Introduction
The tightly controlled proteolytic turnover of
extracellular matrix (ECM) components in the immediate vicinity of
the cell has a significant influence in the development and
maintenance of tissue homeostasis (1). Proteolytic events within this
pericellular microenvironment are mainly executed by extracellular
or cell-surface metalloproteases and serine proteases (2). Among them, the type II transmembrane
serine proteases (TTSPs), are membrane-anchored serine proteases
involved in relevant biological functions (3). Thus, enteropeptidase is produced by
the duodenum and its function is essential to digest food
efficiently (4). Corin contributes
to blood pressure control through activation of atrial natriuretic
peptide (5). Matriptase-2
participates in iron homeostasis (6), and hepsin is implicated in the process
of hearing (7). These and other
examples (8) illustrate the
relevance of TTSPs in normal physiological processes.
The activities of the TTSPs also underlie different
human disorders, including cancer. In fact, these proteases can
cause an active pericellular degradation to breach tissue barriers
thereby facilitating invasion of surrounding tissues (9). Moreover, this pericellular proteolysis
may also trigger the activation of growth factors that promote
tumor cell proliferation and migration (10). However, TTSPs can also display
antitumor effects. For instance, HPN, the gene encoding for
human hepsin, is one of the most highly upregulated in prostate
cancer, and this enzyme is exclusively detected in the surface of
prostate carcinoma cells (11). In
contrast, low expression levels of hepsin correlate with shorter
patient survival in hepatocellular carcinoma (12). Similarly, expression of matriptase
was found to be markedly upregulated in different tumors of
epithelial origin, including breast, lung or pancreatic carcinomas
(13). However, a significant
decrease in its expression was found in gastrointestinal tumors
(14). DESC1 serine protease
exhibits reduced expression levels in head and neck carcinomas in
comparison with matched normal tissues (15), while its exogenous expression
confers pro-tumor effects to MDCK cells and it was found to be
upregulated in kidney, liver, breast and brain tumors (16). Additionally, different studies have
highlighted the functions of different TTSPs as regulators of
cellular signaling pathways and revealed the biochemical mechanisms
underlying their pro-tumor or antitumor effects (9).
Despite the increased understanding of the
functional relevance of TTSPs, some members of this family have
been characterized at the structural level while their roles in
normal or pathological processes remain unknown. This is the case
of polyserase-1 or transmembrane protease, serine 9 (TMPRSS9), a
TTPS widely distributed in human and mouse tissues (17,18).
Structurally, this enzyme shows a complex molecular architecture
containing three tandem serine-protease domains called serase-1,
serase-2 and serase-3 (17). The
third domain is predicted as catalytically inactive due to the
presence of an alanine residue instead of the serine residue
present in the active site of this type of enzyme. Serase-1B is a
splice variant of TMPRSS9 containing only one serase domain which
efficiently activates pro-uPA (18). Active uPA converts plasminogen to
plasmin, an enzyme that cleaves different ECM components and
activates latent collagenases. These effects contribute to the
invasiveness of tumor cells of different origin, including
pancreatic carcinomas. In fact, uPA exhibits marked overexpression
in this type of tumor, and its detection provides prognostic
information (19). Different
reports have emphasized the pro-tumor activities of different TTSPs
in pancreatic cancer (9,20,21).
Activation (22) or upregulation
(23) of pro-uPA may underlie the
pro-tumor effects caused by these serine proteases. In the present
study, we investigated the effects of the exogenous expression of
polyserase-1 in pancreas-derived tumor cells (24,25).
Collectively, our data suggest that polyserase-1 exerts pro-tumor
effects through a mechanism involving activation of pro-uPA and
phosphorylation of ERK.
Materials and methods
Cell lines and culture conditions
The cell line PANC-1 was purchased from Cell Lines
Service and the SK-PC-3 cell line was kindly provided by Dr
Francisco Real (National Cancer Research Centre, Madrid, Spain).
Cells were routinely maintained at 37°C in 5% CO2 in
Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10%
fetal bovine serum (FBS).
Transfection and western blot
analysis
The cell lines were transfected with the plasmid
pcDNA3-FLAG-poly1, which contains the complete sequence of
polyserase-1 and a FLAG epitope at the amino terminal region
(17). Cells transfected with an
empty pcDNA3 vector were employed as a control. Transfections were
performed using the Lipofectamine system (Invitrogen) following the
manufacturer’s recommendations. Cells were selected in the presence
of G-418 (Sigma-Aldrich) at 400 μg/ml. For western blot analysis,
the proteins were separated on SDS/PAGE, and blotted onto PVDF
membranes. Detection of exogenous polyserase-1 was performed using
and an anti-FLAG antibody (Sigma Aldrich), and the H-140 antibody
from Santa Cruz Biotechnology was used to detect uPA. Antibodies to
detect p-ERK and ERK were from Cell Signalling Technology.
qRT-PCR
Total RNA from cell cultures was obtained using the
RNeasy Mini kit (Qiagen) following the manufacturer’s
recommendations. For cDNA synthesis, 2 μg of RNA was used as a
template and the system ThermoScript RT-PCR (Invitrogen) was
employed following the protocol recommended by the manufacturer.
cDNA was then used as a template for quantitative PCR using TaqMan
probe HS00917112_m1, TaqMan Master Mix reagent and the detection
system 7300 Real-Time PCR system (Applied Biosystems). Expression
of human β-actin was employed as endogenous control. Data analysis
was performed using the computer program ABI PRISM 7900HT, and RQ
Manager 1.2 (Applied Biosystems).
Cell invasion assay
In vitro invasion potential was evaluated
using 24-well Matrigel-coated invasion chambers with a 8-μm pore
size (BD Biosciences). Cells (6×104) were allowed to
migrate for 24 h using 10% FBS as a chemoattractant. Cells that
reached the lower surface were fixed and stained with crystal
violet. Three independent experiments were performed with
triplicates for each condition. Cells were counted in eight
randomly selected microscopic fields.
Soft agar colony formation
Anchorage-independent growth was examined using
CytoSelect™ 96-Well In Vitro Tumor Sensitivity Assay kit (Cell
Biolabs) following the manufacturer’s instructions. Cell density
was 6×103 cells/ml, and assays were performed for 8 days
at 37°C and 5% CO2 using 24-well Ultra-low attachment
plates (Costar). Colonies were examined and photographed with a
Leica microscope EZ 2.0 (Leica Microsystems).
Cell adhesion assay
The adhesion capacity of the different cell lines
was analyzed using a colorimetric ECM Cell Adhesion Array kit (EMD
Biosciences) following the manufacturer’s instructions. Briefly,
2×105 cells were incubated for 2 h. The cells were then
lysed, and the absorbance was measured at 485 nm using a Synergy H4
Hybrid reader. All data are the mean of three independent
experiments.
Statistical analysis
Statistical analysis was performed using the
GraphPad Prism 5.0 software. Data are represented as means ± SE.
The occurrence of significant differences was determined with the
Student’s-Welch t-test. P-values <0.05 were considered to
indicate statistically significant differences.
Results
Exogenous expression of
TMPRSS9/polyserase-1 in pancreatic cancer cells
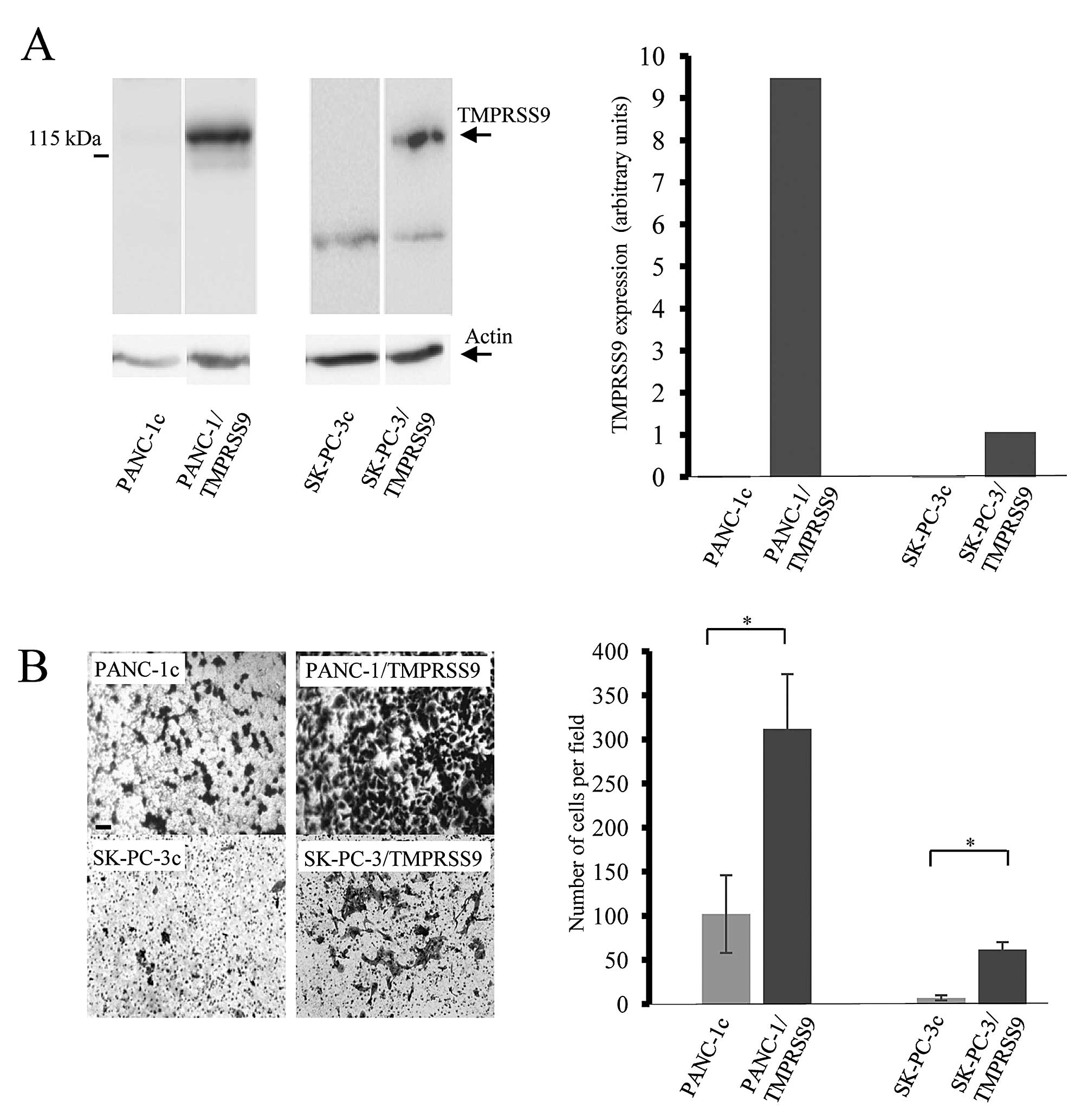
To examine whether polyserase-1 affects the
tumorigenic phenotype of pancreatic cancer cells, we employed two
cell lines, PANC-1 and SK-PC-3, to perform further functional
analysis. These cell lines do not endogenously express TMPRSS9 as
assessed by qRT-PCR; therefore, cells were transfected using the
full-length cDNA for polyserase-1 (17) (Fig.
1A). Expression of exogenous polyserase-1 was assessed using
qRT-PCR and western blot analysis by detection of the FLAG epitope
at the amino-terminal region of the recombinant protein (Fig. 1A). Cells that stably expressed
TMPRSS9, referred to as PANC-1/TMPRSS9 and SK-PC-3/TMPRSS9,
respectively, were chosen for further evaluation of potential
phenotypic changes. PANC-1 and SK-PC-3 cells transfected with an
empty vector, PANC-1c and SK-PC-3c, respectively, did not show
immunoreactivity for the antibodies against FLAG epitope and were
used as negative controls.
Polyserase-1 increases invasive
properties of pancreatic cancer cells
Invasive capacity of the PANC-1/TMPRSS9 and
SK-PC-3/TMPRSS9 cells was examined using Matrigel-coated invasion
chambers (Fig. 1B). Average cell
numbers penetrating the Matrigel per field were 312 in the case of
PANC-1/TMPRSS9 cells and 62 in the case of SK-PC-3/TMPRSS9 cells.
However, only 102 PANC-1c and 10 SK-PC-3c invasive cells per field
were observed in this assay (Fig.
1B). These data indicated that the presence of polyserase-1
markedly increases the invasive capacity of both PANC-1 and SK-PC-3
cells.
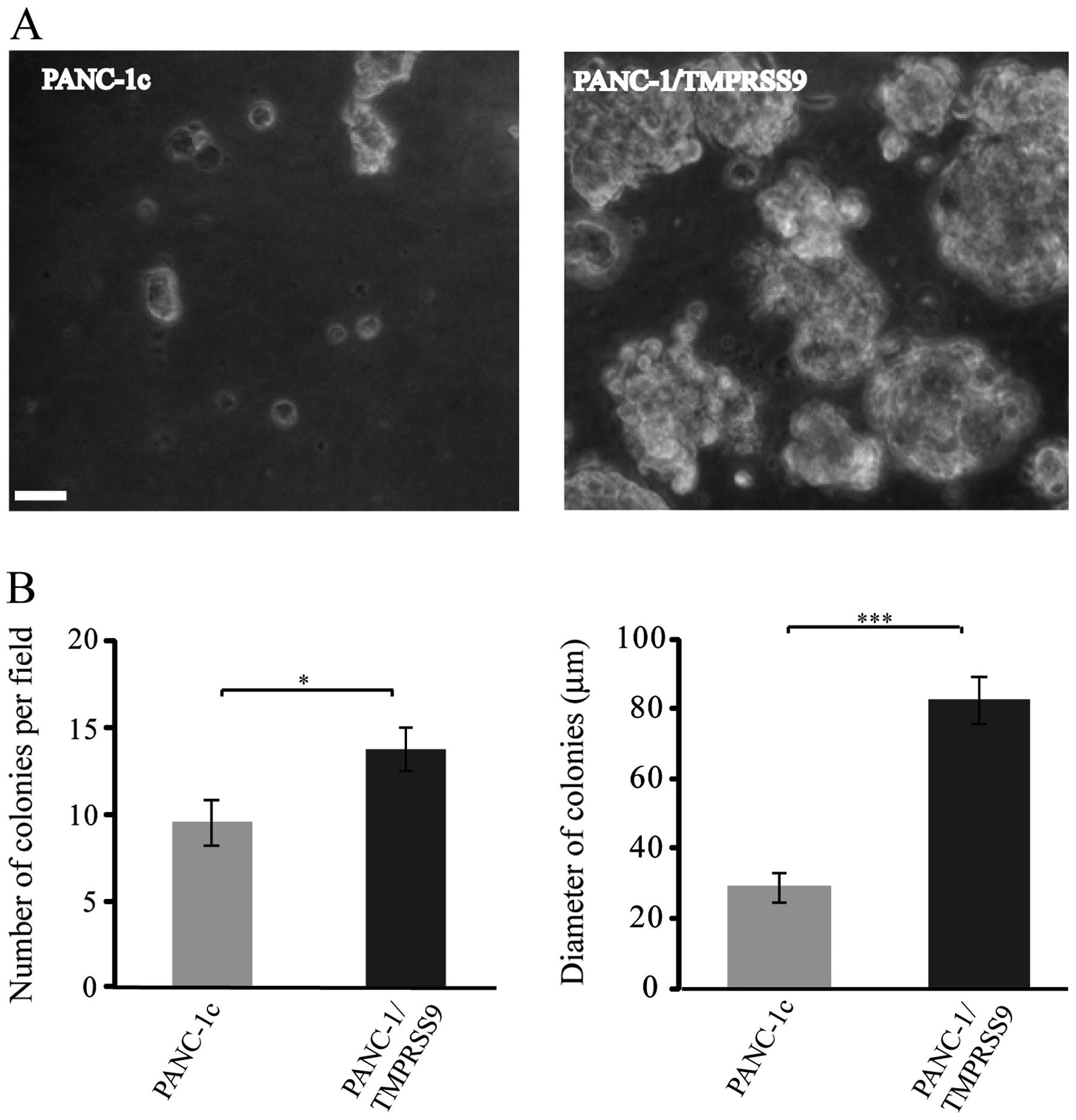
Polyserase-1 enhances
anchorage-independent growth of PANC-1 cells
Results obtained from invasive assays prompted us to
perform new cell-based assays using PANC-1, a cell line commonly
employed as an in vitro model of pancreatic cancer for
tumorigenicity studies since its invasive and proliferative
phenotypes have been widely characterized (24). Thus, we carried out a soft-agar
colony formation assay to compare the PANC-1/TMPRSS9 and PANC-1c
cells and, as shown in Fig. 2A, the
colonies formed by cells expressing polyserase-1 were more numerous
(14 vs. 9) and much larger (82 μm vs. 30 μm in diameter) than those
formed by the PANC-1c cells after 8 days of growth (Fig. 2B). These data are also consistent
with the hypothesis that polyserase-1 acts a pro-tumor
protease.
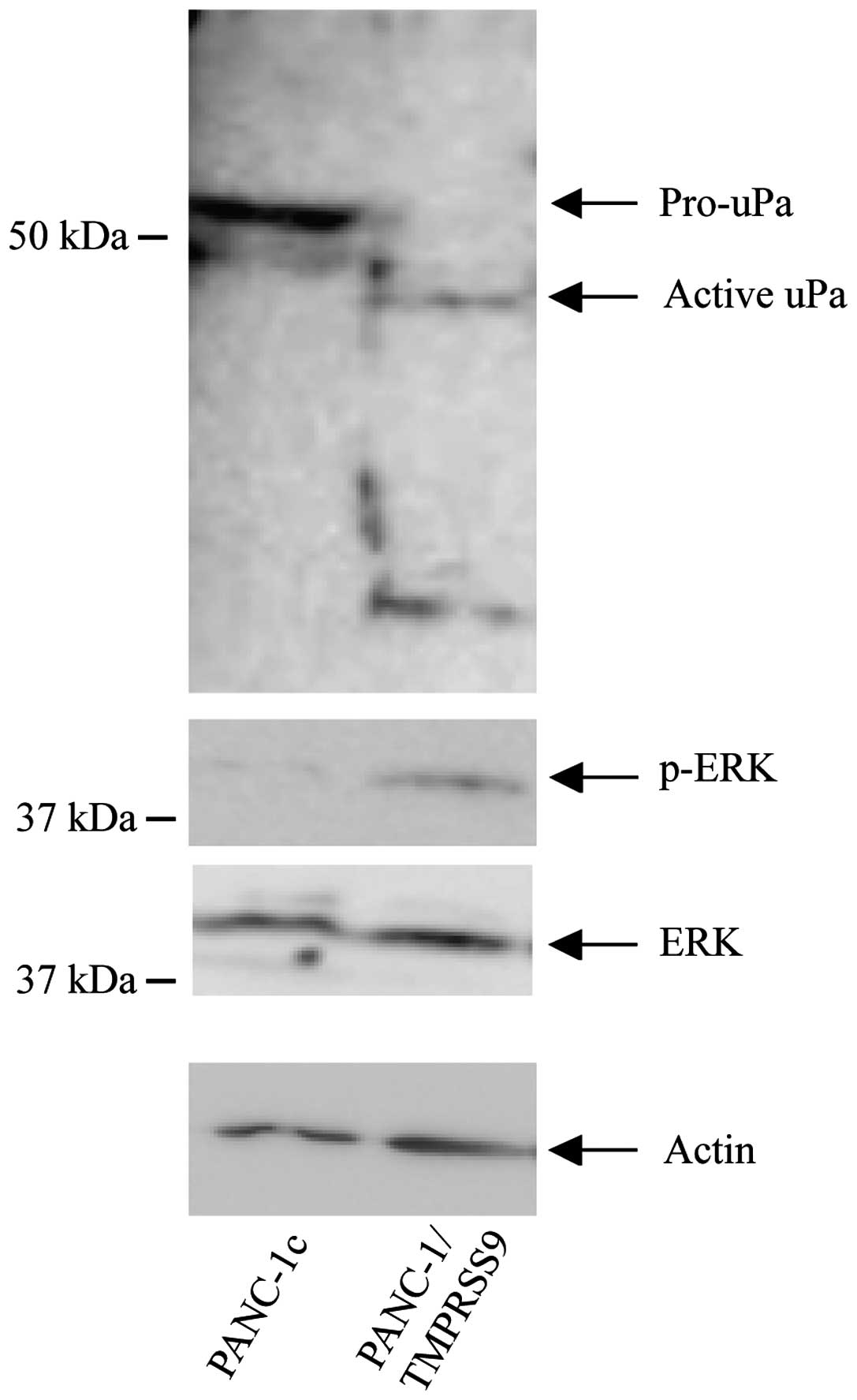
Presence of polyserase-1 activates
pro-uPA and increases phosphorylation levels of ERK
Different mechanisms have been proposed to underlie
the pro-tumor properties associated with the activities of TTSPs,
including activation of pro-uPA or modulation of the ERK signaling
pathway (23). We examined whether
exogenous polyserase-1 also affects these mechanisms in PANC-1
cells. Toward this end, we performed western blot analysis that
revealed a significant activation of pro-uPA and high levels of
phosphorylated ERK (p-ERK) in the PANC-1/TMPRSS9 but not in the
PANC-1c cells (Fig. 3). These
results suggest that TMPRSS9 may contribute to induce cancer cell
invasion by enhancing the activation of survival pathways in tumor
cells.
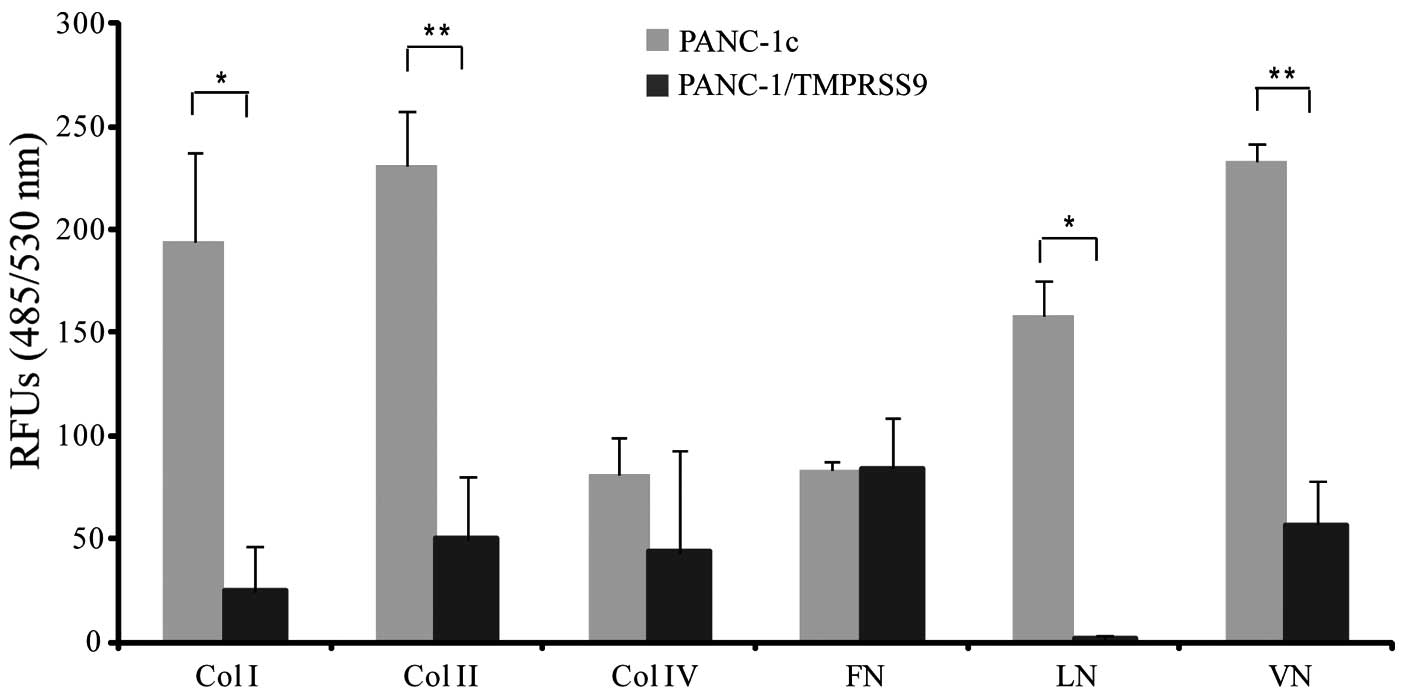
Polyserase-1 alters the adhesive
properties of PANC-1 cells
To evaluate whether polyserase-1 alters adhesion of
PANC-1 cells to different ECM substrates, we performed a
fluorometric assay using PANC-1/TMPRSS9 and PANC-1c cells. The
results clearly indicated that the presence of polyserase-1 reduced
the capacity of PANC-1 cells to bind to collagens as well as to
vitronectin, and completely abolished adherence to laminin
(Fig. 4). However, polyserase-1 did
not alter the binding capacity of PANC-1 cells to fibronectin.
Overall, these results indicated that polyserase-1 induced changes
in cell adhesion which eventually leads to increased tumor
progression.
Discussion
Proteolytic enzymes have been traditionally
associated with tumor progression. In fact, proteases produced by
cancer cells contribute not only to destroy the ECM barrier but
also to actively release growth factors to control cell survival.
However, recently, a growing number of proteases have also been
associated with tumor-protective functions (26). One future challenge includes the
identification of the precise functions of the proteolytic enzymes
related to tumorigenesis. In this regard, we evaluated the
potential contribution of polyserase-1/TMPRSS9 to tumorigenesis
employing cell-based assays. This unique enzyme belongs to the TTSP
family of membrane-anchored serine proteases and is widely
distributed in human and mouse tissues, yet its functional
relevance is largely unknown (18).
Polyserase-1 possesses three serine-protease domains in tandem each
of which shows a high degree of identity with other members of the
TTSP family, particularly with those belonging to the matriptase
subfamily (17). Different TTSPs
such as TMPRSS4, initially referred to as TMPRSS3 (20) and matriptase (21) have been shown to display pro-tumor
activity in various types of tumors, including pancreatic
carcinoma, one of the most aggressive cancers. In the present
study, we demonstrated that polyserase-1 also acts as a pro-tumor
protease. Thus, the presence of this enzyme increased the invasive
properties of both SK-PC-3 and PANC-1 pancreatic cancer cells using
Matrigel-coated invasion chambers. Moreover, different reports have
illustrated the usefulness of PANC-1 to examine the effect of the
overexpression (27) or
downregulation (28) of particular
genes on anchorage-independent growth, a hallmark of malignant
transformation. Using similar approaches, we also found that the
presence of polyserase-1 considerably enhanced this effect in
PANC-1 cells.
Serase-1B, a spliced variant of polyserase-1
containing one serine protease domain, is able to activate pro-uPA
(18), a serine protease largely
involved in tumor progression. PANC-1 cells expressing polyserase-1
were also found to exhibit significant pro-uPA activation when
compared to PANC-1 control cells. Furthermore, the presence of
polyserase-1 also increased levels of ERK phosphorylation in this
cell line. Similar effects have been previously associated to
pro-tumor TTPSs. For example, TMPRSS4 upregulates uPA expression
and activation in the DU145 prostate cancer and the NCI-H322 lung
cancer cell lines by a mechanism involving modulation of the ERK
signaling pathway (23). Matriptase
is also able to activate pro-UPA in the ovarian cancer cell line
HRA (29) and the monocytic
leukemia cell line THP-1 (22). On
the other hand, induction of invasive and migratory capacities of
PANC-1 cells has been linked to activation of ERK by
phosphorylation (30).
Collectively, our data suggest that TMPRSS9 exerts pro-tumor
effects on PANC-1 cancer cells overcoming apoptotic signals induced
in anchorage-dependent cells when detaching from the surrounding
ECM.
ECM components also affect the adhesive properties
of tumor cells. In the case of PANC-1 cells, adhesion capacity to
type I, II and IV collagens, laminin, fibronectin and vitronectin
has been previously assessed (24).
Our data indicated that polyserase-1 reduces the adhesion capacity
of PANC-1 cells to collagens, laminin and vitronectin which may
contribute to the promotion of cell migration and invasion
(31). In reference to other TTSPs,
overexpression of matriptase and TMPRSS4 was also found to alter
the adhesion profile of tumor cells to ECM components thereby
contributing to facilitate their invasive and metastatic properties
(32,33).
In summary, in the present study, we provide initial
evidence suggesting that polyserase-1 induces pro-tumor activities
in pancreatic cancer cells and that uPA and ERK may participate in
these processes. Consequently, polyserase-1 may be involved in
tumor progression, and inhibition of its activity could contribute
to prevent progression of this malignant tumor. Further exhaustive
in vivo functional studies will help to shed light on the
participation of polyserase-1 in tumorigenesis. In this regard,
generation of mice lacking TMPRSS9 may be a useful tool to
understand the precise role of this polyserase-1 not only in
pancreatic carcinoma but also in tumors from different origins.
Acknowledgements
We thank Dr Carlos Lopez-Otin for his comments and
suggestions. The present study was supported by the Grant
PI11/00371 from the Instituto de Investigacion Carlos III,
Ministerio de Economia y Competitividad (Spain).
References
|
1
|
Hornebeck W, Emonard H, Monboisse JC and
Bellon G: Matrix-directed regulation of pericellular proteolysis
and tumor progression. Semin Cancer Biol. 12:231–241. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wilkins-Port CE, Higgins SP, Higgins CE,
Kobori-Hotchkiss I and Higgins PJ: Complex regulation of the
pericellular proteolytic microenvironment during tumor progression
and wound repair: functional interactions between the serine
rrotease and matrix metalloproteinase cascades. Biochem Res Int.
2012:4543682012.
|
|
3
|
Netzel-Arnett S, Hooper JD, Szabo R, et
al: Membrane anchored serine proteases: a rapidly expanding group
of cell surface proteolytic enzymes with potential roles in cancer.
Cancer Metastasis Rev. 22:237–258. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu D, Yuan X, Zheng X and Sadler JE:
Bovine proenteropeptidase is activated by trypsin, and the
specificity of enteropeptidase depends on the heavy chain. J Biol
Chem. 272:31293–31300. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gladysheva IP, Wang D, McNamee RA, et al:
Corin overexpression improves cardiac function, heart failure, and
survival in mice with dilated cardiomyopathy. Hypertension.
61:327–332. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Folgueras AR, de Lara FM, Pendas AM, et
al: Membrane-bound serine protease matriptase-2 (Tmprss6) is an
essential regulator of iron homeostasis. Blood. 112:2539–2545.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guipponi M, Tan J, Cannon PZ, et al: Mice
deficient for the type II transmembrane serine protease,
TMPRSS1/hepsin, exhibit profound hearing loss. Am J Pathol.
171:608–616. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu Q: Type II transmembrane serine
proteases. Curr Top Dev Biol. 54:167–206. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Webb SL, Sanders AJ, Mason MD and Jiang
WG: Type II transmembrane serine protease (TTSP) deregulation in
cancer. Front Biosci. 16:539–552. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hashimoto T, Kato M, Shimomura T and
Kitamura N: TMPRSS13, a type II transmembrane serine protease, is
inhibited by hepatocyte growth factor activator inhibitor type 1
and activates pro-hepatocyte growth factor. FEBS J. 277:4888–4900.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu Q and Parry G: Hepsin and prostate
cancer. Front Biosci. 12:5052–5059. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen CH, Su KY, Tao MH, et al: Decreased
expressions of hepsin in human hepatocellular carcinomas. Liver
Int. 26:774–780. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Szabo R and Bugge TH: Type II
transmembrane serine proteases in development and disease. Int J
Biochem Cell Biol. 40:1297–1316. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kosa P, Szabo R, Molinolo AA and Bugge TH:
Suppression of Tumorigenicity-14, encoding matriptase, is a
critical suppressor of colitis and colitis-associated colon
carcinogenesis. Oncogene. 31:3679–3695. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lang JC and Schuller DE: Differential
expression of a novel serine protease homologue in squamous cell
carcinoma of the head and neck. Br J Cancer. 84:237–243. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Viloria CG, Peinado JR, Astudillo A, et
al: Human DESC1 serine protease confers tumorigenic properties to
MDCK cells and it is upregulated in tumours of different origin. Br
J Cancer. 97:201–209. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cal S, Quesada V, Garabaya C and
Lopez-Otin C: Polyserase-I, a human polyprotease with the ability
to generate independent serine protease domains from a single
translation product. Proc Natl Acad Sci USA. 100:9185–9190. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okumura Y, Hayama M, Takahashi E, et al:
Serase-1B, a new splice variant of polyserase-1/TMPRSS9, activates
urokinase-type plasminogen activator and the proteolytic activation
is negatively regulated by glycosaminoglycans. Biochem J.
400:551–561. 2006. View Article : Google Scholar
|
|
19
|
Cantero D, Friess H, Deflorin J, et al:
Enhanced expression of urokinase plasminogen activator and its
receptor in pancreatic carcinoma. Br J Cancer. 75:388–395. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wallrapp C, Hahnel S, Muller-Pillasch F,
et al: A novel transmembrane serine protease (TMPRSS3)
overexpressed in pancreatic cancer. Cancer Res. 60:2602–2606.
2000.PubMed/NCBI
|
|
21
|
Uhland K, Siphos B, Arkona C, et al: Use
of IHC and newly designed matriptase inhibitors to elucidate the
role of matriptase in pancreatic ductal adenocarcinoma. Int J
Oncol. 35:347–357. 2009.PubMed/NCBI
|
|
22
|
Kilpatrick LM, Harris RL, Owen KA, et al:
Initiation of plasminogen activation on the surface of monocytes
expressing the type II transmembrane serine protease matriptase.
Blood. 108:2616–2623. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Min HJ, Lee Y, Zhao XF, et al: TMPRSS4
upregulates uPA gene expression through JNK signaling activation to
induce cancer cell invasion. Cell Signal. 26:398–408. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deer EL, Gonzalez-Hernandez J, Coursen JD,
et al: Phenotype and genotype of pancreatic cancer cell lines.
Pancreas. 39:425–435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vila MR, Lloreta J, Schussler MH, Berrozpe
G, Welt S and Real FX: New pancreas cancers cell lines that
represent distinct stages of ductal differentiation. Lab Invest.
72:395–404. 1995.PubMed/NCBI
|
|
26
|
Noel A, Gutierrez-Fernandez A, Sounni NE,
et al: New and paradoxical roles of matrix metalloproteinases in
the tumor microenvironment. Front Pharmacol. 3:1402012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ochi N, Tanasanvimon S, Matsuo Y, et al:
Protein kinase D1 promotes anchorage-independent growth, invasion,
and angiogenesis by human pancreatic cancer cells. J Cell Physiol.
226:1074–1081. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo XZ, Xu JH, Liu MP, et al: KAI1
inhibits anchorage-dependent and -independent pancreatic cancer
cell growth. Oncol Rep. 14:59–63. 2005.PubMed/NCBI
|
|
29
|
Suzuki M, Kobayashi H, Kanayama N, et al:
Inhibition of tumor invasion by genomic down-regulation of
matriptase through suppression of activation of receptor-bound
pro-urokinase. J Biol Chem. 279:14899–14908. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu J, Ben QW, Yao WY, et al: BMP2 induces
PANC-1 cell invasion by MMP-2 overexpression through ROS and ERK.
Front Biosci. 17:2541–2549. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Touab M, Villena J, Barranco C, Arumi-Uria
M and Bassols A: Versican is differentially expressed in human
melanoma and may play a role in tumor development. Am J Pathol.
160:549–557. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Welman A, Sproul D, Mullen P, et al:
Diversity of matriptase expression level and function in breast
cancer. PLoS One. 7:e341822012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jung H, Lee KP, Park SJ, et al: TMPRSS4
promotes invasion, migration and metastasis of human tumor cells by
facilitating an epithelial-mesenchymal transition. Oncogene.
27:2635–2647. 2008. View Article : Google Scholar
|