Introduction
Leukemia is a cancer of the blood or bone marrow and
is the result of uncoupling or imbalance of the proliferation and
differentiation of hematopoietic stem cells (HSCs) (1). The malignant cells continue to
proliferate with the loss of differentiation capability, and
abnormalities in the development of HSCs at any stage may result in
leukemia (2). Current treatment
strategies for leukemia include cytotoxic drug therapy,
radiotherapy and bone marrow transplantation. Nevertheless, they
are usually accompanied by numerous adverse side effects. In the
last two decades, remarkable progress has been made in the
treatment of leukemia, yet problems such as cancer cell
heterogeneity, chromosomal instability, lack of selective action of
anti-neoplastic agents and development of multidrug resistance,
still remain to be solved (3).
Therefore, there is a pressing need to develop new compounds with
high therapeutic efficacy on leukemia cells but with minimal
toxicity on normal cells, especially those derived from natural
sources.
Conjugated fatty acids (CFAs) refer to the
positional and geometric isomers of polyunsaturated fatty acids
(PUFAs) in which two carbon-carbon double bonds are separated by
one carbon-carbon single bond (-C=C-C=C-) (4). Naturally occurring CFAs can be
isolated from meat and dairy products of ruminant animals (5), plant seed oils (6) and seaweeds (conjugated tetraenoic
acid, conjugated eicosapentaenoic acid and conjugated
docosahexaenoic acid) (7). Among
all CFAs, conjugated linoleic acid (CLA) has been the most
extensively studied in relation to its occurrence, metabolism and
physiological effects (8).
Nevertheless, the occurrence of CLA in natural food is found to be
<1%, while conjugated linolenic acid (CLNA) is present at a
higher concentration in natural food (30–70%), especially in some
plant seed oils (6,9). Previous research has demonstrated the
various beneficial effects of CLNA on human health, including
anti-obesity, hypolipidemic, anti-inflammatory, immunomodulatory
and cancer chemopreventive activities (9–11). A
previous study showed that conjugated trienoic fatty acids produced
by alkali isomerization of α-linolenic acid at concentrations above
25 μM displayed potent antitumor effects on a wide range of human
cancer cell lines in vitro, including hepatoma (HepG2), lung
adenocarcinoma (A549), breast adenocarcinoma (MCF-7), stomach
tubular adenocarcinoma (MKN-7) and colon carcinoma (DLD-1)
(12). Interestingly, it has been
shown that CLNA exhibits a stronger antitumor effect than CLA on
various types of human cancer cells (12,13).
In another study, it was found that α-eleostearic acid
(9Z,11E,13E-octadecatrienoic acid), an isomer
of CLNA, induced DNA fragmentation, increased caspase activity and
expression of caspase mRNA in human colon cancer DLD-1 cells
(13). Moreover, Grossmann et
al (14) showed that the
growth-inhibitory and apoptosis-inducing effects of α-eleostearic
acid on human breast cancer cells might be mediated by an
oxidation-dependent mechanism. These earlier results suggest that
α-eleostearic acid is a CLNA isomer that might have potential in
the treatment of breast and colon cancers. Yet, the antitumor
effects of CLNA isomers on various types of human myeloid leukemia
cells and their action mechanisms remain poorly understood, both
in vitro and in vivo.
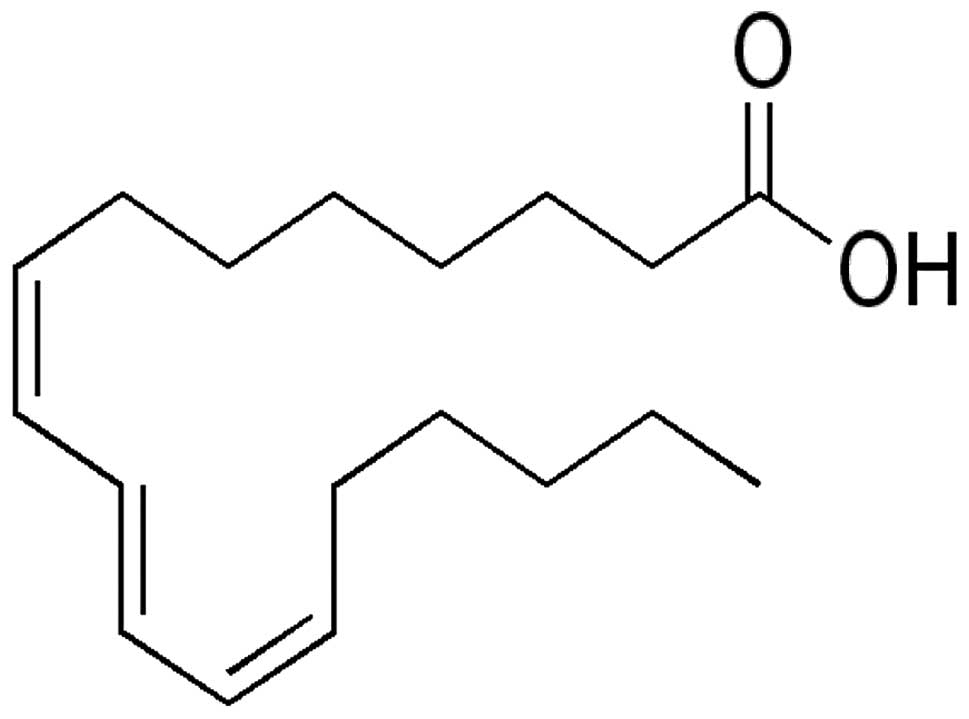
In the present study, we found that jacaric acid
(8Z,10E,12Z-octadecatrienoic acid; Fig. 1) is the most potent CLNA isomer that
can significantly inhibit the growth of human eosinophilic leukemia
EoL-1 cells in vitro. Mechanistic studies indicated that
jacaric acid exerts its anti-leukemic action through triggering of
cell cycle arrest, and by inducing the apoptosis and
differentiation of leukemia cells.
Materials and methods
Chemicals and reagents
CLNAs used in the study, which include α-eleostearic
acid, β-eleostearic acid, β-calendic and jacaric acid, with an
estimated purity >97%, were purchased from Larodan Fine
Chemicals AB, Sweden. The stock solution (0.2 M) was prepared by
dissolving the powder in sterile, cell culture-tested ethanol
(Sigma-Aldrich Co., USA). All other chemicals were purchased from
Sigma-Aldrich unless otherwise stated.
Culture of the tumor cell line
The human eosinophilic leukemia EoL-1 cell line
obtained from the Riken Cell Bank (Tsukuba Science City, Japan) was
originally established from the peripheral blood of a 33-year-old
male with eosinophilic leukemia (15). The cells were maintained in
RPMI-1640 medium (Gibco, USA) supplemented with 10% fetal bovine
serum (FBS; Gibco) and 1% antibiotics (100 U/ml penicillin G, 100
μg/ml streptomycin sulfate and 0.25 μg/ml amphotericin B in 0.85%
saline) in 5% humidified air at 37°C.
In vitro assays for the
anti-proliferative activity
Cell proliferation was measured using both the
colorimetric MTT assay and fluorometric cell proliferation assay.
Briefly, EoL-1 cells (1×105/ml) seeded in 96-well
microtiter plates were incubated at 37°C with different
concentrations of jacaric acid for various periods of time. The
anti-proliferative response was measured by a standard MTT
colorimetric assay (16) and
recorded by a Benchmark microplate reader (Bio-Rad Laboratories,
USA). The result was also confirmed by using the
CyQuant® NF Cell Proliferation Assay kit (Molecular
Probes; Invitrogen Corp., USA) (17) and recorded using a fluorescence
plate reader (Tecan Polarion, USA).
Analysis of cell cycle profile
EoL-1 cells (1×105/ml) were synchronized
with 0.5% heat-inactivated FBS (HI-FBS) overnight and incubated at
37°C with different concentrations of jacaric acid. Afterwards, the
cells were stained by propidium iodide (PI) (Sigma-Aldrich), and
the cell cycle profile was analyzed by flow cytometry (FACSCanto™
flow cytometer; BD BioSciences, USA) using the software ModFit LT
V3.0 (Verity Software House).
Measurement of DNA fragmentation by Cell
Death Detection ELISAPLUS kit
The measurement was performed according to the
manufacturer’s instructions in the Cell Death Detection
ELISAPLUS kit (Roche Applied Science, USA). Briefly,
EoL-1 cells (1×105/ml) were incubated with different
concentrations of jacaric acid at 37°C for various periods of time.
The supernatant was transferred to the well of a
streptavidin-coated 96-well microtiter plate, and the absorbance
was measured at 405 nm. The degree of apoptosis was expressed as
the enrichment factor, which was calculated as follows: Enrichment
factor = DNA fragments in treated sample/DNA fragments in the
control.
Analysis of Annexin V-GFP/PI dual
staining profile
Induction of apoptosis can also be measured using
Annexin V-GFP/PI dual staining method. Briefly, EoL-1 cells
(1×105/ml) were incubated with different concentrations
of jacaric acid at 37°C for 24 h. The cells were then resuspended
in Annexin V binding buffer (BD Biosciences) supplemented with
Annexin V-GFP fusion protein and PI. The samples were incubated for
30 min at room temperature and were analyzed for PerCP-Cy5.5 with
an emission wavelength of 695 nm versus FITC fluorescence with an
emission wavelength of 525 nm by the FACSCanto flow cytometer. The
percentages of cells in the four quadrants were calculated by
WinMDI (version 2.9) software.
Determination of mitochondrial membrane
potential by JC-1 staining
EoL-1 cells (1×105/ml) were incubated
with different concentrations of jacaric acid at 37°C for 24 h.
Afterwards, the cells were resuspended in PBS supplemented with
JC-1 dye (Molecular Probes; Invitrogen). The samples were incubated
for 30 min at 37°C and then analyzed for red fluorescence (FL-2)
with an emission wavelength of 575 nm vs. green fluorescence (FL-1)
with an emission wavelength of 525 nm by the FACSCanto flow
cytometer. The percentages of cells with membrane depolarization
were calculated by WinMDI (version 2.9) software.
Western blot analysis
Protein expression was determined by western blot
technique using a panel of specific antibodies. EoL-1 cells
(1×105/ml) were incubated with different concentrations
of jacaric acid at 37°C for 72 h. Cell pellets were collected and
total proteins were extracted using cell lysis buffer. The protein
concentration was measured using Bradford reagent (Sigma-Aldrich),
and the protein samples were resolved on 12% polyacrylamide gels
and transferred to PVDF membranes. The membranes were first
incubated with the following primary antibodies: mouse anti-human
Bax, Bcl-2, caspase-3, CDK2 and rabbit anti-human cyclin E
antibodies (all from Santa Cruz Biotechnology, USA), rabbit
anti-human pp53 antibody (Cell Signaling) and mouse anti-human
β-actin antibody (Sigma-Aldrich), followed by incubation with
HRP-conjugated secondary antibodies (GE Healthcare Ltd., UK) and
finally developed with ECL reagent (Santa Cruz).
Cell morphological study
The procedures were similar as previously described
with slight modifications (18). In
brief, EoL-1 cells (1×105/ml) were incubated with
different concentrations of jacaric acid at 37°C for 9 days. At day
4 and day 7 of the treatment, treated cells were washed with plain
medium, and the cells (1×105/ml) were further incubated
with fresh medium supplemented with jacaric acid. Cell morphology
was then determined by the preparation of cytospin smears. The
EoL-1 cells (5×104) were fixed onto a microscopic slide
by cytocentrifugation at 500 rpm for 5 min using the Shandon
Cytospin 3 centrifuge (Shandon Scientific Ltd., UK). The cells were
air-dried and then stained with Hemacolor staining solutions
(Diagnostica Merck, USA) for 15 sec and destained under de-ionized
water. Finally, the air-dried cells on the slides were mounted with
neutral mounting medium, Canada balsam (Sigma-Aldrich), and
morphology was examined under a light microscope.
Detection of eosinophil peroxidase (EPO)
and major basic protein (MBP) expression by flow cytometry
The procedures of Lung et al (18) were followed with slight
modifications. EoL-1 cells (1×105/ml) were incubated
with different concentrations of jacaric acid at 37°C for 9 days.
At day 4 and day 7 of the treatment, treated cells were washed with
plain RPMI medium, and the cells (1×105/ml) were further
incubated with fresh medium supplemented with jacaric acid. The
cells were harvested and fixed with 100% ice-cold methanol for 10
min at 4°C and washed once with PBS. Afterwards, the cells were
incubated with normal mouse IgG (1 mg/ml; Sigma-Aldrich) for 30 min
before staining with mouse anti-human EPO or mouse anti-human MBP
monoclonal antibodies (0.5 mg/ml; Pharmingen Inc., USA) for 2 h.
Lastly, the cells were incubated with FITC-labeled goat anti-mouse
IgG (0.5 mg/ml; Sigma-Aldrich) for 1 h. The stained cells were
analyzed for FITC fluorescence with an emission wavelength of 525
nm by the FACSCanto flow cytometer.
Statistical analysis
Each experiment was repeated at least three times
and only the results of the most representative experiments are
shown. The data are expressed as the arithmetic mean ± standard
error (SE). One-way analysis of variance (ANOVA) or unpaired
Student’s t-test was used for statistical analysis and the
differences were considered as statistically significant at
P<0.05.
Results
Anti-proliferative effect of conjugated
linolenic acid (CLNA) isomers on human eosinophilic leukemia EoL-1
cells
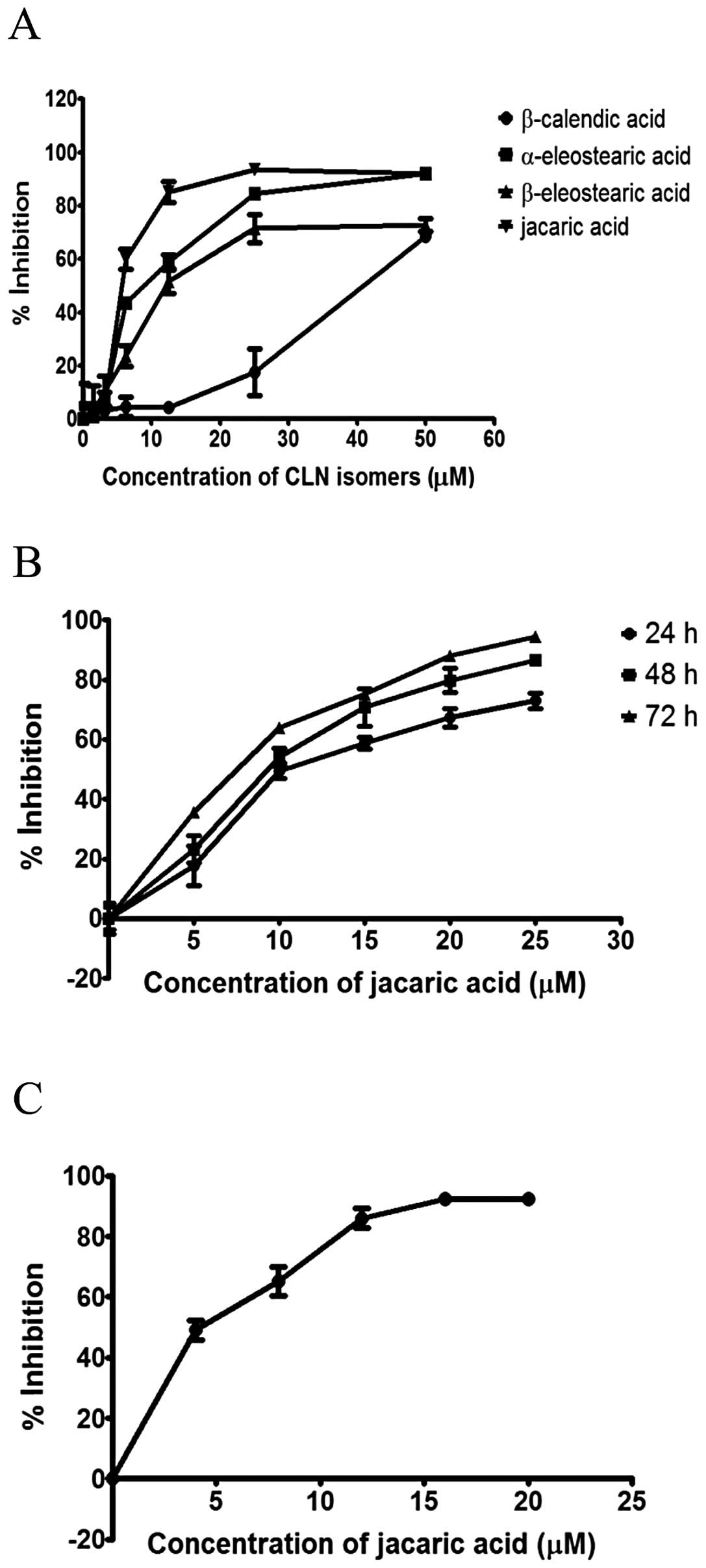
To determine the anti-proliferative effect of CLNA
isomers on the EoL-1 cells, the MTT reduction assay was performed.
As shown in Fig. 2A, all four CLNA
isomers were found to exhibit an inhibitory effect on the
proliferation of EoL-1 cells in a concentration-dependent manner.
In contrast, the solvent control (up to 0.1% v/v ethanol) did not
exert any significant inhibitory effect on EoL-1 cells (<5%
inhibition, data not shown). Jacaric acid was found to be the most
potent isomer among all the CLNA isomers tested and therefore it
was chosen as the specific target for further investigation in the
present study. Fig. 2B shows that
jacaric acid inhibited the growth of EoL-1 cells in a time- and
concentration-dependent manner, and the estimated 50% inhibitory
concentration (IC50) after a 48-h treatment was found to
be 5.33±0.86 μM. The growth-inhibitory effect of jacaric acid on
EoL-1 cells was confirmed by the CyQuant® NF Cell
Proliferation Assay kit (Fig. 2C).
Notably, jacaric acid exhibited no direct cytotoxicity on normal
primary myeloid cells such as murine peritoneal macrophages, as the
percentage of cell viability of the macrophages remained >90%
even when the cells were incubated with 100 μM jacaric acid for 48
h (data not shown).
Jacaric acid triggers cell cycle arrest
at the G0/G1 phase and modulates the
expression of cell cycle-regulatory proteins in EoL-1 cells
To determine the possible mechanisms of the
anti-proliferative effect of jacaric acid on EoL-1 cells, cells
were stained with propidium iodide after incubation with jacaric
acid for 72 h, and the cell cycle profile was analyzed by flow
cytometry. As shown in Fig. 3A,
jacaric acid triggered cell cycle arrest at the
G0/G1 phase, accompanied by a decrease in the
percentage of cells at the S phase. To further elucidate the
underlying mechanisms, western blotting was carried out in order to
examine the protein expression levels of cyclin E, CDK2 and pp53
(Fig. 3B), which are known to be
involved in the transition of G0/G1 to S
phase (19,20). Our results showed that the protein
expression levels of cyclin E and CDK2 decreased in the jacaric
acid-treated EoL-1 cells, whereas there was an increase in the
expression level of the pp53 protein (Fig. 3C–E). Collectively, the results
indicate that jacaric acid treatment of EoL-1 cells leads to cell
cycle arrest at the G0/G1 phase, and
modulates the expression of certain cell cycle-regulatory proteins
such as cyclin E, CDK2 and pp53.
Jacaric acid induces apoptosis of EoL-1
cells as revealed by the Cell Death Detection ELISAPLUS
kit, Annexin V assay and JC-1 dye staining
Apart from triggering cell cycle arrest, another
possible mechanism for the growth-inhibitory effect of jacaric acid
is the induction of apoptosis. To examine whether jacaric acid
induces apoptosis in the EoL-1 cells, the Cell Death Detection
ELISAPLUS kit was used according to the manufacturer’s
instructions. It was found that jacaric acid induced DNA
fragmentation in the EoL-1 cells, as revealed by the increase in
the enrichment factor in the jacaric acid-treated EoL-1 cells in a
time- and concentration-dependent manner (Fig. 4A), suggesting that jacaric acid
induced apoptosis in the EoL-1 cells. To gain further insight into
the molecular mechanism underlying the induction of apoptosis, the
effects of jacaric acid on altering the expression levels of
different apoptosis-regulatory proteins were examined by western
blot analysis (Fig. 4B). The
expression level of the anti-apoptotic protein Bcl-2 was decreased
whereas the expression levels of Bax and caspase-3 proteins were
increased (Fig. 4C–E). Apart from
the Cell Death Detection ELISA, the Annexin V assay was used to
detect the externalization of phosphatidylserine (PS) which is a
hallmark of early apoptosis (21).
In addition, JC-1 dye staining was used to measure the
mitochondrial membrane potential which is known to decrease prior
to PS externalization and DNA fragmentation (22,23).
Our results showed that jacaric acid induced PS externalization in
the EoL-1 cells at 24 h of incubation in a concentration-dependent
manner as demonstrated by the Annexin V assay (Fig. 5), and reduced the mitochondrial
membrane potential of the EoL-1 cells after staining with JC-1 dye
(Fig. 6). Together these results
suggest that jacaric acid inhibits the growth of EoL-1 cells by
inducing apoptosis.
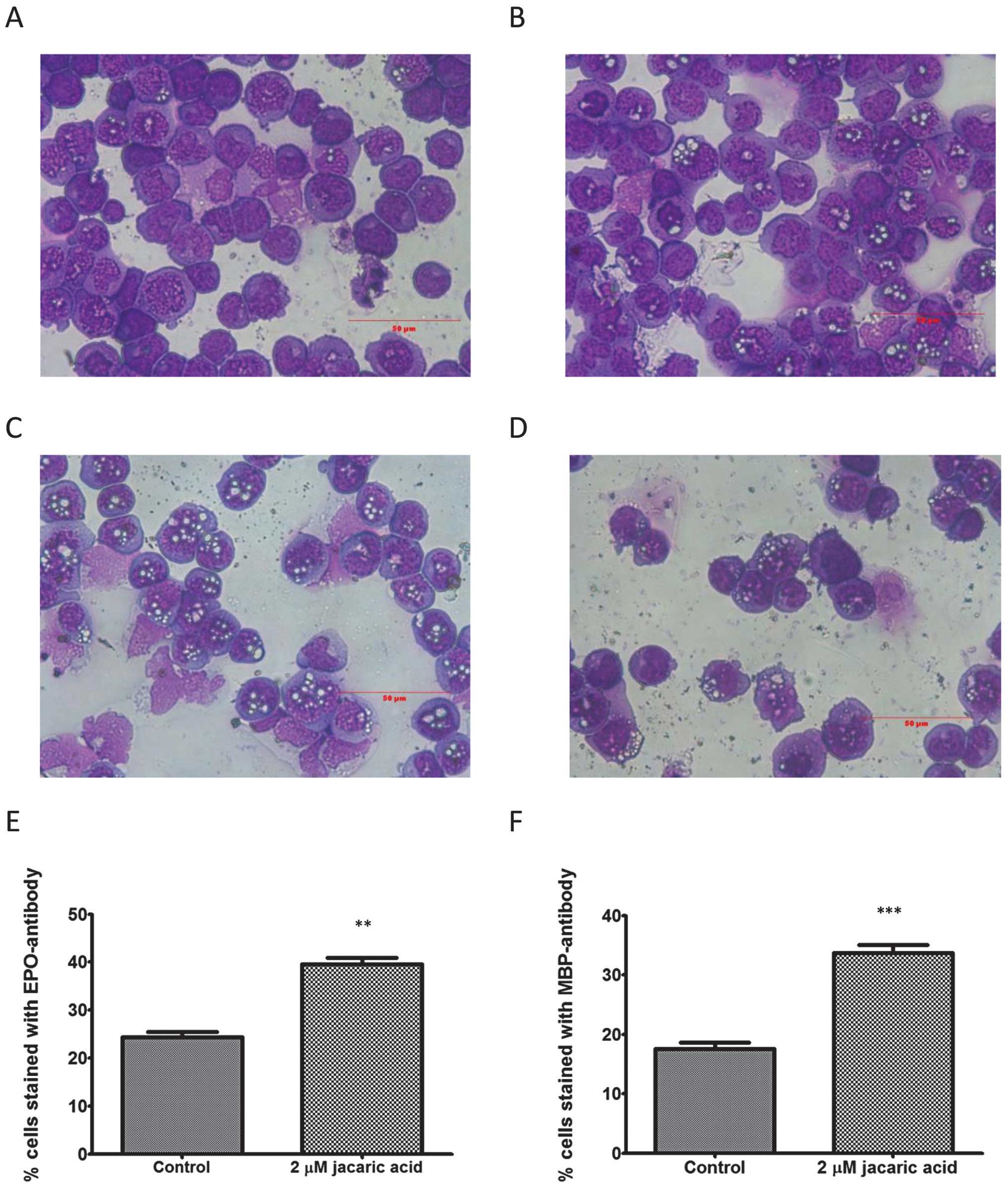
Jacaric acid induces morphological
differentiation of EoL-1 cells and increases the expression of
mature eosinophil markers EPO and MBP
Induction of cellular differentiation can also lead
to suppression of cell growth (24). As shown in Fig. 7B–D, after treatment with different
concentrations of jacaric acid for 9 days, there were more
refractive vacuoles in the treated-EoL-1 cells than those in the
control (Fig. 7A). This suggests
that the granule matrix was altered leading to the presence of
small refractive vacuoles when observed under a light microscope.
In order to confirm the induction of eosinophilic differentiation
of EoL-1 cells by jacaric acid, the expression levels of two
eosinophil-specific proteins, eosinophil peroxidase (EPO) and the
major basic protein (MBP), were measured by flow cytometry. As
shown in Fig. 7E and F, a
significant increase in the expression levels of EPO and MBP
proteins was noted after 9 days of treatment with 2 μM jacaric
acid. Collectively, our results indicate that prolonged exposure (9
days) of EoL-1 cells to a low concentration (1–2 μM) of jacaric
acid induces eosinophilic differentiation of the cells as
represented by morphological changes and an elevation in EPO and
MBP proteins.
Discussion
Clonally derived human leukemia cell lines are
important research tools for the study of cell transformation,
hematopoietic cell differentiation and apoptosis (25,26).
The EoL-1 cell line is a well-established human chronic
eosinophilic leukemia cell line which has been commonly used as an
in vitro model for the study of human eosinophil functions
and their regulation, and is particularly useful for analyzing
leukemic cell differentiation (27,28).
In the present study, we found that jacaric acid, one of the CLNA
isomers, inhibited the growth of EoL-1 cells in a time- and
concentration-dependent manner, as measured by MTT reduction
colorimetric assay and the CyQuant® NF Cell
Proliferation fluorometric assay. This is similar to a previous
report which demonstrated that CLNA produced by alkali
isomerization of α-linolenic acid displayed a potent antitumor
effect on a wide range of human cancer cell lines in vitro
(12). Our results indicate that
among the four CLNA isomers tested, jacaric acid exhibited the most
potent antitumor effect on EoL-1 cells and this is in line with the
finding of Shinohara et al (29) who showed that jacaric acid also
exerted the most potent in vitro cytotoxic effect on human
adenocarcinoma DLD-1 cells. To investigate whether jacaric acid
inhibits the growth of EoL-1 cells through triggering cell cycle
arrest, the cells were stained with PI, and the cell cycle profile
was analyzed by flow cytometry. Our results showed that jacaric
acid triggered cell cycle arrest at the G0/G1
phase, accompanied by a decrease in the percentage of cells at the
S phase. Cell cycle progression is known to be regulated by
different cyclins and cyclin-dependent kinases (CDK) (30). Some studies have shown that cell
cycle arrest at the G0/G1 phase is regulated
by CDK2, CDK4 and cyclin E (30–32).
Other studies have demonstrated that an increase in the protein
expression levels of the p21, p27 and p53 proteins caused cell
cycle arrest at the G0/G1 phase in human
breast carcinoma and human lung cancer A549 cells (32,33).
Our present study showed that the protein expression levels of
cyclin E and CDK2 were decreased in the jacaric acid-treated EoL-1
cells, whereas there was an increase in the expression level of the
pp53 protein. Taken together, our results demonstrated that jacaric
acid led to cell cycle arrest at the G0/G1
phase, which was accompanied by alteration of the cell
cycle-regulatory proteins controlling the G1 phase
mitotic checkpoint.
Induction of apoptosis is another mechanism which
may account for the observed anti-proliferative effect of jacaric
acid on EoL-1 cells. Previous studies have demonstrated that
α-eleostearic acid, another isomer of CLNA, induced DNA
fragmentation, increased caspase activity and expression of caspase
mRNA in human colon cancer DLD-1 cells and these were shown to be
associated with lipid peroxidation (13). A recent report showed that jacaric
acid could induce apoptosis in human prostate cancer cells
(34). Using the Cell Death
Detection ELISAPLUS kit, we found that there was an
increase in DNA fragmentation in the jacaric acid-treated EoL-1
cells, suggesting the occurrence of apoptosis in the EoL-1 cells.
Western blotting indicated that the expression level of the
anti-apoptotic Bcl-2 protein was decreased. On the other hand, the
expression levels of several proteins that are involved in the
apoptotic pathway, including Bax and caspase-3, were increased, and
these results are in line with previous findings (35). In addition, an elevation in PS
externalization and increased mitochondrial membrane depolarization
in the jacaric acid-treated EoL-1 cells further supported our
finding that jacaric acid induces apoptosis in EoL-1 cells.
It has been reported that EoL-1 cells can be induced
to differentiate into mature, eosinophilic granule-containing cells
in response to a number of stimuli, including cytokines such as
granulocyte-colony stimulating factor and tumor necrosis factor-α
(36), chemokines such as
leukotactin (28), and small
molecules such as butyrate (37),
dibutyryl cyclic AMP, prostaglandin E2 and forskolin (38). In addition, previous research from
our laboratory demonstrated that the green tea catechin
epigallocatechin-3-gallate (18)
inhibited the growth of EoL-1 cells in vitro by inducing
cellular differentiation. To the best of our knowledge, the ability
of CLNA to induce differentiation in human myeloid leukemia cells
has not yet been reported. In the present study, EoL-1 cells
treated with a lower concentration (1–2 μM) of jacaric acid for a
longer period of time (9 days) had more refractive vacuoles in
their cytoplasm than those in the control, suggesting that EoL-1
cells could be triggered to undergo morphological differentiation
into mature eosinophil-like cells. Moreover, jacaric acid also
upregulated the expression levels of two eosinophil-specific
granule proteins, EPO and MBP, in the EoL-1 cells under the
prescribed experimental conditions. The results, when taken
together, suggest that jacaric acid can exhibit anti-proliferative
activity on human eosinophilic EoL-1 cells by triggering cell cycle
arrest at the G0/G1 phase, by inducing
leukemic cell apoptosis, and by inducing eosinophilic
differentiation of leukemia cells. Since a recent report showed
that jacaric acid did not exhibit any significant toxicity in
vivo (39), further elucidation
of the anti-leukemic efficacy and action mechanisms of jacaric acid
in vivo may provide a better insight into the development of
jacaric acid as a potential candidate for the treatment of certain
forms of myeloid leukemia with minimal toxicity and few side
effects.
Acknowledgements
We thank Miss Ada Kong for the excellent technical
assistance for this reserach.
References
|
1
|
Jude CD, Gaudet JJ, Speck NA and Ernst P:
Leukemia and hematopoietic stem cells: balancing proliferation and
quiescence. Cell Cycle. 7:586–591. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leung KN, Mak NK and Fung MC: Cytokines in
the differentiation therapy of leukemia: from laboratory
investigations to clinical applications. Crit Rev Clin Lab Sci.
42:473–514. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsiftsoglou AS, Pappas IS and Vizirianakis
IS: Mechanisms involved in the induced differentiation of leukemia
cells. Pharmacol Ther. 100:257–290. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nagao K and Yanagita T: Conjugated fatty
acids in food and their health benefits. Biosci Bioeng.
100:152–157. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Griinari JM, Corl BA, Lacy SH, Chouinard
PY, Nurmela KV and Bauman DE: Conjugated linoleic acid is
synthesized endogenously in lactating dairy cows by
Delta(9)-desaturase. J Nutr. 130:2285–2291. 2000.PubMed/NCBI
|
|
6
|
Takagi T and Itabashi Y: Occurrence of
mixtures of geometrical-isomers of conjugated octadecatrienoic
acids in some seed oils: Analysis by open-tubular gas liquid
chromatography and high performance liquid chromatography. Lipids.
16:546–551. 1981. View Article : Google Scholar
|
|
7
|
Bhaskar N, Kinami T, Miyashita K, Park SB,
Endo Y and Fujimoto K: Occurrence of conjugated polyenoic fatty
acids in seaweeds from the Indian Ocean. Z Naturforsch C.
59:310–314. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dilzer A and Park Y: Implication of
conjugated linoleic acid (CLA) in human health. Crit Rev Food Sci
Nutr. 52:488–513. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tanaka T, Hosokawa M, Yasui Y, Ishigamori
R and Miyashita K: Cancer chemopreventive ability of conjugated
linolenic acids. Int J Mol Sci. 12:7495–7509. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koba K, Akahoshi A, Yamasaki M, et al:
Dietary conjugated linolenic acid in relation to CLA differently
modifies body fat mass and serum and liver lipid levels in rats.
Lipids. 37:343–350. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hennessy AA, Ross RP, Devery R and Stanton
C: The health promoting properties of the conjugated isomers of
α-linolenic acid. Lipids. 46:105–119. 2011.
|
|
12
|
Igarashi M and Miyazawa T: Newly
recognized cytotoxic effect of conjugated trienoic fatty acids on
cultured human tumor cells. Cancer Lett. 148:173–179. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsuzuki T, Tokuyama Y, Igarashi M and
Miyazawa T: Tumor growth suppression by alpha-eleostearic acid, a
linolenic acid isomer with a conjugated triene system, via lipid
peroxidation. Carcinogenesis. 25:1417–1425. 2004. View Article : Google Scholar
|
|
14
|
Grossmann ME, Mizuno NK, Dammen ML,
Schuster T, Ray A and Cleary MP: Eleostearic acid inhibits breast
cancer proliferation by means of an oxidation-dependent mechanism.
Cancer Prev Res. 2:879–886. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saito H, Bourinbaiar A, Ginsburg M, et al:
Establishment and characterization of a new human eosinophilic
leukemia cell line. Blood. 66:1233–1240. 1985.PubMed/NCBI
|
|
16
|
Liao X and Leung KN: Tryptanthrin induces
growth inhibition and neuronal differentiation in the human
neuroblastoma LA-N-1 cells. Chem Biol Interact. 203:512–521. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liao XM and Leung KN: Indirubin-3′-oxime
induces mitochondrial dysfunction and triggers growth inhibition
and cell cycle arrest in human neuroblastoma cells. Oncol Rep.
29:371–379. 2013.
|
|
18
|
Lung HL, Ip WK, Wong CK, Mak NK, Chen ZY
and Leung KN: Anti-proliferative and differentiation-inducing
activities of the green tea catechin epigallocatechin-3-gallate
(EGCG) on the human eosinophilic leukemia EoL-1 cell line. Life
Sci. 72:257–268. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wilken R, Veena MS, Wang MB and Srivatsan
ES: Curcumin: A review of anti-cancer properties and therapeutic
activity in head and neck squamous cell carcinoma. Mol Cancer.
10:122011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li L, Dai HJ, Ye M, et al: Lycorine
induces cell-cycle arrest in the G0/G1 phase in K562 cells via HDAC
inhibition. Cancer Cell Int. 12:492012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martin SJ, Finucane DM, Amarante-Mendes
GP, O’Brien GA and Green DR: Phosphatidylserine externalization
during CD95-induced apoptosis of cells and cytoplasts requires
ICE/CED-3 protease activity. J Biol Chem. 271:28753–28756. 1996.
View Article : Google Scholar
|
|
22
|
Castedo M, Macho A, Zamzami N, et al:
Mitochondrial perturbations define lymphocytes undergoing apoptotic
depletion in vivo. Eur J Immunol. 25:3277–3284. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zamzami N, Marchetti P, Castedo M, et al:
Sequential reduction of mitochondrial transmembrane potential and
generation of reactive oxygen species in early programmed cell
death. J Exp Med. 182:367–377. 1995. View Article : Google Scholar
|
|
24
|
Hansen CM, Mathiasen IS and Binderup L:
The anti-proliferative and differentiation-inducing effects of
vitamin D analogs are not determined by the binding affinity for
the vitamin D receptor alone. J Investig Dermatol Symp Proc.
1:44–48. 1996.PubMed/NCBI
|
|
25
|
Drexler HG, Matsuo AY and MacLeod RA:
Continuous hematopoietic cell lines as model systems for
leukemia-lymphoma research. Leuk Res. 24:881–911. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mayani H, Flores-Figueroa E and
Chavez-Gonzalez A: In vitro biology of human myeloid leukemia. Leuk
Res. 33:624–637. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cools J, Quentmeier H, Huntly BJ, et al:
The EOL-1 cell line as an in vitro model for the study of
FIP1L1-PDGFRA-positive chronic eosinophilic leukemia. Blood.
103:2802–2805. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee JS and Kim IS: Leukotactin-1/CCL15
induces cell migration and differentiation of human eosinophilic
leukemia EoL-1 cells through PKCdelta activation. Mol Biol Rep.
37:2149–2156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shinohara N, Tsuduki T, Ito J, et al:
Jacaric acid, a linolenic acid isomer with a conjugated triene
system, has a strong antitumor effect in vitro and in vivo. Biochim
Biophys Acta. 1821:980–988. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nigg EA: Cyclin-dependent protein kinases:
key regulators of the eukaryotic cell cycle. Bioessays. 17:471–480.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Geng Y, Whoriskey W, Park MY, et al:
Rescue of cyclin D1 deficiency by knockin cyclin E. Cell.
97:767–777. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liang YC, Lin-Shiau SY, Chen CF and Lin
JK: Inhibition of cyclin-dependent kinases 2 and 4 activities as
well as induction of Cdk inhibitors p21 and p27 during growth
arrest of human breast carcinoma cells by
(−)-epigallocatechin-3-gallate. J Cell Biochem. 75:1–12.
1999.PubMed/NCBI
|
|
33
|
Lu HF, Chen YS, Yang JS, et al:
Gypenosides induced G0/G1 arrest via inhibition of cyclin E and
induction of apoptosis via activation of caspases-3 and -9 in human
lung cancer A-549 cells. In vivo. 22:215–221. 2008.PubMed/NCBI
|
|
34
|
Gasmi J and Sanderson JT: Jacaric acid and
its octadecatrienoic acid geoisomers induce apoptosis selectively
in cancerous human prostate cells: a mechanistic and 3-D
structure-activity study. Phytomedicine. 20:734–742. 2013.
View Article : Google Scholar
|
|
35
|
Sun Y, Lin Y, Li H, Liu J, Sheng X and
Zhang W: 2,5-Hexanedione induces human ovarian granulosa cell
apoptosis through BCL-2, BAX, and CASPASE-3 signaling pathways.
Arch Toxicol. 86:205–215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shintaku N, Ohshima Y, Jung EY, et al:
Induction of eosinophilic granules, nonspecific esterase activity
and CD14 expression in the human eosinophilic leukemia cell line,
EOL-1. Hematol Oncol. 12:129–139. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Izumi T, Kishimoto S, Takano T, et al:
Expression of human platelet-activating factor receptor gene in
EoL-1 cells following butyrate-induced differentiation. Biochem J.
305:829–835. 1995.
|
|
38
|
Tai G, Eun-Young J, Yuji H, et al:
Different effects of cyclic AMP and butyrate on eosinophilic
differentiation, apoptosis and bcl-2 expression of a human
eosinophilic leukemia cell line, EoL-1. Hematol Oncol. 14:181–192.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shinohara N, Ito J, Tsuduki T, et al:
Jacaric acid, a linolenic acid isomer with a conjugated triene
system, reduces stearoyl-CoA desaturase expression in liver of
mice. J Oleo Sci. 61:433–441. 2012. View Article : Google Scholar : PubMed/NCBI
|