Introduction
Glioblastoma multiforme (GBM, grade IV astrocytoma)
is considered to be the most highly malignant brain tumor (1). GMB is highly aggressive and currently
incurable. While many patients with low-grade gliomas survive for
many years, the median survival time of GBM patients is only 12
months (2). The poor prognosis is
mainly attributed to the highly diffusive growth pattern of GBM
cells into surrounding brain tissue, thereby preventing complete
surgical removal (3). There has
been a negligible decrease in the mortality rate of GBM patients
over the past few decades, indicating that GBM is a complex disease
and not easy to cure. Therefore, the development of more effective
therapies for GBM must cope with recurrence through elimination of
residual single cells or microscopic cell clusters.
Cancer stem cells (CSCs) are a subpopulation of
tumor cells with the ability to undergo self-renewal and propagate
the tumor population (4).
Increasing evidence suggests that CSCs also exist in the GBM
population and this CSC subpopulation is referred to as glioma stem
cells (GSCs) (5–9). GSCs possess the abilities for
self-renewal and multiple differentiation, but also have greater
tumorigenic potential than matched non-stem tumor cells when
xenotransplanted into immunocompromised rats (7). In addition, GSCs are resistant to
chemotherapy and radiotherapy (10,11).
Conventional cancer treatments often fail to eliminate GSCs
completely, leading to tumor repopulation and relapse (12,13).
Thus, targeting GSCs is considered a novel therapeutic avenue to
more effectively eradicate malignant tumors and reduce the risk of
relapse.
Tanshinone IIA is a lipophilic diterpene isolated
from Salvia miltiorrhiza (Danshen), a widely used Chinese
herbal medicine (14). A number of
studies have shown that tanshinone IIA possesses anticancer
(15,16), anti-inflammatory (17,18)
and anti-oxidative (19) activities
in vitro and in vivo through the inhibition of
special signaling pathways (20,21),
suggesting it may be an effective chemotherapeutic agent for
malignant tumor therapy.
Previous studies have shown that CSCs are maintained
by inflammatory signaling pathways and the microenvironment
(22,23) in which inflammatory cytokine
interleukin 6 (IL6) (22) plays an
important role. Tanshinone IIA was found to exhibit a potent
inhibitory effect on the expression of inflammatory cytokines
(21,24). Therefore, the anti-inflammatory
activity of tanshinone IIA may be a potential therapeutic strategy
for tumor inhibition through the downregulation or attenuation of
the expression of inflammatory cytokines.
In the present study, we investigated the effect of
serum-free medium (SFM) on GSC formation and examined the effects
of tanshinone IIA on human GSCs in vitro and in vivo,
and aimed to elucidate the potential molecular mechanisms of its
antitumor effect.
Materials and methods
Cell culture and clone formation
Human WJ1 (25)
cells were cultured in DMEM/F12 supplemented with 10% FBS (SCM),
penicillin (100 U/ml) and streptomycin (100 μg/ml) in a humidified
atmosphere of 50 μg/ml CO2 at 37°C until formation of an
adherent monolayer of GBM cells. Then cells from the serum-grown
cultures were dissociated and cultured with serum-free DMEM/F12
medium supplemented with 20 ng/ml EGF, 20 ng/ml bFGF, and 1X B27
(Invitrogen, Carlsbad, CA, USA), until primary neurosphere
formation (26). Neurospheres were
photographed using an inverted microscope after neurosphere
formation.
RT-PCR
Total RNA was harvested from cells using TRIzol
reagent (CWBIO). PCR was performed on cDNA generated by HIFI-MMLV
reverse transcriptase (CWBIO) in a total reaction volume of 20 μl
according to the manufacturer’s instructions. The sequences of
forward and reverse oligonucleotide primers, specific to the chosen
candidates and housekeeping genes, were used as follows: CD133
forward, 5′-CGACTGAGACCCAACATC-3′ and reverse, 5′-CCCTTTT
GATACCTGCTACGAC-3′; GFAP forward, 5′-CGATCAACT CACCGCCAACA-3′ and
reverse, 5′-GTGGCTTCATCTGCT TCCTGTC-3′; GAPDH forward,
5′-ACCACAGTCCATGCCA TCAC-3′ and reverse,
5′-TCCACCACCCTGTTGCTGTA-3′. All data were normalized to GAPDH
transcript levels.
Western blotting
Western blotting was performed as previously
described (3). Rabbit polyclonal
antibodies for human CD133 (1:500 dilution; Signalway Antibody,
Preland, TX, USA), nestin, GFAP, βIII-tubulin, IL6, Bax, cleaved
caspase-3, Bcl2, signal transducer and activator of transcription 3
(STAT3), phospho-STAT3(tyrosine705) and phospho-STAT3(serine727)
(1:500 dilution; Bios Biotechnology, Beijing, China) were used
according to the manufacturer’s instructions. Antibody recognition
was detected with the peroxidase-conjugated goat anti-rabbit IgG
(H+L) secondary antibody (Zhongshan Goldenbridge Biotechnology,
Beijing, China) using a 1:3,000 dilution. Antibody-bound proteins
were detected by the BeyoECL Plus kit (Beyotime Institute of
Biotechnology, Shanghai, China) and western blotting system
(Universal Hood II, Bio-Rad, USA), and normalized to β-actin and
quantified using the ChemiDoc™ XRS (Bio-Rad).
Tumorigenicity assay
To evaluate the tumorigenicity of the GBMS cells,
1×104, 1×105, 1×106 of GBM cells
and 1×102, 1×103, 1×104 of GBMS
cells were suspended in 50 μl Matrigel (BD Biosciences) and 50 μl
PBS, and then cells were implanted bilaterally into the flank of
BALB/c nu/nu mice. Animals were maintained under standard
conditions according to the guidelines of the Institutional Animal
Care and Use Committee of Sichuan University. Tumor incidence was
recorded 3 times weekly after the injection. Mice were maintained
on a standard rodent chow and had free access to water. Tumors of
the euthanized mice were collected, and their size and weight were
measured.
Inhibition of colony formation
Single-cell suspensions of GBMS were seeded in a
96-well plate at a density of 100 cells per well. Cells were
treated with tanshinone IIA at concentrations of 0.25, 0.5 and 1.0
μg/ml respectively. Each concentration was repeated in 6 wells.
After a 7-day incubation at 37°C in a humidified 5% CO2
atmosphere, the number of neurospheres in each well was quantified
under a microscope. Three independent experiments were
performed.
MTT assay
The 3-[4,5-dimethylthiazol-2-yl]-2, 5
diphenyltetrazolium bromide (MTT) assay was performed as previously
described (27). GBMS, GBM and LO2
(normal control) cells (2×103/well) were seeded in 100
μl of medium/well in 96-well plates. After a 24-h incubation,
tanshinone IIA was added at various concentrations (0.125, 0.25,
0.5, 1.0 and 2.0 μg/ml); 5 wells were included for each
concentration. The effects of tanshinone IIA on the viability of
GBMS, GBM and normal cells was expressed as the % cytoviability,
using the following formula: % cytoviability = A490 of the treated
cells/A490 of the control cells (without tanshinone IIA) × 100%
(28). Three independent
experiments were performed.
Annexin V-FITC assay
In order to determine apoptosis in GBMS cells, the
Annexin V-FITC apoptosis detection kit (KeyGen Biotech, Nanjing,
China) was used according to the manufacturer’s instructions. The
extent of apoptosis in the samples was measured by flow cytometry
using BD FACSCalibur flow cytometer and CellQuest software. Three
independent experiments were performed.
In vivo inhibition assay
The flank xenografts were established by
subcutaneous injections of 104 GSCs per flank (5 mice
per group). Animals were maintained under standard conditions
according to the guidelines of the Institutional Animal Care and
Use Committee of Sichuan University. After one week of inoculation,
the tumor-bearing mice were randomized into four groups, each
having 5 mice. The mice of the three treatment groups were injected
with 10, 20 and 40 mg/kg of tanshinone IIA i.p. 3 times a week for
8 weeks, respectively, and the control mice were injected with an
equal volume of blank dissolvant. In the experimental process, mice
were weighed, and tumor volumes were assessed each week. At the end
of the experiment, the mice were sacrificed by carbon dioxide
asphyxiation and tumors were harvested, weighed and examined. The
tumor inhibitory rate was calculated using the following formula:
Tumor inhibitory rate (%) = (mean tumor weight of the control mice
− mean tumor weight of the treated mice)/average tumor weight of
the control mice × 100%.
Statistical analysis
Results are expressed as means ± standard deviation.
To evaluate the significant differences between two groups, the
means were compared using the Student’s t-test. Multiple group
comparisons were performed using one-way analysis of variance. The
level of statistical significance was defined as P<0.05 for all
tests. All statistical analyses were performed using SPSS 16.0
(SPSS, Inc., Chicago, IL, USA)
Results
GBMS exhibit characteristics of GSCs in
vitro
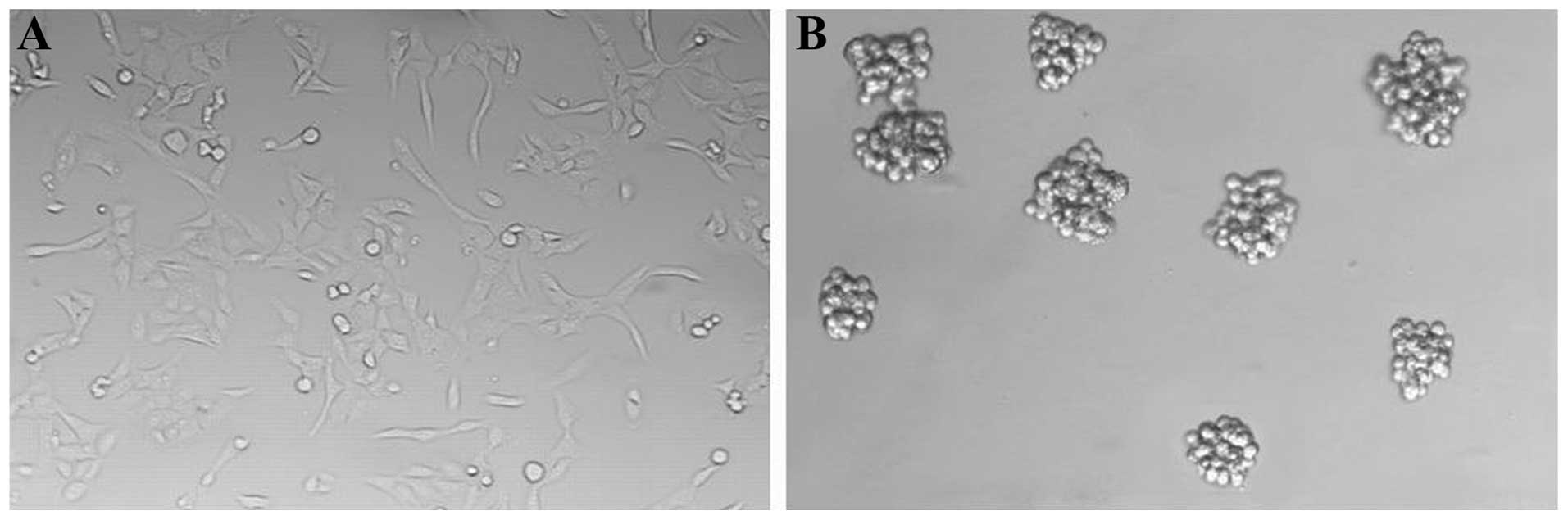
GBM cells exhibited adherent growth in SCM (Fig. 1A). We switched the GBM cells into
culture conditions for GSC proliferation in vitro. Primary
neurosphere-like colonies (GBM neurospheres; GBMS) were observed in
the SFM after 10–14 days (Fig. 1B).
We next digested these neurosphere-like colonies into single-cell
suspensions and were seeded at a clonal density in the SFM in a
24-well plate. Subspheres appeared after culturing for 7–10 days.
This result indicated that GBMS contained individual stem-like
cells with the ability to self-renew and form new spheres.
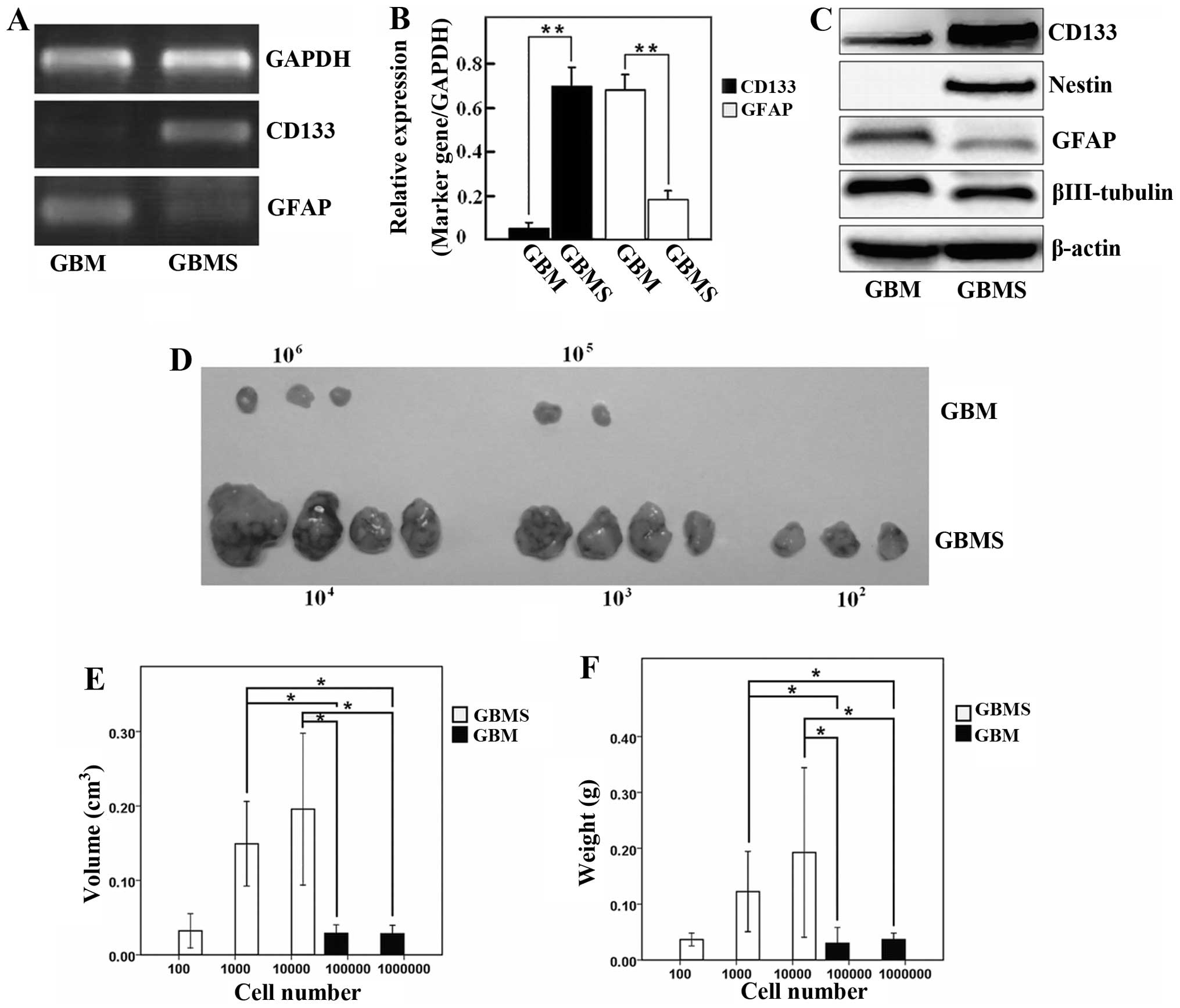
The level of the CD133 transcript was significantly
higher in the GBMS when compared with the GBM cells. In contrast,
the GFAP transcript was obviously lower in the spheres than that in
the GBM cells (Fig. 2A and B;
P<0.05). Western blotting data showed that the protein levels of
stemness and differentiation markers of GSCs were in agreement with
the immunofluorescence and gene expression (Fig. 2C). These results indicated that the
stem-like characteristic of GBMS were enriched when grown in
SFM.
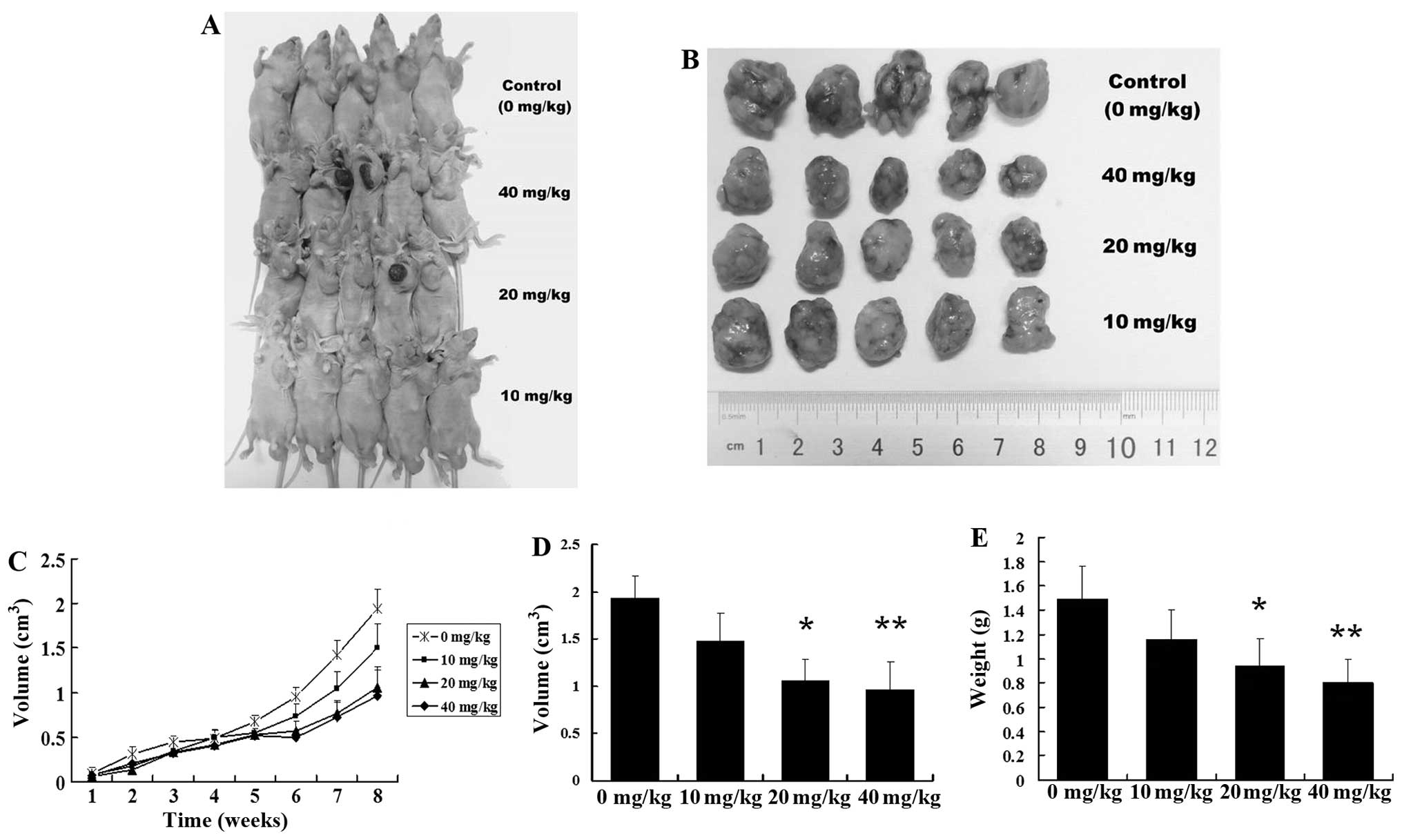
GBMS display higher tumorigenicity than
GBM cells in vivo
To confirm the different tumor-initiating
capabilities between GBMS and GBM cells in vivo, both
spheres (1×102, 1×103, 1×104
cells) and adherent monolayer cells (1×104,
1×105, 1×106 cells) were implanted
bilaterally into the flank of BALB/c nu/nu mice for analysis of
transplanted tumorigenicity. GBMS cells generated tumors when only
1×102 cells were injected into mice. In contrast, at
least 1×105 GBM cells were needed to generate tumors,
suggesting that GBMS contain more tumor-initiating cells by at
least 1,000-fold when compared to this number in the parental
cells. A comparative analysis of gross appearance revealed the
presence of significant differences regarding the size and mass
between the tumors newly generated from the GBM and GBMS cells
after implantation for 6 weeks (Fig.
2D–F; P<0.05). This result indicates that the tumorigenicity
of GBMS cells was significantly higher than that of the GBM
cells.
Tanshinone IIA inhibits GSC (GBMS)
proliferation in vitro
To determine whether tanshinone IIA suppresses GSC
proliferation, we compared the inhibitory effect of tanshinone IIA
on human GSCs, GBM and normal control (LO2) cells and under proper
drug concentrations after treatment for 1–5 days, respectively. The
inhibition of tanshinone IIA at various concentrations in these
cells was determined as the ratio of the number of viable treated
cells to the number of viable cells of the untreated controls.
Tanshinone IIA exhibited a dose- and time-dependent inhibitory
effect on GSCs, GBM and normal cells (Fig. 3A–C). The IC50 value was
2.542±0.34 μg/ml for normal cells, 2.066±0.135 μg/ml for GBM cells
and 1.812±0.196 μg/ml for GSCs, respectively, after 72 h of
treatment (Fig. 3D; P<0.05). Our
results showed that, compared with GBM and normal cells, GSCs were
more sensitive to tanshinone IIA in vitro, suggesting that
tanshinone IIA possesses stronger inhibitory activity against
GSCs.
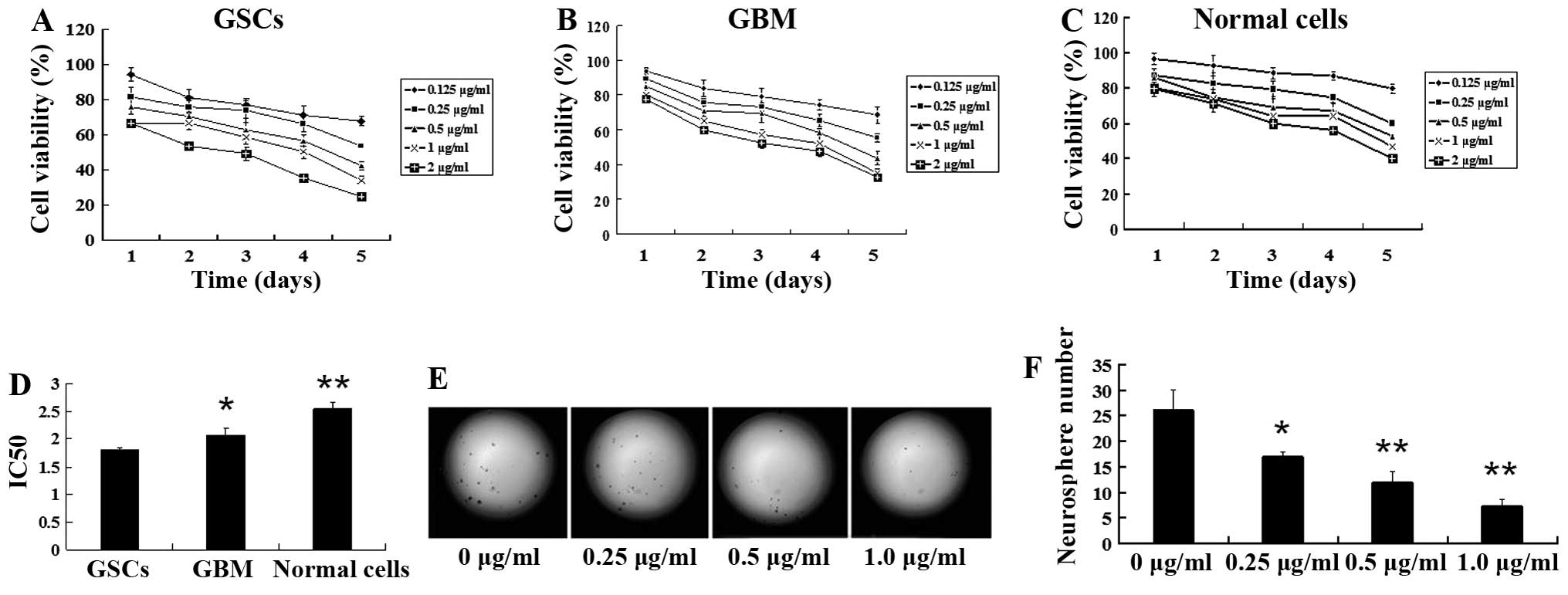 | Figure 3Tanshinone IIA suppresses neurosphere
formation and proliferation of GSCs. GBMS, GBM and LO2 (normal
control) cells were treated with 0.125, 0.25, 0.5, 1.0 and 2.0
μg/ml of tanshinone IIA and incubated for 1, 2, 3, 4 and 5 days,
respectively. Cell viability was detected by MTT assay, using the
method indicated in Materials and methods. Tanshinone IIA exhibited
a dose- and time-dependent inhibitory effect on (A) GBMS, (B) GBM
and (C) normal control cells. Tanshinone IIA exhibited a more
significant inhibitory effect in the GBMS cells than in the GBM and
LO2 cells. (D) The half inhibitory concentration (IC50)
values for the GSCs, GBM and normal cells after treatment with
tanshinone IIA for 3 days are shown (72 h; *P<0.05,
**P<0.01). (E) GSCs were plated in 96-well plates at
a density of 100 cells/well and treated with the indicated
concentrations of tanshinone IIA. Representative examples of
neurospheres from GSCs on day 7 are shown. Tanshinone IIA decreased
neurosphere number in a dose-dependent manner (magnification ×200).
(F) The histogram shows that there was a significant decrease in
the number of neurospheres after tanshinone IIA treatment in a
dose-dependent manner; **P<0.01. Results are mean
values ± SD of independent experiments performed in triplicate. |
Tanshinone IIA inhibits neurosphere
formation
To test the inhibitory effect of tanshinone IIA on
neurosphere formation in GSCs, we incubated single-cell suspensions
of GBMS with escalating concentrations of tanshinone IIA and
evaluated neurosphere formation on day 7. We found that there was a
dose-dependent reduction in neurosphere formation after treatment
with tanshinone IIA (Fig. 3E). The
mean number of neurospheres in the control were 26.0±4.0 after
seeding 100 cells per well, whereas the mean numbers of
neurospheres treated with 0.25, 0.5 and 1.0 μg/ml of tanshinone IIA
for 48 h were 17.0±1.0 (P<0.05), 12.0±2.0 (P<0.01) and
7.33±1.15 (P<0.01), respectively (Fig. 3F). A significant reduction in the
number of neurospheres was observed at concentrations of tanshinone
IIA as low as 0.25 μg/ml. Following exposure to tanshinone IIA at a
dose of 1.0 μg/ml, neurosphere formation of GSC decreased
sharply.
Tanshinone IIA inhibits the growth of
GSC-derived tumors
To investigate the inhibitory activity of tanshinone
IIA on human GSCs in vivo, human GSC-bearing mice were
injected with 10, 20 or 40 mg/kg of tanshinone IIA i.p. 3 times a
week for 8 weeks. Tanshinone IIA potently inhibited the growth of
GSC-derived xenografts with a reduction in tumor weight and volume
(Fig. 4A–C). In contrast, the
controls were not significantly inhibited in terms of tumor growth.
The mean tumor volume of the control mice was 1.92±0.40
cm3, and those of the mice injected with 10, 20, and 40
mg/kg of tanshinone IIA were 1.34±0.49, 1.02±0.38 (P<0.05) and
0.90±0.36 cm3 (P<0.01), respectively (Fig. 4D). The mean tumor weight of the
control mice was 1.49±0.27 g, and those of the mice injected with
10, 20 and 40 mg/kg of tanshinone IIA were 1.16±0.24, 0.94±0.22
(P<0.05) and 0.80±0.20 g (P<0.01), respectively (Fig. 4E). The tumor inhibitory rates were
22.14, 36.91 (P<0.05) and 46.30% (P<0.05), respectively. No
evidence of toxicity was identified in the treated animals by
comparing the body weight increase, histopathological changes in
major organs, and blood biochemistry analysis of both the control
and the treated group animals (data not shown).
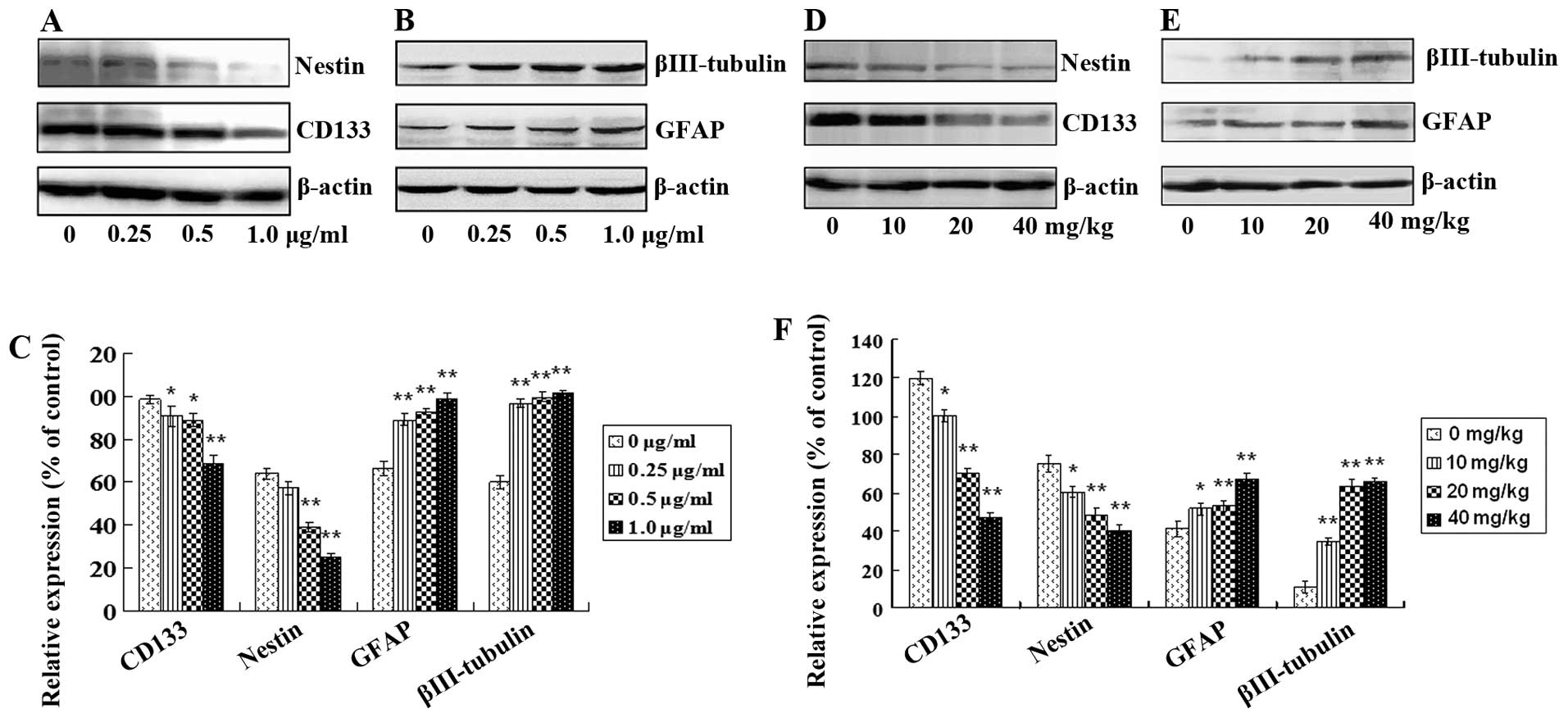
Tanshinone IIA attenuates GSC stemness
and induces GSC apoptosis
After treatment with tanshinone IIA, protein
expression levels of GSC markers including CD133 and nestin and
differentiation and neural lineage markers including GFAP for
astrocytes and βIII-tubulin for neurons in vitro and in
vivo were analyzed. CD133 and nestin in GSCs were decreased
both in vitro and in vivo in a dose-dependent manner
(Fig. 5A and D; Fig. 5C and F; P<0.05). In contrast, the
levels of GFAP and βIII-tubulin were increased in a dose-dependent
manner (Fig. 5B and E; Fig. 5C and F; P<0.05). Furthermore,
tanshinone IIA treatment resulted in th apoptosis of GSCs. As shown
in Fig. 6A, the number of apoptotic
cells was significantly increased following treatment with
tanshinone IIA. Tanshinone IIA exposure resulted in a more than
3-fold increase in Annexin V-positive cells (Fig. 6B), which was highly significant
(P<0.01). Moreover, expression levels of apoptosis-related
proteins were examined in vitro and in vivo after
treatment with tanshinone IIA. Bax and cleaved caspase-3 (apoptotic
proteins) were increased and anti-apoptotic protein Bcl-2 was
decreased in a dose-dependent manner after treatment with 1.0, 2.0
or 4.0 μg/ml of tanshinone IIA for 24 h in vitro (Fig. 6C and D; P<0.05).
Apoptosis-related proteins in tumors from human GSC bearing-mice
were examined after treatment with 10, 20 and 40 mg/kg of
tanshinone IIA for 8 weeks. Bax and cleaved caspase-3 were
increased, and Bcl-2 was decreased in a dose-dependent manner
(Fig. 6E and F; P<0.05), which
was in consistent with the in vitro results. These results
indicate that tanshinone IIA has the potential not only to
attenuate GSC stemness, but also to induce GSC apoptosis in
vitro and in vivo.
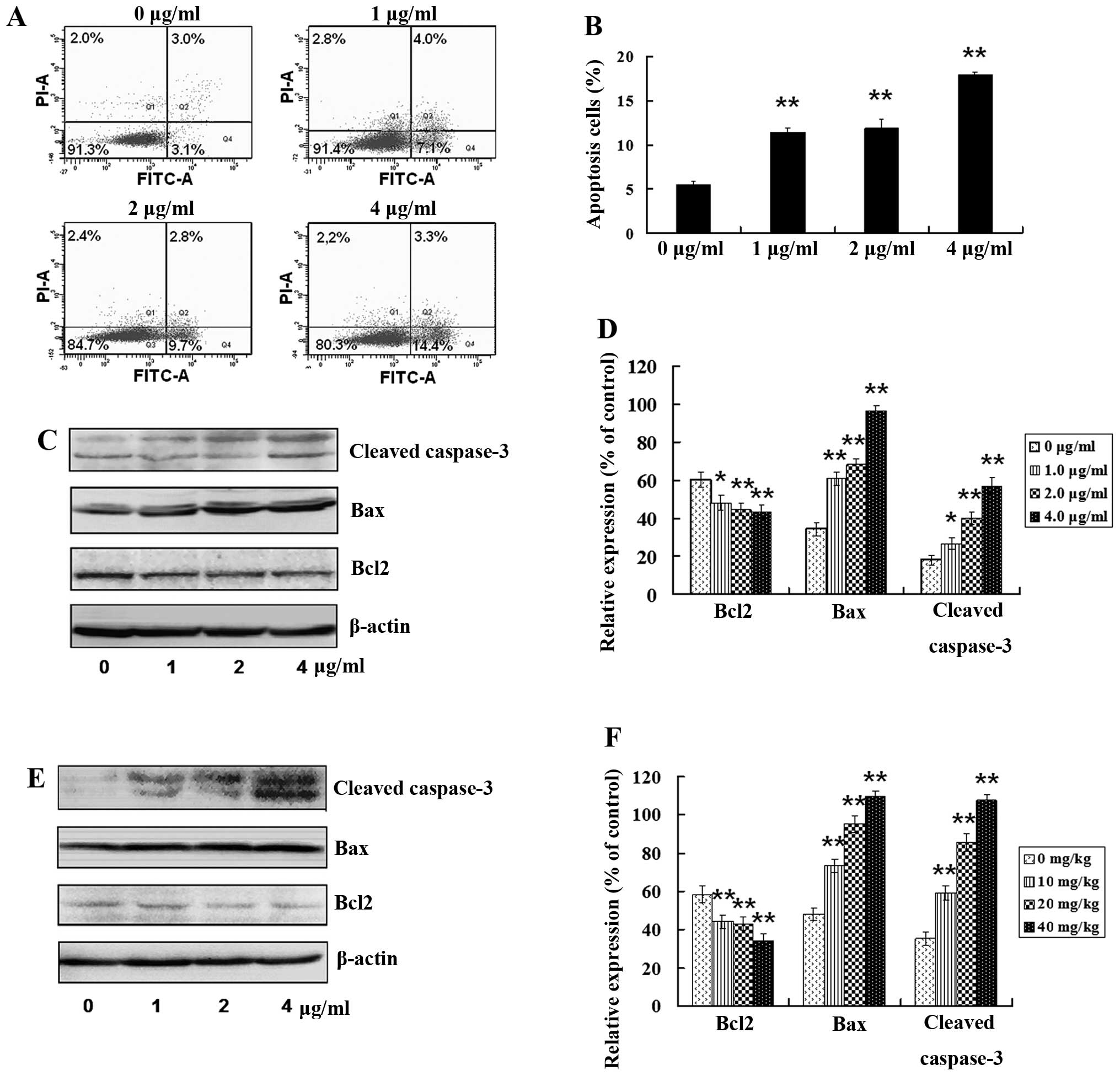 | Figure 6Tanshinone IIA induces GSC apoptosis.
(A) Following treatment with tanshinone IIA (1.0, 2.0 and 4.0
μg/ml) for 24 h, respectively, apoptosis in GSCs was detected by
flow cytometry. (B) Apoptotic rate was calculated. (C) GSCs were
treated with tanshinone IIA (1.0, 2.0 and 4.0 μg/ml) for 24 h,
respectively. The levels of apoptotic proteins Bax and cleaved
caspase-3 were upregulated, while the expression of anti-apoptosis
protein Bcl2 was downregulated in a dose-dependent manner. (D) The
histogram shows that there was a significant increase in Bax and
cleaved caspase-3, and a significant decrease in Bcl2 in
vitro in a dose-dependent manner after tanshinone IIA
treatment; *P<0.05, **P<0.01. (E) The
GSC-bearing mice were injected with 10, 20 or 40 mg/kg of
tanshinone IIA for 8 weeks, respectively, and the levels of the
apoptotic proteins were examined in tumor tissues. Bax, cleaved
caspase-3 were upregulated, while Bcl2 was downregulated in a
dose-dependent manner. (F) There was a significant increase in Bax
and cleaved caspase-3, and a significant decrease in Bcl2 in
vivo in a dose-dependent manner after tanshinone IIA treatment;
*P<0.05, **P<0.01. |
Tanshinone IIA regulates protein
expression mediating inflammatory signaling pathways in GSCs
Inflammatory cytokines play important roles in the
maintenance and progression of CSCs. To understand the molecular
mechanisms underlying the inhibitory effect of tanshinone IIA on
GSCs, we examined protein expression profiles mediating
inflammatory signaling pathways. Inflammatory cytokine IL6, STAT3
and the active form of STAT3 [(phospho-STAT3(tyrosine705) and
phospho-STAT3(serine727)] in GSCs in vitro and in
vivo were analyzed using western blotting. Expression levels of
IL6, phospho-STAT3(tyrosine705) and phospho-STAT3(serine727) were
higher in the GSCs from cells and tumor tissues (Fig. 7A and C, respectively). IL6,
phospho-STAT3(tyrosine705) and phospho-STAT3(serine727) were
decreased in vitro after treatment with 0.25, 0.5 and 1.0
μg/ml of tanshinone IIA for 48 h and in vivo after treatment
with 10, 20 and 40 mg/kg of tanshinone IIA for 8 weeks (Fig. 7B and D; P<0.05). However, no
significant expression change in STAT3 was observed after treatment
with different concentrations of tanshinone IIA (Fig. 7B and D; P>0.05). These results
suggest that IL6 and downstream activated STAT3 play roles in GSC
growth, and tanshinone IIA has the potential to block inflammatory
signaling pathways in GSCs by downregulating pathway-related
protein expression.
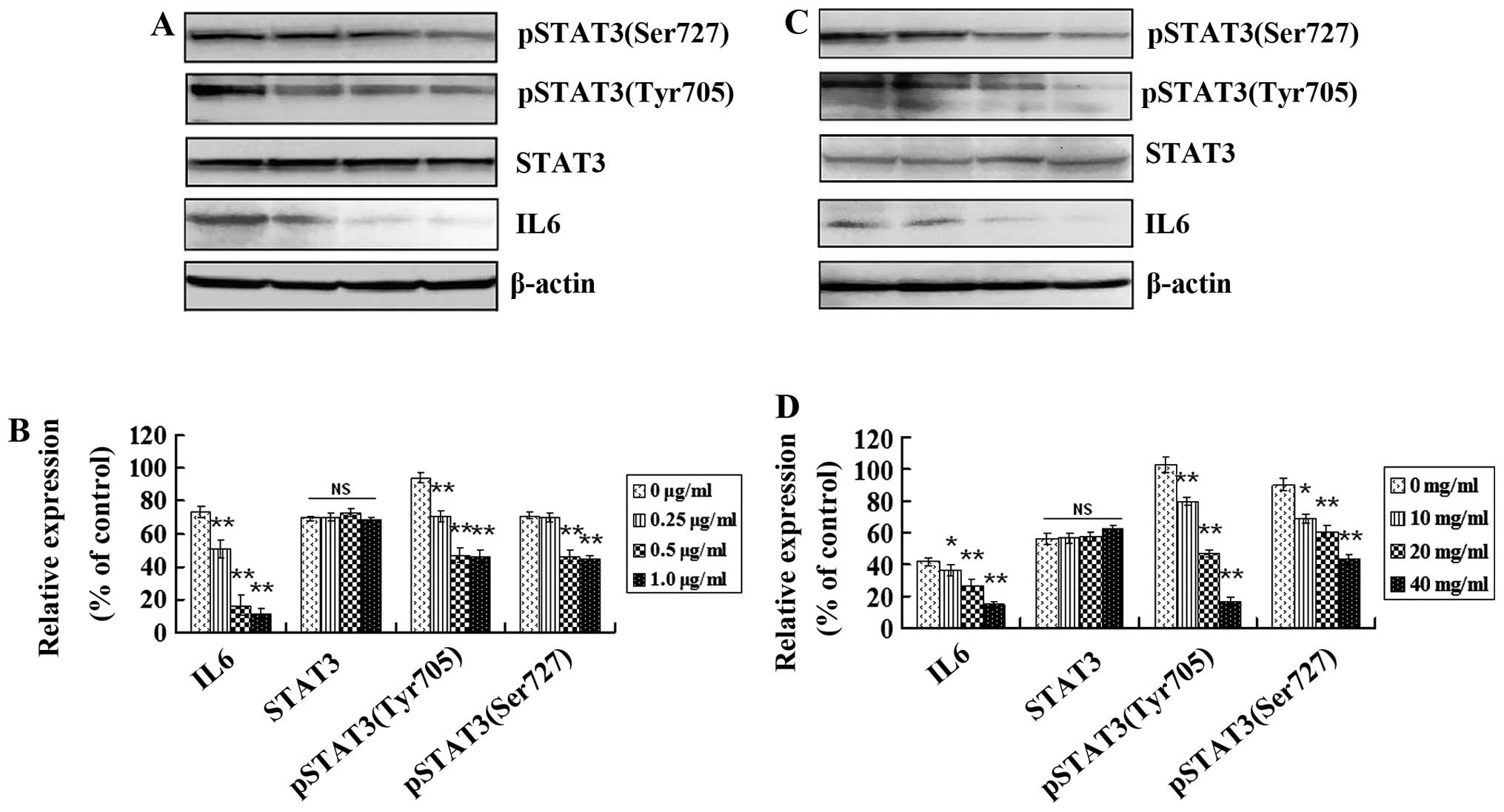 | Figure 7Tanshinone IIA regulates protein
expression mediating inflammatory signaling pathways in GSCs. (A)
Western blot analysis of IL6, STAT3, phospho-STAT3(tyrosine705) and
phospho-STAT3(serine727) proteins before and after tanshinone IIA
(0.25, 0.5 and 1.0 μg/ml) treatment for 48 h. (B) The histogram
shows that there was no change in STAT3 but a significant decrease
in IL6, phospho-STAT3(tyrosine705) and phospho-STAT3(serine727)
in vitro in a dose-dependent manner after tanshinone IIA
treatment; *P<0.05, **P<0.01. (C)
Western blot analysis of IL6, STAT3, phospho-STAT3(tyrosine705) and
phospho-STAT3(serine727) proteins before and after tanshinone IIA
treatment (10, 20, and 40 mg/kg) in vivo. (D) There was no
change in STAT3 but a significant decrease in IL6,
phospho-STAT3(tyrosine705) and phospho-STAT3(serine727) in
vivo in a dose-dependent manner after tanshinone IIA treatment;
*P<0.05, **P<0.01. |
Discussion
GBM is an extremely lethal tumor that is highly
resistent to therapy, and achieving a cure through surgical
intervention is difficult. Previous research has shown that
therapeutic resistance of GBM is caused by the selective survival
of highly tumorigenic GSCs (9). It
is critical for cancer therapy that treatments target and eliminate
these resistent population of stem-like cells (29). Therefore, it is necessary to culture
and enrich GSCs in GBM cells in order to identify their aggressive
mechanisms and then aim to eliminate GSCs in patients.
In the present study, we successfully isolated GBMS
from the human GBM cell line WJ1. GBMS exhibited properties of
GSCs, including the ability for self-renewal, exhibited pale with
toluidine blue, expression of known GSC markers and high
tumorigenicity in vivo. These lines of evidence confirmed
the isolation of GSCs from WJ1.
A previous study showed that GSCs play core roles in
the therapeutic resistance of GBM (9). Thus, targeting malignant GSCs using
therapeutic drugs that have the potential to eliminate the most
aggressive GSCs but preserve neural cell populations should be the
preferred treatment measure in experimental research. Tanshinone
IIA has anticancer (30,31) and anti-inflammatory (14,17,18)
activities. In the present study, following GSC exposure to
tanshinone IIA, the formation of neurospheres was reduced in a
dose-dependent manner and the cell viability of GSCs was suppressed
in a dose- and time-dependent manner. These results suggest that
tanshinone IIA has strong anti-GSC activity. In an in vivo
experiment, tanshinone IIA slowed the growth of human brain cancer
derived from GSCs, and significantly reduced the weight and volume
of tumor masses without any untoward toxicity. These findings
demonstrated that using tanshinone IIA has a potential inhibitory
effect on GSCs in vitro and in vivo.
Since CSCs play a core role in cancer recurrence,
metastasis and high patient mortality, targeting and attenuating
CSC stemness, induction of CSC differentiation and apoptosis are
promising treatment avenues other than targeting tumor masses in
the treatment of a malignant tumors. Friedman et al
(32) demonstrated that
all-trans retinoic acid (ATRA), a derivative of retinol,
causes differentiation of CSCs as well as normal neural progenitor
cells by the loss of the stem cell marker nestin. In the present
study, expression levels of stemness markers (CD133 and nestin) in
GSCs were decreased, and differentiation and neural lineage markers
(GFAP and βIII-tubulin) were increased in vitro and in
vivo in a dose-dependent manner after treatment with tanshinone
IIA. This indicates that the stemness of GSCs was attenuated after
treatment with tanshinone IIA. The Annexin V analysis and western
blot analysis of apoptotic proteins Bax and cleaved caspase-3 and
anti-apoptotic protein Bcl-2 indicated that tanshinone IIA
treatment resulted in apoptotic cell death of GSCs. These results
suggest that tanshinone IIA has an anti-GSC activity through
induction of GSC apoptosis. Taken together, all these data strongly
demonstrated that tanshinone IIA has the potential not only to
attenuate GSC stemness, but also to induce GSC apoptosis.
It was well recognized that inflammatory cytokines
and signaling pathways play pivotal roles in maintaining stem-like
properties in human glioma cells (33). Wang et al (34) demonstrated that GSCs preferentially
express the IL6 receptors α (IL6Rα) and glycoprotein 130 (gp130),
and the targeting of IL6Rα or IL6 ligand expression in GSCs
significantly reduces growth and neurosphere formation capacity
while increasing apoptosis. Guryanova et al (35) demonstrated that the activation of
STAT3 signaling is necessary for maintaining self-renewal and the
tumorigenic potential of GSCs. STAT3 is a downstream mediator of
pro-survival IL6 signals in GSCs and is constitutively activated in
glioma cells. Perturbation of IL6 signaling in GSCs attenuates
STAT3 activation, and small molecule inhibitors of STAT3 potently
induce GSC apoptosis (34). Thus,
it can be seen that the IL6/STAT3 signaling axis plays an important
role for GSC maintanence and activity. In the present study, the
expression level of IL6 was higher in GSCs and tumor tissue derived
from GSC bearing-mice. IL6 levels were downregulated after
treatment with tanshinone IIA, indicating that tanshinone IIA has a
significant inhibitory effect on IL6 expression in GSCs. The
oncogenic potential of STAT3 depends on its phosphorylation on
tyrosine705 and serine727 that leads to activation of target gene
transcription. Phosphorylated STATs form dimers, translocate to the
nucleus using the karyopherin-β nucleocytoplasmic transport system,
bind DNA, and activate transcription (36,37).
In the present study, activated STAT3 [(STAT3(tyrosine705) and
STAT3(serine727)] had high levels in GSCs. Tanshinone IIA not only
downregulated the expression levels of IL6 but also decreased
activated STAT3 in GSCs in vitro and in vivo in a
dose-dependent manner. This result suggests that the IL6/STAT3
signaling axis might mediate the growth and progression of GSCs.
Disturbing IL6/STAT3 signaling pathways by tanshinone IIA is
closely associated with growth inhibition of GSCs,
Based on the findings in this experimental study,
tanshinone IIA inhibits proliferation, attenuates stemness and
induces apoptosis in GSCs in vitro and in vivo. Its
partial mechanism of activity may be associated with attenuating
inflammatory cytokine production and blocking the IL6/STAT3
signaling pathway.
Acknowledgements
This research was supported by the National Natural
Science Foundation of China (grant no. 31071197).
References
|
1
|
Daumas-Duport C, Scheithauer B, O’Fallon J
and Kelly P: Grading of astrocytomas, a simple and reproducible
method. Cancer. 62:2152–2165. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smith JS and Jenkins RB: Genetic
alterations in adult diffuse glioma: occurrence, significance, and
prognostic implications. Front Biosci. 5:D213–D231. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang L, Liu Z, Balivada S, Shrestha T,
Bossmann S, Pyle M, Pappan L, Shi J and Troyer D: Interleukin-1β
and transforming growth factor-β cooperate to induce neurosphere
formation and increase tumorigenicity of adherent LN-229 glioma
cells. Stem Cell Res Ther. 3:52012.
|
|
4
|
Jordan CT: Cancer stem cells:
controversial or just misunderstood? Cell Stem Cell. 4:203–205.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ignatova TN, Kukekov VG, Laywell ED,
Suslov ON, Vrionis FD and Steindler DA: Human cortical glial tumors
contain neural stem-like cells expressing astroglial and neuronal
markers in vitro. Glia. 39:193–206. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Galli R, Binda E, Orfanelli U, Cipelletti
B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F and Vescovi
A: Isolation and characterization of tumorigenic, stem-like neural
precursors from human glioblastoma. Cancer Res. 64:7011–7021. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bao S, Wu Q, Sathornsumetee S, Hao Y, Li
Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD and Rich JN: Stem
cell-like glioma cells promote tumor angiogenesis through vascular
endothelial growth factor. Cancer Res. 66:7843–7848. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eramo A, Ricci-Vitiani L, Zeuner A,
Pallini R, Lotti F, Sette G, Pilozzi E, Larocca LM, Peschle C and
De Maria R: Chemotherapy resistance of glioblastoma stem cells.
Cell Death Differ. 13:1238–1241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rich JN: Cancer stem cells in radiation
resistance. Cancer Res. 67:8980–8984. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu G, Yuan X, Zeng Z, Tunici P, Ng H,
Abdulkadir IR, Lu L, Irvin D, Black KL and Yu JS: Analysis of gene
expression and chemoresistance of CD133+ cancer stem
cells in glioblastoma. Mol Cancer. 5:672006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singh SK, Clarke ID, Hide T and Dirks PB:
Cancer stem cells in nervous system tumors. Oncogene. 23:7267–7273.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yin X, Yin Y, Cao FL, Chen YF, Peng Y, Hou
WG, Sun SK and Luo ZJ: Tanshinone IIA attenuates the inflammatory
response and apoptosis after traumatic injury of the spinal cord in
adult rats. PLoS One. 7:e383812012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dai ZK, Qin JK, Huang JE, Luo Y, Xu Q and
Zhao HL: Tanshinone IIA activates calcium-dependent apoptosis
signaling pathway in human hepatoma cells. J Nat Med. 66:192–201.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chiu TL and Su CC: Tanshinone IIA induces
apoptosis in human lung cancer A549 cells through the induction of
reactive oxygen species and decreasing the mitochondrial membrane
potential. Int J Mol Med. 25:231–236. 2010.PubMed/NCBI
|
|
17
|
Ren ZH, Tong YH, Xu W, Ma J and Chen Y:
Tanshinone IIA attenuates inflammatory responses of rats with
myocardial infarction by reducing MCP-1 expression. Phytomedicine.
17:212–218. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fan GW, Gao XM, Wang H, Zhu Y, Zhang J, Hu
LM, Su YF, Kang LY and Zhang BL: The anti-inflammatory activities
of tanshinone IIA, an active component of TCM, are mediated by
estrogen receptor activation and inhibition of iNOS. J Steroid
Biochem Mol Biol. 113:275–280. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen W, Tang F, Xie B, Chen S, Huang H and
Liu P: Amelioration of atherosclerosis by tanshinone IIA in
hyperlipidemic rabbits through attenuation of oxidative stress. Eur
J Pharmacol. 674:359–364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Wei Y, Yuan S, Liu G, Lu Y, Zhang
J and Wang W: Potential anticancer activity of tanshinone IIA
against human breast cancer. Int J Cancer. 116:799–807. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Su CC and Lin YH: Tanshinone IIA inhibits
human breast cancer cells through increased Bax to Bcl-xL ratios.
Int J Mol Med. 22:357–361. 2008.PubMed/NCBI
|
|
22
|
Iliopoulos D, Hirsch HA, Wang G and Struhl
K: Inducible formation of breast cancer stem cells and their
dynamic equilibrium with non-stem cancer cells via IL6 secretion.
Proc Natl Acad Sci USA. 108:1397–1402. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Korkaya H, Liu S and Wicha MS: Regulation
of cancer stem cells by cytokine networks: attacking cancer’s
inflammatory roots. Clin Cancer Res. 17:6125–6129. 2011.PubMed/NCBI
|
|
24
|
Jang SI, Jeong SI, Kim KJ, Kim HJ, Yu HH,
Park R, Kim HM and You YO: Tanshinone IIA from Salvia
miltiorrhiza inhibits inducible nitric oxide synthase
expression and production of TNF-alpha, IL-1beta and IL6 in
activated RAW 264.7 cells. Planta Med. 69:1057–1059.
2003.PubMed/NCBI
|
|
25
|
Wang J, Wang X, Jiang S, Lin P, Zhang J,
Wu Y, Xiong Z, Ren JJ and Yang H: Establishment of a new human
glioblastoma multiforme cell line (WJ1) and its partial
characterization. Cell Mol Neurobiol. 27:831–843. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee J, Kotliarova S, Kotliarov Y, Li A, Su
Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, Park JK
and Fine HA: Tumor stem cells derived from glioblastomas cultured
in bFGF and EGF more closely mirror the phenotype and genotype of
primary tumors than do serum-cultured cell lines. Cancer Cell.
9:391–403. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
van Meerloo J, Kaspers GJ and Cloos J:
Cell sensitivity assays: the MTT assay. Methods Mol Biol.
731:237–245. 2011.PubMed/NCBI
|
|
28
|
Kim MJ, Kim YJ, Park HJ, Chung JH, Leem KH
and Kim HK: Apoptotic effect of red wine polyphenols on human colon
cancer SNU-C4 cells. Food Chem Toxicol. 44:898–902. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yuan X, Curtin J, Xiong Y, Liu G,
Waschsmann-Hogiu S, Farkas DL, Black KL and Yu JS: Isolation of
cancer stem cells from adult glioblastoma multiforme. Oncogene.
23:9392–9400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kapoor S: Tanshinone IIA: a potent,
natural anti-carcinogenic agent for the management of systemic
malignancies. Chin J Integr Med. 15:1532009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma H, Fan Q, Yu J, Xin J and Zhang C:
Novel microemulsion of tanshinone IIA, isolated from Salvia
miltiorrhiza Bunge, exerts anticancer activity through inducing
apoptosis in hepatoma cells. Am J Chin Med. 41:197–210.
2013.PubMed/NCBI
|
|
32
|
Friedman MD, Jeevan DS, Tobias M, Murali R
and Jhanwar-Uniyal M: Targeting cancer stem cells in glioblastoma
multiforme using mTOR inhibitors and the differentiating agent
all-trans retinoic acid. Oncol Rep. 30:1645–1850.
2013.PubMed/NCBI
|
|
33
|
Jin X, Kim SH, Jeon HM, Beck S, Sohn YW,
Yin J, Kim JK, Lim YC, Lee JH, Kim SH, Kang SH, Pian X, Song MS,
Park JB, Chae YS, Chung YG, Lee SH, Choi YJ, Nam DH, Choi YK and
Kim H: Interferon regulatory factor 7 regulates glioma stem cells
via interleukin-6 and Notch signalling. Brain. 135:1055–1069. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang H, Lathia JD, Wu Q, Wang J, Li Z,
Heddleston JM, Eyler CE, Elderbroom J, Gallagher J, Schuschu J,
MacSwords J, Cao Y, McLendon RE, Wang XF, Hjelmeland AB and Rich
JN: Targeting interleukin 6 signaling suppresses glioma stem cell
survival and tumor growth. Stem Cells. 27:2393–2404. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guryanova OA, Wu Q, Cheng L, Lathia JD,
Huang Z, Yang J, MacSwords J, Eyler CE, McLendon RE, Heddleston JM,
Shou W, Hambardzumyan D, Lee J, Hjelmeland AB, Sloan AE, Bredel M,
Stark GR, Rich JN and Bao S: Nonreceptor tyrosine kinase BMX
maintains self-renewal and tumorigenic potential of glioblastoma
stem cells by activating STAT3. Cancer Cell. 19:498–511. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bromberg J and Darnell JE Jr: The role of
STATs in transcriptional control and their impact on cellular
function. Oncogene. 19:2468–2473. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schindler C, Levy DE and Decker T:
JAK-STAT signaling: from interferons to cytokines. J Biol Chem.
282:20059–20063. 2007. View Article : Google Scholar
|