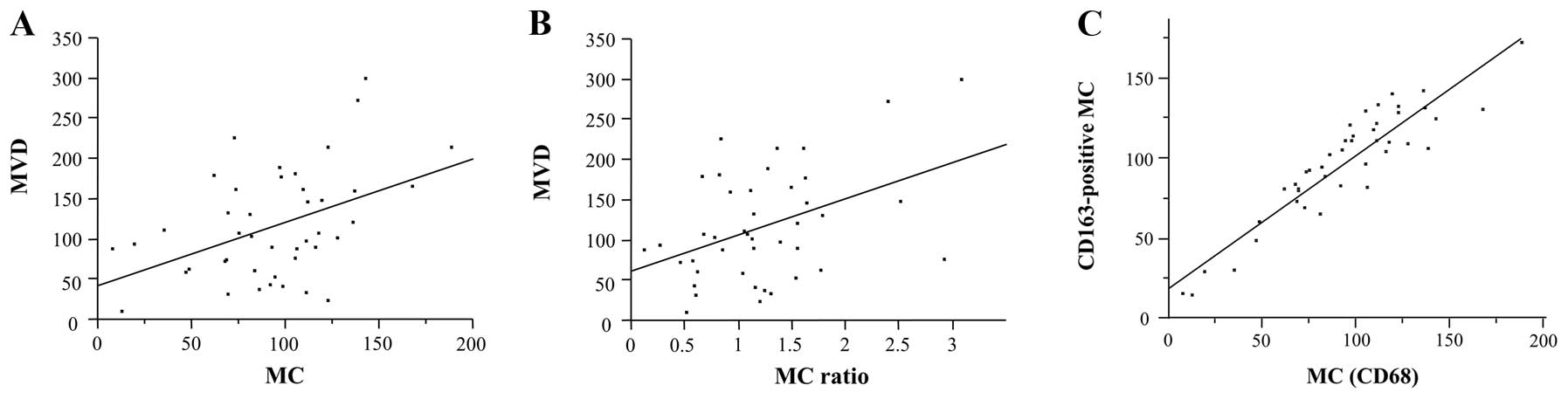
1.2±0.6. CD163-positive MC was 96.8±34.5. A significant correlation
was again obtained between MVD and MC (P=0.0026, r=0.4486; Fig. 1A) as well as between MVD and the MC
ratio (P=0.0039, r=0.4308; Fig.
1B). In addition, a significant correlation was obtained
between MC and CD163-positive MC (P<0.0001, r=0.9173; Fig. 1C), and MC and CD163-positive MC
showed an excellent agreement with an intraclass correlation
coefficient of 0.913.
Of the 26 wHCCs evaluated by CTHA, 12 wHCCs showed
hypoattenuation (n=11) or isoattenuation (n=1) and 14 wHCCs
contained a hyperattenuation area on the first phase. The MVD and
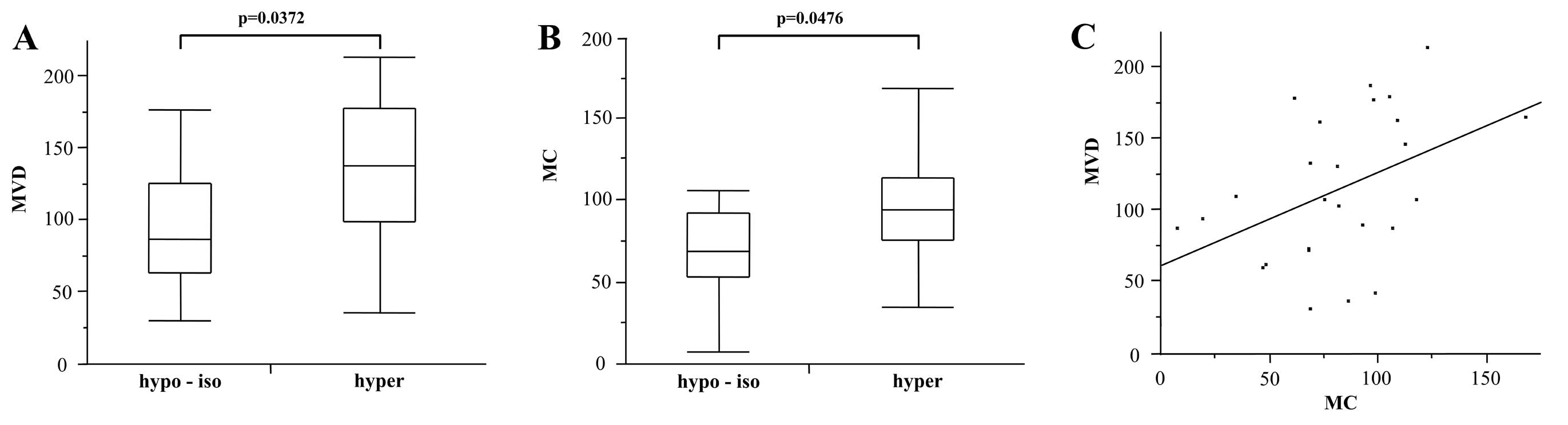
MC of these lesions were 115.2±51.8 and 81.3±34.3, respectively.
The MVD of the hyperattenuation group (132.4±52.4) was
significantly higher than that of the hypo- or isoattenuation group
(95.1±45.0; P=0.0372; Fig. 2A). In
addition, the MC of the hyperattenuation group (92.8±36.0) was
significantly higher than that of the hypo- or isoattenuation group
(67.8±27.8; P=0.0476; Fig. 2B). In
these lesions, a significant correlation was obtained between MVD
and MC (P=0.0270, r=0.4334; Fig.
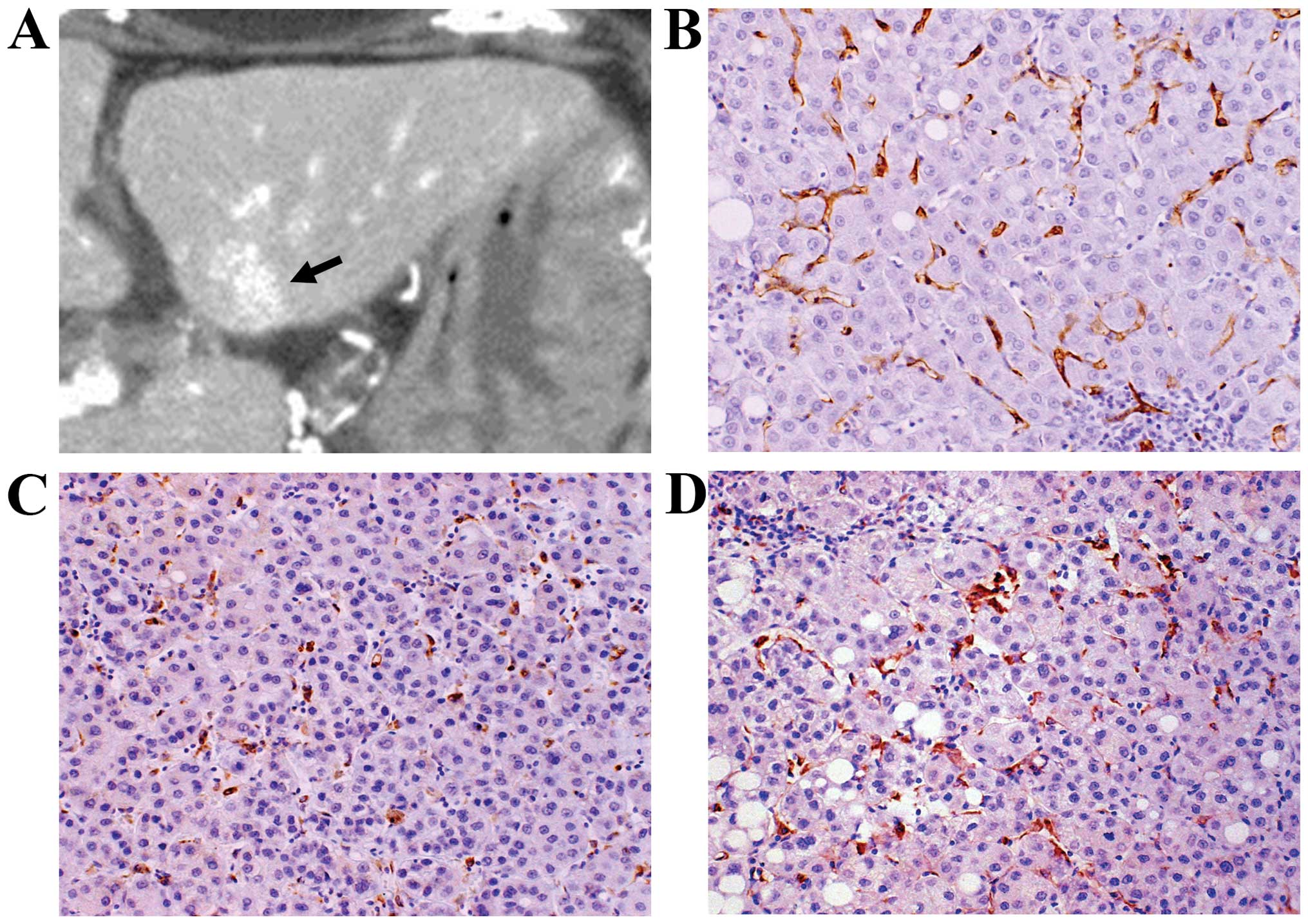
2C). Two typical cases are shown in Figs. 3 and 4.
Pathological and imaging analysis of the
wmHCCs
The MVD of the moderately differentiated components
(223.1±77.6) was significantly higher than that of the
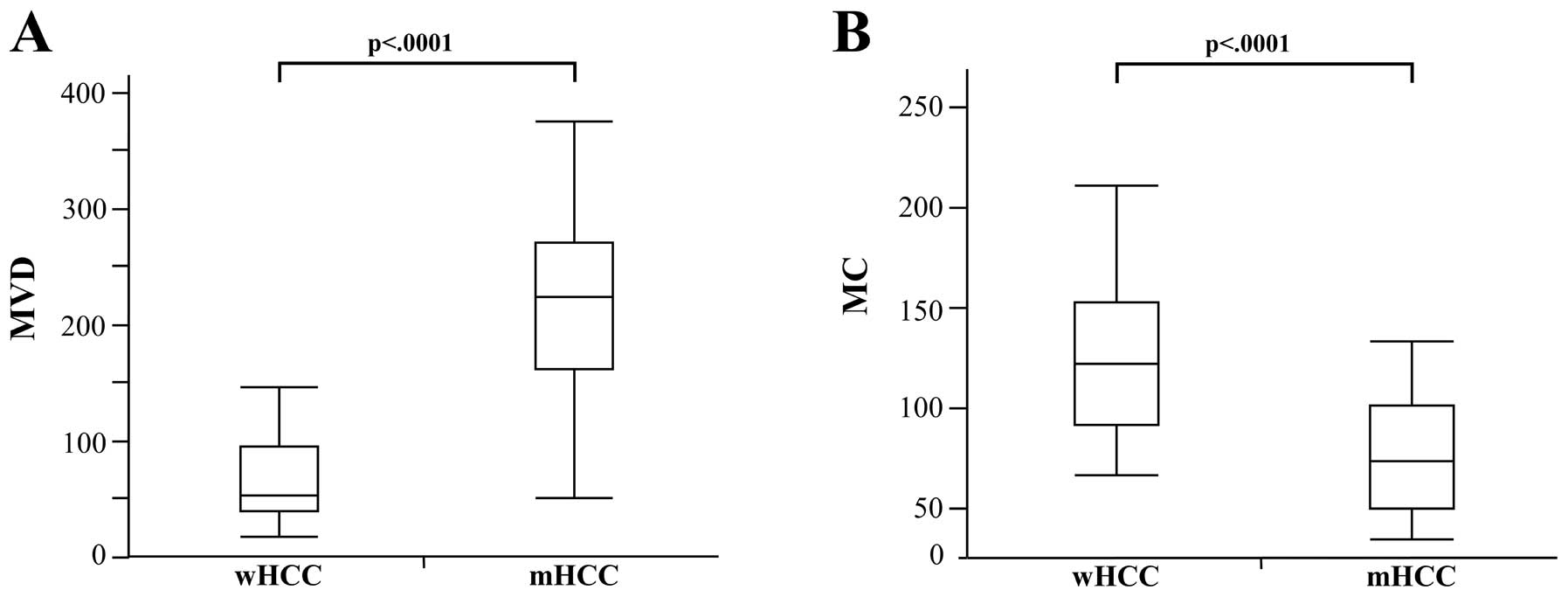
well-differentiated components (65.9±35.9; P<.0001; Fig. 5A). In contrast, the MC of the
moderately differentiated components (76.8±29.5) was significantly
lower than that of the well-differentiated components (127.6±42.6,
P<.0001; Fig. 5B). On the first
phase of CTHA, all the moderately differentiated components showed
marked hyperattenuation. Regarding the outer well-differentiated
components, 12 lesions showed hypo- (n=10) or isoattenuation (n=2)
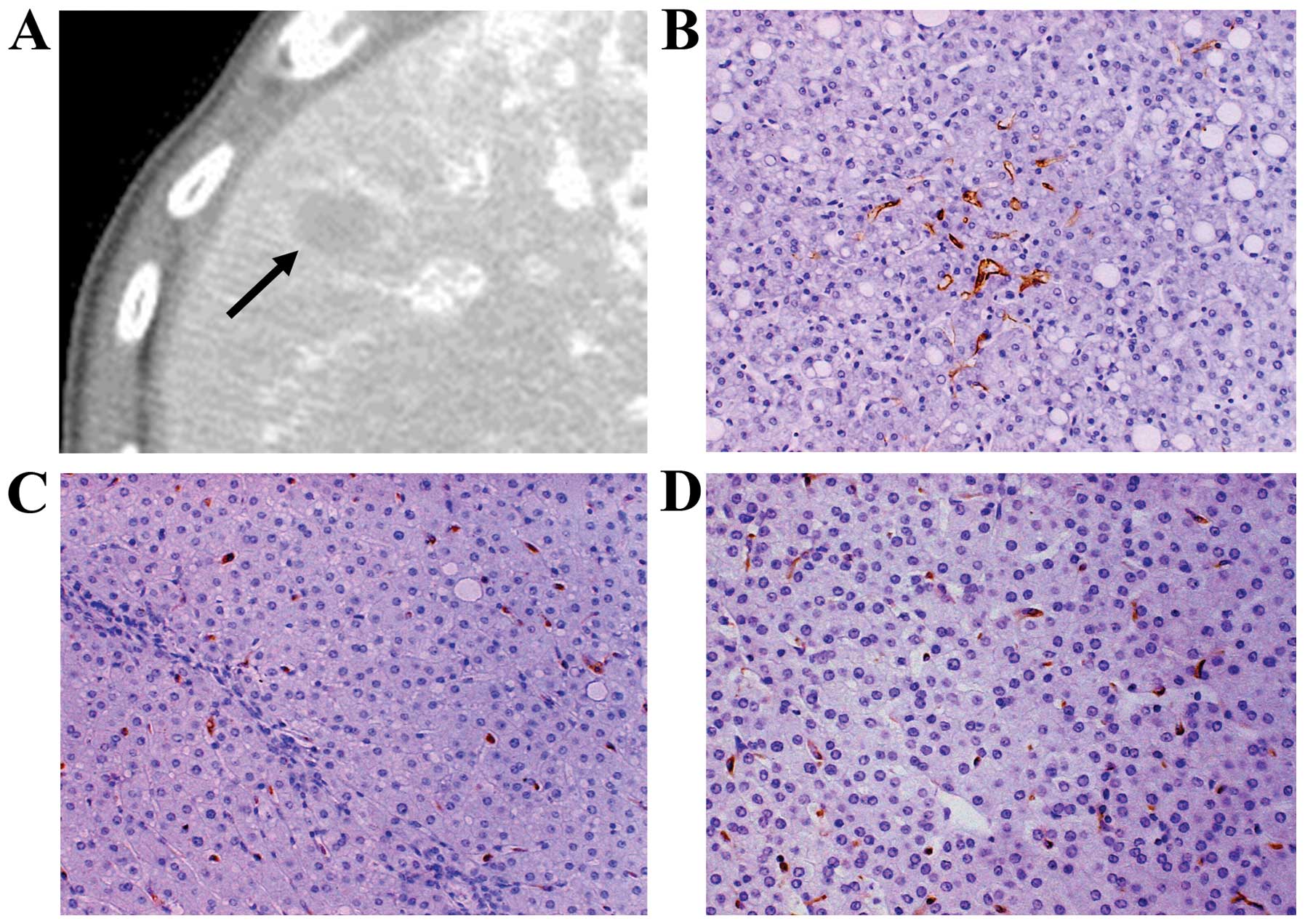
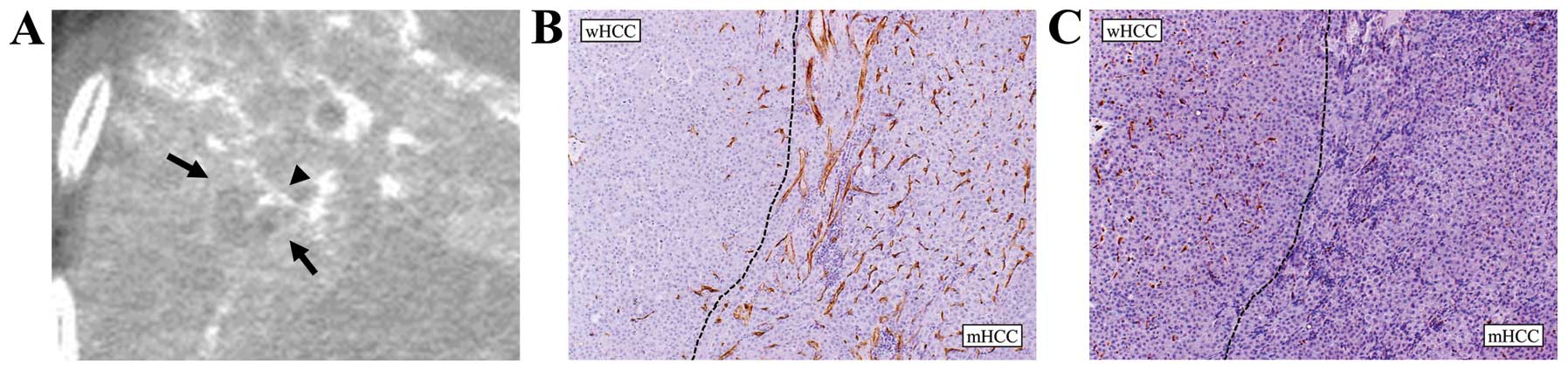
and 18 lesions showed hyperattenuation. A representative case is
shown in Fig. 6.
Discussion
The molecular mechanism of tumor angiogenesis has
been well described (25,26). Vascular endothelial growth factor
(VEGF) and the angiopoietin (Ang) family are vascular
endothelium-specific growth factors (25). In HCCs, the correlation between such
growth factors and MVD has been revealed in several studies
(27–29). In addition, another study
demonstrated that the stromal cells in solid tumors play an
important role in tumor progression (30).
TAMs are particularly important components of the
tumor microenvironment (12,13).
Macrophages are released from bone marrow, then circulate in the
blood stream and migrate into tissues such as Kupffer cells in the
liver. These macrophages are capable of lysing tumor cells,
presenting antigens to T cells, and expressing immunostimulatory
cytokines (12). In contrast, TAMs
which are exposed to tumor-derived molecules such as interleukin
(IL)-4 and IL-10 and are alternatively activated have poor
antigen-presenting capability, and they produce factors that
suppress T-cell proliferation and activity (12,31).
TAMs also release growth factors, cytokines and chemokines that
regulate tumor growth, tumor invasion, and angiogenesis, such as
VEGF (12). The role of TAMs in
angiogenesis has been reported in many solid tumors (14–17).
In light of our present results, we hypothesize that
TAMs play a crucial role in angiogenesis in wHCC, but not in
moderately differentiated HCC. The mechanisms that control such
changes of TAMs remain controversial. Stromal invasion, which means
tumor cell invasion into the intratumoral portal tracts, is one of
the most important pathologic features in wHCC (20). Stromal invasion decreases the number
of portal veins and arteries, and may cause hypoxia. Murdoch et
al (32) reported that TAMs
accumulate in large numbers in avascular and hypoxia areas.
During the multistep development of wHCC, stromal
invasion increases and causes increasing areas of hypoxia. We
suspect that such hypoxic conditions recruit TAMs and promote
angiogenesis in wHCC. In the present study, the wHCCs showed
various attenuation levels on CTHA and various MVDs. The increasing
stromal invasion during tumor development in wHCCs can explain the
finding that the wHCCs showed such varying vascularity. However, in
moderately differentiated HCC, VEGF and the Ang family that express
in carcinoma cells play a central role in tumor angiogenesis, as
previously described (28,29). It no longer seems that TAMs play an
important role in angiogenesis. A previous finding that the
macrophage count in the wHCC is higher than that in the classical
(moderately and poorly differentiated) HCC may also support our
hypothesis (18). We assume that
TAMs may induce an ‘angiogenetic switch’ at an early stage of
HCC.
One issue that we should consider is whether
macrophages in HCC are classical (M1) macrophages or
immunosuppressive M2 macrophages, as they both express CD68. We,
therefore, compared the MC between the tumors and the non-tumorous
tissue. Twenty-eight of the 43 wHCCs (65.1%) had higher MC in the
tumor than in the non-tumorous tissue. In addition, a significant
correlation was found between MVD and the MC ratio. We also
compared the correlation between CD68- and CD163-positive
macrophage counts. CD163 is a transmembrane scavenger receptor for
haptoglobin-hemoglobin complexes and is expressed in M2 macrophages
(22–24). In the present study, a strong
correlation was found between CD68- and CD163-positive macrophage
counts. In addition, MC and CD163-positive MC showed an excellent
agreement. Therefore, we consider that TAMs in HCC are M2
phenotype. Also, in previous studies, CD68-positive macrophages in
the tumor were regarded as TAMs (14–16)
which possess the immunosuppressive M2 macrophage phenotype.
Only a few radiological investigations have reported
a correlation between imaging findings and these molecular
mechanisms in tumor angiogenesis. Some reports described the
relationship between imaging findings and VEGF expression in HCC
(33,34). Regarding TAMs, a role of macrophages
in tumor progression in HCC under treatment with sorafenib, an oral
multikinase inhibitor of VEGF, has been reported (35). Such molecular imaging studies will
increase our understanding of the radiological imaging findings
related to the molecular biologic environment and, in addition,
should be used along with new target therapies for molecular
mechanisms in tumor progression.
The present study has a few limitations. First, we
divided the wHCCs into two groups (hyperattenuation group vs. iso-
or hypoattenuation group) in the evaluation of vascularity on CTHA
as only one lesion showed isoattenuation. Second, our results
cannot be compared with SPIO-enhanced MR imaging findings. Only two
of our 43 patients with wHCC underwent SPIO-enhanced MR imaging.
Since the uptake of SPIO in the tumor reflects the number and
function of TAMs, we may be able to predict angiogenetic changes in
tumors in the future using this method.
In conclusion, TAMs may have an important role in
promoting angiogenesis in wHCCs, but not in moderately
differentiated HCCs. Our findings suggest a role of TAMs in
promoting angiogenesis at an early stage of HCC during its
multistep development.
Acknowledgements
We thank Dr Yoshihiko Maehara, Department of Surgery
and Science, Kyushu University, for providing the clinical
information for this manuscript. The present study was supported by
a Grant-in-Aid for Scientific Research (C) (24591814) from the
Japanese Ministry of Education, Culture, Sports, Science, and
Technology.
References
|
1
|
Okuda K: Hepatocellular carcinoma:
clinicopathological aspects. J Gastroenterol Hepatol. 12:S314–S318.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bosch FX, Ribes J, Díaz M and Cléries R:
Primary liver cancer: worldwide incidence and trends.
Gastroenterology. 127:S5–S16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar
|
|
4
|
Feitelson MA, Sun B, Satiroglu Tufan NL,
Liu J, Pan J and Lian Z: Genetic mechanisms of
hepatocarcinogenesis. Oncogene. 21:2593–2604. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kudo M: Multistep human
hepatocarcinogenesis: correlation of imaging with pathology. J
Gastroenterol. 44(Suppl 19): 112–118. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ueda K, Terada T, Nakanuma Y and Matsui O:
Vascular supply in adenomatous hyperplasia of the liver and
hepatocellular carcinoma: a morphometric study. Hum Pathol.
23:619–626. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fujita N, Aishima S, Iguchi T, et al:
Down-regulation of artery in moderately differentiated
hepatocellular carcinoma related to tumor development. Hum Pathol.
41:838–847. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tajima T, Honda H, Taguchi K, et al:
Sequential hemodynamic change in hepatocellular carcinoma and
dysplastic nodules: CT angiography and pathologic correlation. AJR
Am J Roentgenol. 178:885–897. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hayashi M, Matsui O, Ueda K, et al:
Correlation between the blood supply and grade of malignancy of
hepatocellular nodules associated with liver cirrhosis: evaluation
by CT during intraarterial injection of contrast medium. AJR Am J
Roentgenol. 172:969–976. 1999. View Article : Google Scholar
|
|
10
|
Biswas SK and Mantovani A: Macrophage
plasticity and interaction with lymphocyte subsets: cancer as a
paradigm. Nat Immunol. 11:889–896. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Martinez FO, Gordon S, Locati M and
Mantovani A: Transcriptional profiling of the human
monocyte-to-macrophage differentiation and polarization: new
molecules and patterns of gene expression. J Immunol.
177:7303–7311. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lewis CE and Pollard JW: Distinct role of
macrophages in different tumor microenvironments. Cancer Res.
66:605–612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shirabe K, Mano Y, Muto J, et al: Role of
tumor-associated macrophages in the progression of hepatocellular
carcinoma. Surg Today. 42:1–7. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leek RD, Lewis CE, Whitehouse R, Greenall
M, Clarke J and Harris AL: Association of macrophage infiltration
with angiogenesis and prognosis in invasive breast carcinoma.
Cancer Res. 56:4625–4629. 1996.PubMed/NCBI
|
|
15
|
Koide N, Nishio A, Sato T, Sugiyama A and
Miyagawa S: Significance of macrophage chemoattractant protein-1
expression and macrophage infiltration in squamous cell carcinoma
of the esophagus. Am J Gastroenterol. 99:1667–1674. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hanada T, Nakagawa M, Emoto A, Nomura T,
Nasu N and Nomura Y: Prognostic value of tumor-associated
macrophage count in human bladder cancer. Int J Urol. 7:263–269.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lissbrant IF, Stattin P, Wikstrom P,
Damber JE, Egevad L and Bergh A: Tumor associated macrophages in
human prostate cancer: Relation to clinicopathological variables
and survival. Int J Oncol. 17:445–451. 2000.PubMed/NCBI
|
|
18
|
Imai Y, Murakami T, Yoshida S, et al:
Superparamagnetic iron oxide-enhanced magnetic resonance images of
hepatocellular carcinoma: correlation with histological grading.
Hepatology. 32:205–212. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Theise ND, Ishak KG, Kojiro M, et al:
Hepatocellular carinoma. World Health Organization Classification
of the Digestive System. Bosman FT, Carneiro F, Hruban RH and
Theise ND: IARC Press; Lyon: pp. 205–216. 2010
|
|
20
|
International Consensus Group for
Hepatocellular Neoplasia. Pathologic diagnosis of early
hepatocellular carcinoma: a report of theInternational Consensus
Group for Hepatocellular Neoplasia. Hepatology. 49:658–664. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis - correlation in invasive
breast carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hasita H, Komohara Y, Okabe H, et al:
Significance of alternatively activated macrophages in patients
with intrahepatic cholangiocarcinoma. Cancer Sci. 101:1913–1919.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fujiwara Y, Komohara Y, Ikeda T and Takeya
M: Corosolic acid inhibits glioblastoma cell proliferation by
suppressing the activation of signal transducer and activator of
transcription-3 and nuclear factor-kappa B in tumor cells and
tumor-associated macrophages. Cancer Sci. 102:206–211. 2011.
View Article : Google Scholar
|
|
24
|
Komohara Y, Ohnishi K, Kuratsu J and
Takeya M: Possible involvement of the M2 anti-inflammatory
macrophage phenotype in growth of human gliomas. J Pathol.
216:15–24. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yancopoulos GD, Davis S, Gale NW, Rudge
JS, Wiegand SJ and Holash J: Vascular-specific growth factors and
blood vessel formation. Nature. 407:242–248. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Holash J, Wiegand SJ and Yancopoulos GD:
New model of tumor angiogenesis: dynamic balance between vessel
regression and growth mediated by angiopoietins and VEGF. Oncogene.
18:5356–5362. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
El-Assal ON, Yamanoi A, Soda Y, et al:
Clinical significance of microvessel density and vascular
endothelial growth factor expression in hepatocellular carcinoma
and surrounding liver: possible involvement of vascular endothelial
growth factor in the angiogenesis of cirrhotic liver. Hepatology.
27:1554–1562. 1998. View Article : Google Scholar
|
|
28
|
Wada H, Nagano H, Yamamoto H, et al:
Expression pattern of angiogenic factors and prognosis after
hepatic resection in hepatocellular carcinoma: importance of
angiopoietin-2 and hypoxia-induced factor-1α. Liver Int.
26:414–423. 2006.PubMed/NCBI
|
|
29
|
Mitsuhashi N, Shimizu H, Ohtsuka M, et al:
Angiopoietins and Tie-2 expression in angiogenesis and
proliferation of human hepatocellular carcinoma. Hepatology.
37:1105–1113. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
de Visser KE, Eichten A and Coussens LM:
Paradoxical roles of the immune system during cancer development.
Nat Rev Cancer. 6:24–37. 2006.
|
|
31
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization: tumor-associated macrophages
as a paradigm for polarized M2 mononuclear phagocytes. Trends
Immunol. 23:549–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Murdoch C, Giannoudis A and Lewis CE:
Mechanisms regulating the recruitment of macrophages into hypoxic
areas of tumors and other ischemic tissues. Blood. 104:2224–2234.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kanematsu M, Osada S, Amaoka N, et al:
Expression of vascular endothelial growth factor in hepatocellular
carcinoma and the surrounding liver: correlation with
angiographically assisted CT. AJR Am J Roentgenol. 183:1585–1593.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kanematsu M, Osada S, Amaoka N, et al:
Expression of vascular endothelial growth factor in hepatocellular
carcinoma and the surrounding liver and correlation with MRI
findings. AJR Am J Roentgenol. 184:832–841. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang W, Zhu XD, Sun HC, et al: Depletion
of tumor-associated macrophages enhances the effect of sorafenib in
metastatic liver cancer models by antimetastatic and antiangiogenic
effects. Clin Cancer Res. 16:3420–3430. 2010. View Article : Google Scholar : PubMed/NCBI
|