Introduction
Chemotherapy is a key method of treatment in the
primary and palliative care of patients with lung cancer, of which
non-small cell lung cancer (NSCLC) accounts for the majority of
cases. Cisplatin is one of the most common chemotherapeutic drugs
for lung cancer treatment, particularly for NSCLC; however, many
patients have resistance to cisplatin initially and secondarily
(1,2). To overcome drug resistance, patients
with NSCLC are administered large doses of drugs, which induce
numerous adverse effects and fail to improve the clinical
prognosis. Therefore, a better understanding of the molecular
mechanisms underlying cisplatin resistance is warranted to further
clarify the exact mechanisms underlying chemoresistance and to find
or design efficient drugs to improve individual chemotherapy
strategies for NSCLC patients.
Increasing studies have shown that the active
effluence of chemotherapeutic drugs from cancer cells is one of the
main mechanisms of drug resistance. Cancer cells often exhibit drug
resistance with the overexpression of membrane transport proteins,
which effectively pump antitumor drugs out (3). The ATP-binding cassette (ABC)
multi-drug transporters, such as ABCG2 (BCRP/MXR/ABCP), are
considered to be responsible for the bulk of drug efflux in human
cancer (4). Moreover, the
overexpression of ABCG2 has been reported to confer drug resistance
upon NSCLC to various chemotherapeutic drugs (5). Furthermore, a previous study also
showed ABCG2 to be closely associated with clinical outcome in
platinum-based chemotherapy for advanced NSCLC patients. For
example, ABCG2-positive patients showed a lower response effect to
chemotherapy and a shorter progression-free survival and low
survival rate than ABCG2-negative patients (6).
However, the potential function of ABCG2 for cancer
has not been completely elucidated. A number of recent reports have
suggested that novel functions exist for this transporter besides
drug efflux. A recent study demonstrated that ABCG2 was involved in
the proliferation of cancer cells and suppression of ABCG2
inhibited cancer cell proliferation (10), which indicated that ABCG2 was
involved in cancer cell proliferation. Notably, a recent study
demonstrated that ABCG2 could directly regulate the conversion
between symmetric and asymmetric cell division in a cardiac side
population with determination of progenitor cell fate decisions
(7). Although asymmetric cell
division is a proposed characteristic of cancer stem cells, a
recent study revealed that the majority of glioma stem cells were
passaged through expansive symmetric cell division instead of
asymmetric cell division (8).
Moreover, there is emerging evidence that asymmetric division could
function as a mechanism of tumor suppression in Drosophila
neuroblasts. Loss-of-function mutations of cell polarity and cell
fate determinants induce neuroblasts to divide symmetrically,
leading to an increase in number, tissue overgrowth and
transplantable tumors, which was ultimately similar to the
generation of mammalian cancer (9).
Whether or not there is a direct relationship between ABCG2 and
cell division in drug-resistant cancer cells remains to be
determined. To date, the role of ABCG2 in cancer cell division
remains unclear.
To explore the potential relationship between ABCG2
expression and the pattern of cancer cell division, we established
cisplatin-resistant NSCLC cell lines and found that these cell
lines have significantly increased expression of ABCG2. Markedly,
as evidenced by PKH-26 staining, we found that these drug-resistant
cell lines divided symmetrically more frequently than the parental
cells. We therefore speculated that ABCG2 could regulate cell
division in cisplatin-resistant NSCLC cells. To verify this
hypothesis, we observed the cell division patterns of parental
NSCLC cells that overexpressed ABCG2 and drug-resistant NSCLC cells
with decreased ABCG2 expression by RNA interference. The result
showed that ABCG2 regulated cell division in drug-resistant cancer
cells.
Materials and methods
Cell lines and reagent
The human lung cancer cell lines, A549 and H460,
were obtained from the American Type Culture Collection (ATCC) and
routinely maintained in RPMI-1640 medium supplemented with 10%
fetal bovine serum, 100 U/ml of penicillin, 100 mg/ml of
streptomycin and 2 mM L-glutamine. All cells were cultured as
monolayer cultures and maintained in a humidified atmosphere of 5%
CO2 in air at 37°C. Cisplatin,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
propidium iodide (PI) and RNase A were purchased from Sigma-Aldrich
Chemical Company (St. Louis, MO, USA). Antibodies against ABCG2
were purchased from Abcam, Inc. (Cambridge, MA, USA).
Drug sensitivity assay (MTT)
Cells (3×103) were seeded in 96-well
plates and allowed to adhere overnight at 37°C. The attached cells
were then exposed to various concentrations of cisplatin for 72 h.
The MTT reagent [5 mg/ml (20 μl/well)] was added to each well for 4
h. Subsequently, the medium was discarded and the purple MTT
formazan was dissolved by DMSO (100 μl/well). Then, the absorbance
value was measured at 490 nm and the concentration of cisplatin
that induced 50% inhibition (IC50) was calculated by
GraphPad Prism software (version 5; GraphPad Software, Inc., San
Diego, CA, USA).
Generation of drug-resistant cell
lines
A549 and H460 cisplatin-resistant cell lines were
derived from each original parental cell line by exposure to
cisplatin following the IC50 value for 72 h, and were
then designated cisplatin-resistant (CisR) cells. The medium was
removed and cells were cultured for an additional 72 h in normal
medium. Cells were cultured with or without media containing
cisplatin for 72 h, repeatedly. This cycle was carried out for ~6
months, after which time the IC50 concentrations were
re-assessed in each resistant cell line.
Establishment of stable
ABCG2-overexpressing or shRNA-ABCG2 knockdown cell lines
A549 cells were transfected with the recombinant
lentivirus of ABCG2 or control lentivirus (GFP-lentivirus), which
was constructed by GeneChem Co. Ltd. (Shanghai, China). To
establish A549/CisRshABCG2 cells, A549/CisR cells were transfected
with SMART vector shRNA lentiviral particles targeted against the
ABCG2 gene with 5 μg/ml of Polybrene (Sigma-Aldrich) to inhibit
ABCG2 gene expression. An empty SMART vector expressing TurboGFP
was used as control. The stable shRNA-ABCG2 cell line,
A549/CisRshABCG2, was established and the expression of ABCG2 was
evaluated by western blotting and PCR.
Western blot analysis
For western blot analysis, 40 μg of total protein
was separated by sodium dodecyl sulphate-polyacrylamide gel
electrophoresis (SDS-PAGE), and the proteins were then transferred
electrophoretically onto a polyvinylidene difluoride membrane.
After blocking with 5% skimmed milk in TBST buffer for 1 h at 37°C,
the membranes were incubated overnight at 4°C with specific primary
antibody (rat anti-BRCP/ABCG2 antibody, 1:500 dilution; Abcam). The
protein levels were normalized against β-actin from the same sample
(1:3,000 dilution; Boster, China). For detection, the membrane was
incubated with goat anti-rat IgG antibody (1:10,000) in TBST for 1
h at room temperature. The immunoblots were imaged by an enhanced
chemiluminescence (ECL) detection system, followed by exposure to
ECL Hyperfilm (Beyotime, China).
Reverse transcription and quantitative
real-time PCR assay
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen) following the manufacturer’s instructions. The
mRNAs were reverse-transcribed into cDNA using a PrimeScript™ RT
reagent kit (Takara Bio, Shiga, Japan). Then, a real-time
quantitative PCR (RT-qPCR) assay was performed using the Applied
Biosystems 7500 Sequence Detection system (Applied Biosystems,
Foster City, CA, USA), and SYBR® Premix Ex Taq™
II (Takara Bio). The primer sequences used in real-time RT-PCR
were: ABCG2 forward, 5′-GAAACCTGGTCTCAACGCCATCC-3′ and reverse,
5′-CGTCAGAGTGCCCATCACAACAT-3′; β-actin forward,
5′-CCTGGCACCCAGCACAAT-3′ and reverse, 5′-GCC GATCCACACGGAGTACT-3′.
The threshold cycle (Ct) was used to determine the relative level
of expression of each gene by normalizing to the Ct of β-actin. All
genes were tested in 3 independent experiments.
PKH-26 staining
The cells from each group were washed with PBS and
resuspended in PKH diluents with PKH dye labeling. The cells were
incubated at room temperature for 5 min with periodic mixing. To
stop the reaction, 2 ml of serum was added, and the cells were
incubated for 1 min to allow binding of excess dye. The samples
were centrifuged and washed twice with 10 ml of complete medium to
remove the unbound dye. Finally, the cells were resuspended in DMEM
serum-free medium supplemented with 20 ng/ml of EGF, 20 ng/ml of
bFGF and 4 μg/ml of insulin, and plated on to ultra-low attachment
96-well at a density of 1,000/ml. PKH-26-stained cells were
observed with fluorescence microscopy and images were acquired with
an Olympus camera or confocal microscopy.
Statistical analysis
All data are presented as the means ± standard
deviation (SD) of at least 3 independent experiments. Analysis of
variance (ANOVA) and two-tailed Student’s t-tests were used to
identify significant differences in the growth of sorted cells.
Differences were considered to indicate a statistically significant
result when the P-value was <0.05.
Results
Establishment and identification of
cisplatin-resistant NSCLC cell lines
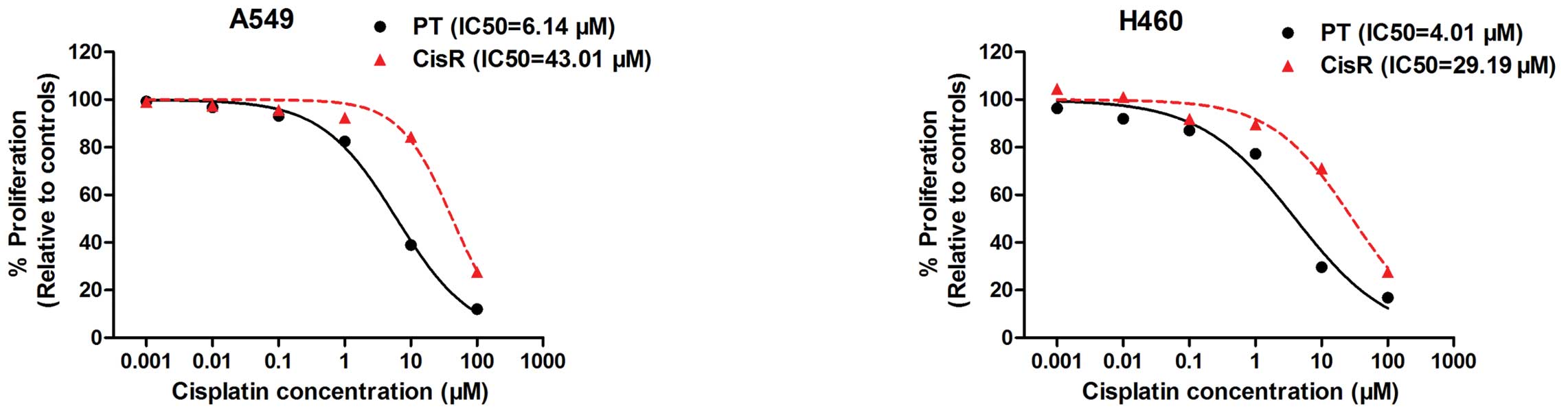
To determine IC50 values, cells were
incubated with concentrations of cisplatin ranging from 0.001 to
100 μM. Dose-response curves were generated and IC50
concentrations were calculated (Fig.
1). Then, we established two cisplatin-resistant lung cancer
cell lines (H460/CisR and A549/CisR) by repeatedly treating
parental H460 and A549 cells with the IC50 concentration
of cisplatin. At 6 months, the IC50 values of H460/CisR
and A549/CisR were also measured and calculated. The
IC50 concentration of cisplatin-resistant cells
displayed a significant increase compared to the corresponding
parental cells (Fig. 1). In A549
cells, the IC50 concentration of cisplatin-resistant
cells was determined to be 43.01 μM compared to 6.14 μM in the
original parental cell line; a 7-fold increase in the concentration
of cisplatin was required to obtain a 50% inhibition in cell
growth. A significant increase in IC50 concentrations
was also observed in H460/CisR cells (29.19 vs. 4.01 μM),
indicating a 7.28-fold increase in the H460/CisR cell lines
compared to the parental cells. Taken together, the results
demonstrated a cisplatin-resistant phenotype in two NSCLC cell
lines following continuous exposure to cisplatin in
vitro.
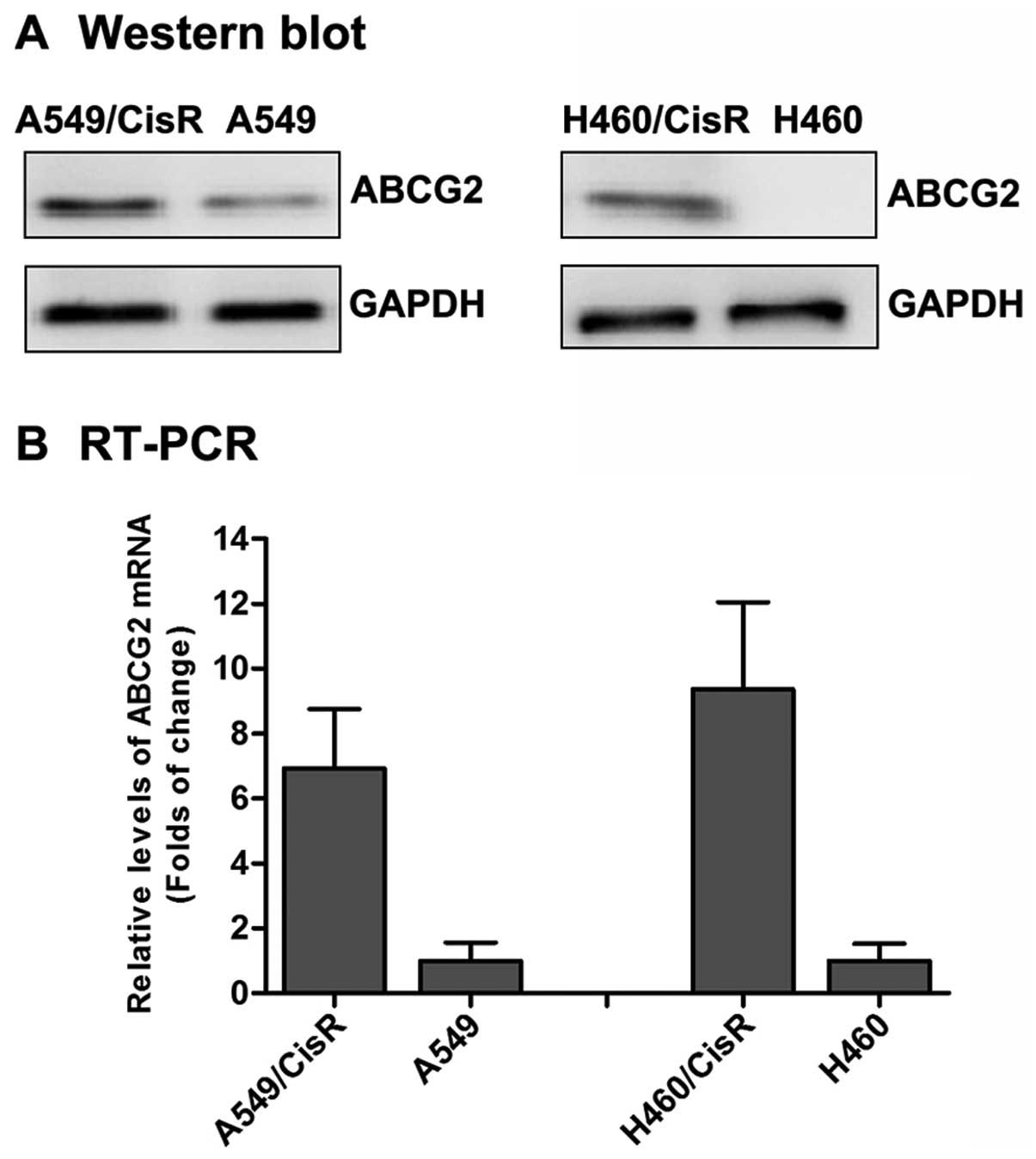
Overexpression of ABCG2 in
cisplatin-resistant NSCLC cells
To observe the difference of the cisplatin-resistant
cells and parental cells, we determined the level of expression of
ABCG2 in A549, A549/CisR, H460 and H460/CisR cells by western
blotting and real-time quantification PCR. The level of expression
of ABCG2 in the A549/CisR cells was higher than in the parental
cells. Similar results were also observed in H460/CisR cells
(Fig. 2). Moreover, a higher level
of expression of ABCG2 mRNA was observed in A549/CisR cells and
H460/CisR cells as compared to the parental cells (Fig. 2). The relative level of expression
of ABCG2 mRNA in A549/CisR and H460/CisR cells was 6.92- or
9.36-fold higher than the corresponding parental cells,
respectively. These results are consistent with numerous other
studies, which demonstrated that ABCG2 had increased expression in
human cancer cell lines screened by various anticancer drugs
(11,12).
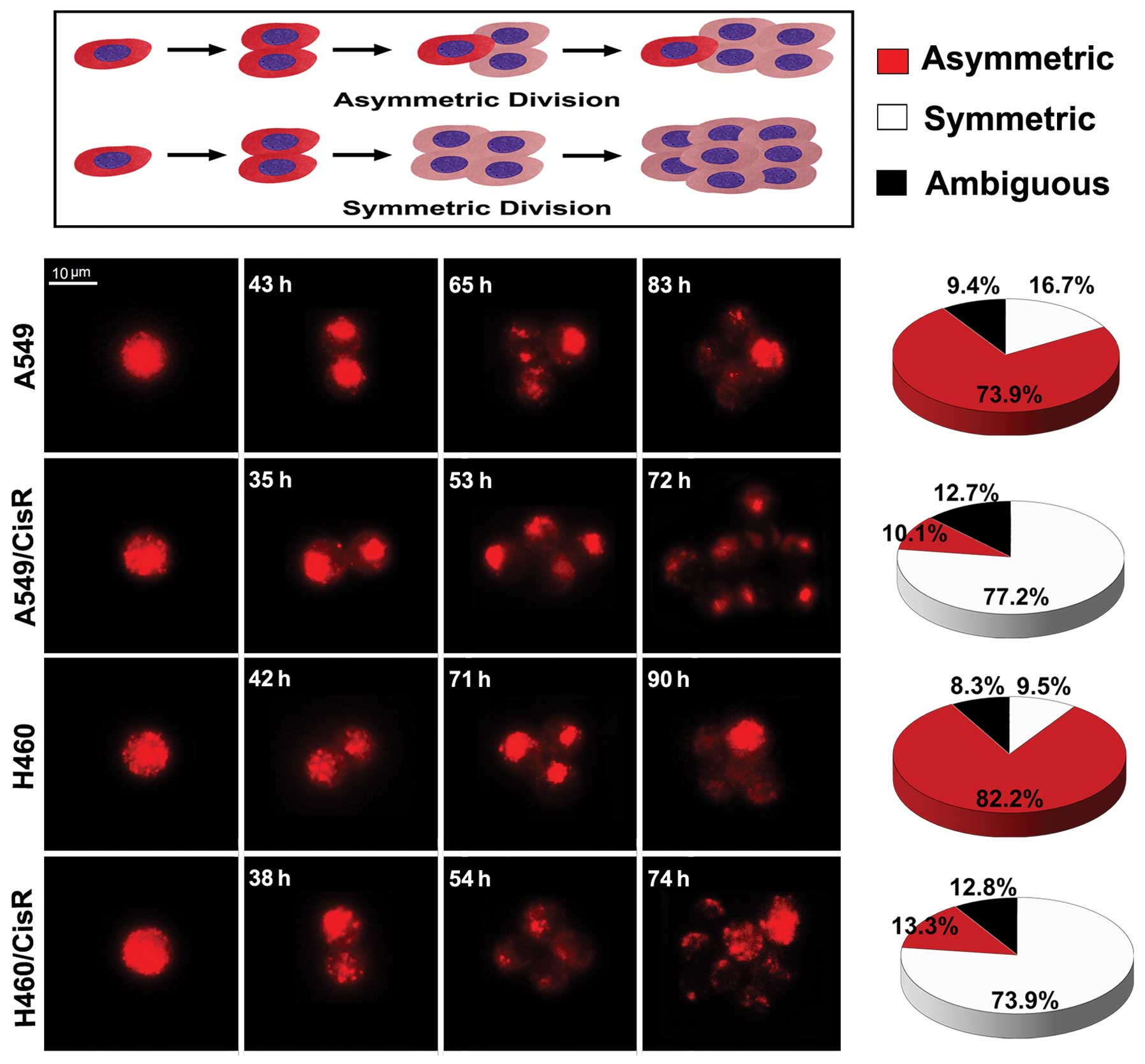
Increased symmetric division in
cisplatin-resistant NSCLC cells
To determine the pattern of cell self-renewing
divisions in cisplatin-resistant cells (A549/CisR and H460/CisR
cells) and the parental cells (A549 and H460 cells), we stained the
four cell lines with PKH-26. PKH-26 is a fluorescent dye that binds
to cell membranes and segregates in daughter cells after each cell
division. This method is commonly used to detect the pattern of
cell division (13). The results
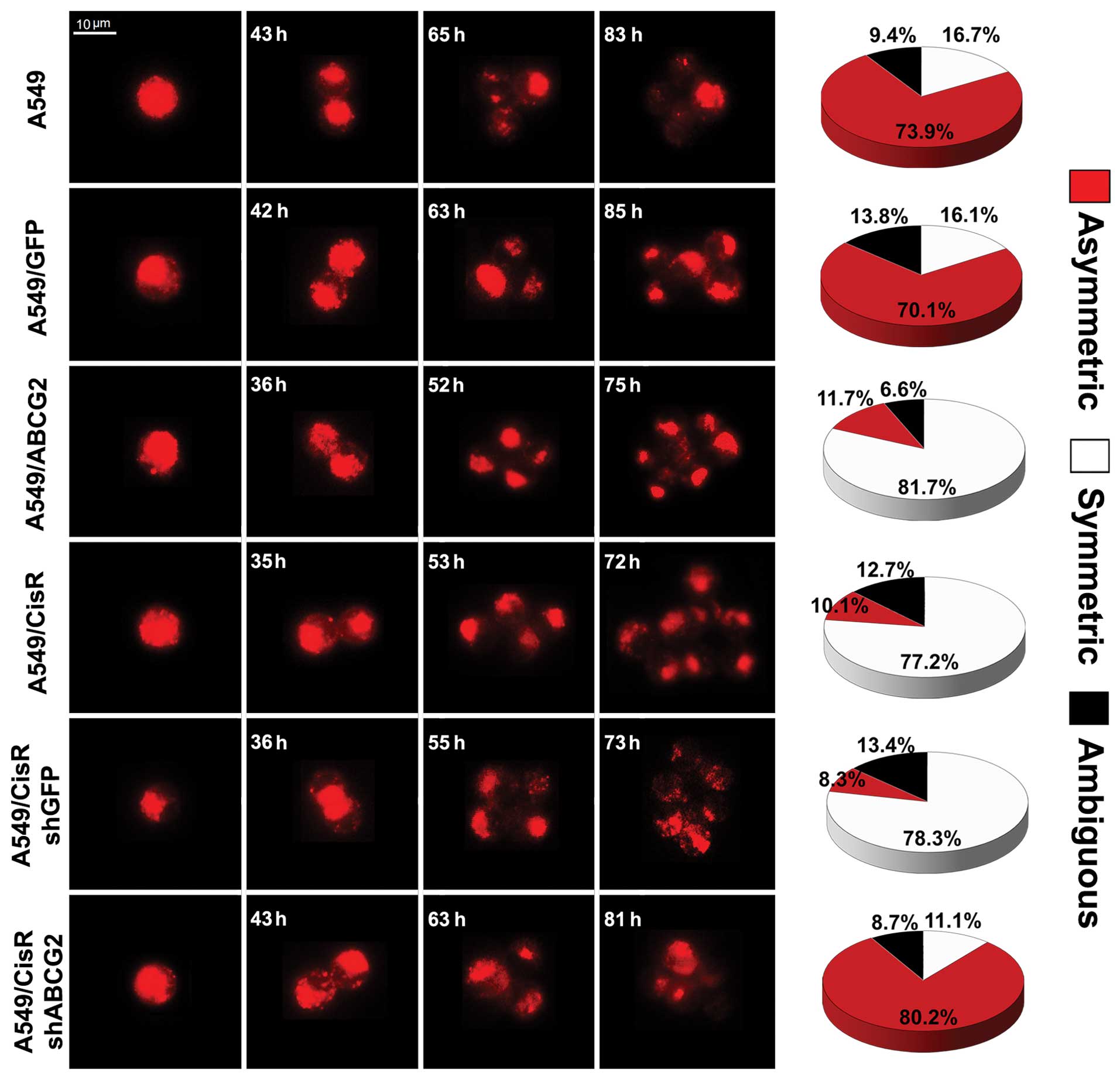
showed that the pattern of cell division in A549 parental cells was
73.9% asymmetric, 16.7% symmetric, and 9.4% undefined (according to
PHK-26 staining which could not be classified by PKH-26 dye). The
pattern of cell division in H460 parental cells was similar to the
pattern of A549 parental cells, including 82.2% asymmetric, 9.5%
symmetric and 8.3% undefined. However, the patterns of cell
division in cisplatin-resistant NSCLC cells were significantly
different from the pattern of NSCLC parental cells (Fig. 3). The pattern of cell division in
A549/CisR cells was 77.2% symmetric, 10.1% asymmetric and 12.7%
undefined. The pattern of cell division in H460/CisR cells
comprised of 73.9% symmetric, 13.3% asymmetric and 12.8% undefined.
In summary, these results suggest that symmetric and asymmetric
divisions co-exist in cisplatin-resistant NSCLC cells and the
parental cells, but with different proportions. Thus, parental
NSCLC cells mainly divide asymmetrically, whereas
cisplatin-resistant NSCLC cells mainly divide symmetrically.
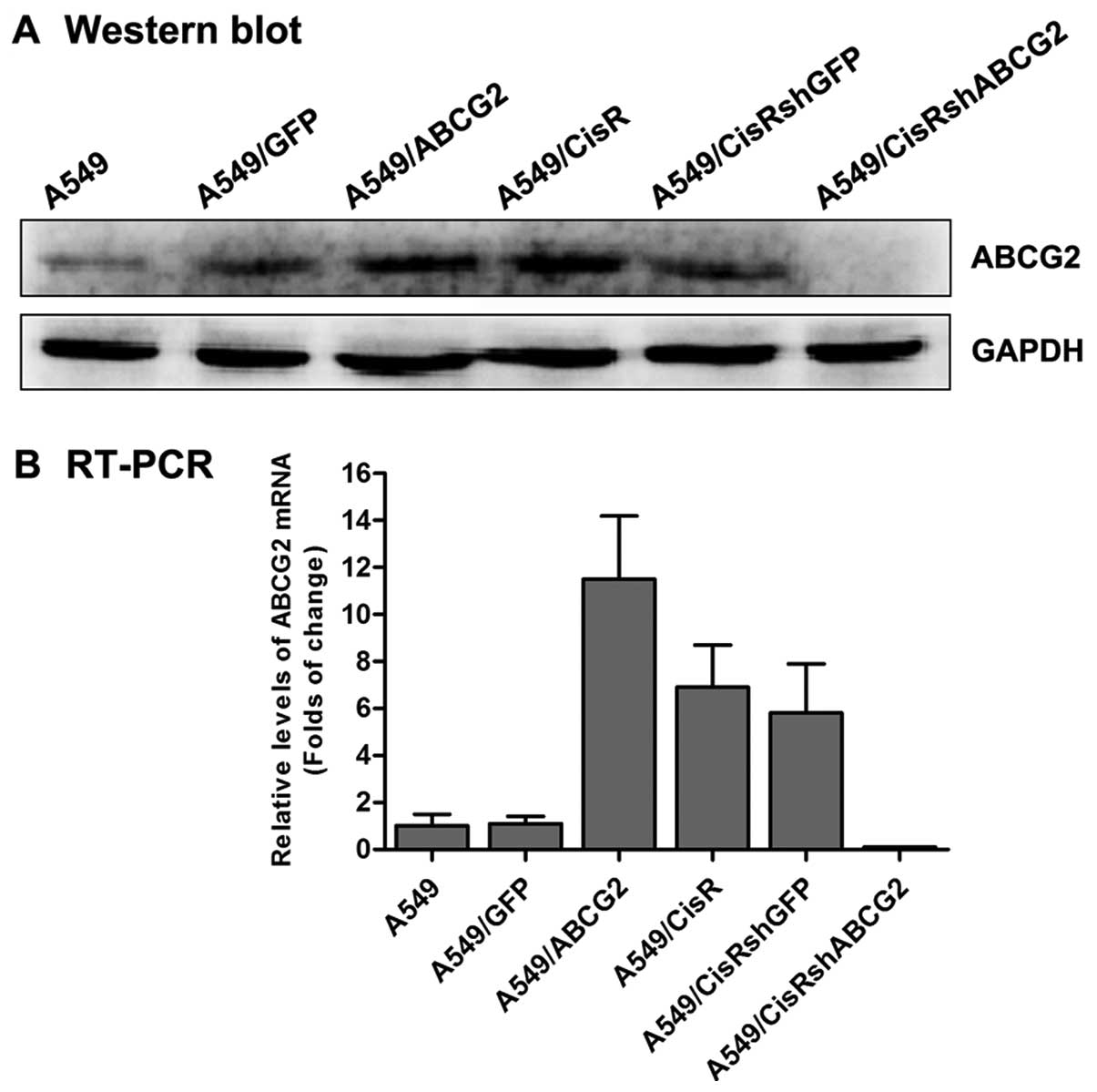
Increased symmetric division in
ABCG2-overexpressing NSCLC cell lines
The experiments described above indicated that
cisplatin-resistant cells have increased symmetric division and
display a higher level of ABCG2 expression. We next hypothesized
that ABCG2 is involved in cell division in drug-resistant cells. To
determine the relationship between ABCG2 and cell division in
cisplatin-resistant NSCLC cells, we transfected the recombinant
lentivirus of ABCG2 into A549 cells to establish a stable
ABCG2-overexpressing cell line (A549/ABCG2). As shown in Fig. 4, the results of western blotting and
real-time quantification PCR showed that the expression of ABCG2 in
A549/ABCG2 cells was clearly increased. Moreover, PKH-26 staining
showed that a significantly greater proportion of A549/ABCG2 cells
(81.7%) divided symmetrically than A549 parental cells (16.7%;
Fig. 5). These data indicate that
ABCG2 overexpression increases the potential for symmetric division
in A549 cells.
Increased asymmetric division in the
ABCG2-inhibition cisplatin-resistant NSCLC cell line
To further confirm that the characteristics of ABCG2
described above could regulate cell division in NSCLC cells, we
transfected A549/CisR cells with SMART vector shRNA lentiviral
particles targeted against ABCG2 to inhibit expression of the gene.
As shown in Fig. 4, the level of
expression of ABCG2 in A549/CisRshABCG2 cells was significantly
lower than A549/CisR cells. Furthermore, PKH-26 staining showed
that a significantly lower percentage of A549/CisRshABCG2 cells
(11.1%) divided symmetrically than A549/CisR cells (77.2%; Fig. 5). These findings, in addition to the
observation that ABCG2 overexpression increased the percentage of
symmetric division, indicate that ABCG2 regulates the pattern of
cell self-renewing divisions in cisplatin-resistant NSCLC cell
lines.
Discussion
ABCG2 is widely expressed in various normal tissues
and stem cells, as well as cancer cells. Previous studies have
suggested that ABCG2 plays a critical role in the maintenance of
the stem cell phenotype and multidrug resistance of cancer cells
(14,15). However, to date, there have been no
studies reporting the role of ABCG2 expression in cancer cell
division. Hence, in the present study, we attempted to determine
the possible role of ABCG2 in cell division in cisplatin-resistant
NSCLC cells. Our data showed that ABCG2 is overexpressed in
cisplatin-resistant NSCLC cells, and those cells were more prone to
divide symmetrically, while silencing of ABCG2 in
cisplatin-resistant NSCLC cells led to increased asymmetric
division. Our study demonstrated that ABCG2 could regulate the
pattern of cell division in cisplatin-resistant NSCLC cell
lines.
Platinum-based chemotherapeutics, such as cisplatin,
remain the standard first-line chemotherapy for NSCLC with good
performance status and have shown significant improvement in
overall survival and quality of life. However, cisplatin induces a
variety of problems, such as cisplatin resistance, which is a major
obstacle for successful cancer treatment (16). Furthermore, cisplatin-resistant
tumors also fail to respond to other drugs (17). Thus, it is critical to gain a better
understanding of the molecular mechanisms underlying the
drug-resistant phenotype associated with cisplatin resistance. In
the present study, we generated a clinically-relevant, isogenic
model of cisplatin resistance in a panel of NSCLC cell lines from
original, age-matched parent cell lines, and characterized these
cell lines in terms of the fold-changes in IC50 values.
Using IC50 concentrations, cisplatin-resistant cell
lines were established over time through chronic in vitro
exposure to cisplatin, after which time the IC50 values
were re-assessed in cisplatin-treated cell lines. The
IC50 of cisplatin-treated cells was shown to be
significantly higher, demonstrating a more resistant phenotype of
these cells. Although the exact mechanisms underlying cisplatin
resistance are unclear, multidrug resistance has emerged as a
significant cellular mechanism to explain clinical drug resistance
and ABCG2 has been implicated in multidrug resistance in cancer
chemotherapy (18). ABCG2 is a
member of the ABC family and functions as an ATP-binding cassette
discharge pump. ABCG2 prevents the intracellular accumulation of
substrate compounds, including anticancer drugs, by limiting the
influx into and facilitating the efflux out of cells. In cancer
cells, high expression of ABCG2 prevents the intracellular
accumulation of anticancer drugs, which results in drug resistance
(19). In NSCLC, several studies
have demonstrated that there is an association between high ABCG2
expression with platinum-based regimens and lower response rate,
shorter overall survival and progression-free survival (6,20). In
agreement with previous findings, our western blotting and PCR
results showed that the level of ABCG2 expression in the
cisplatin-resistant NSCLC cells was significantly higher than in
the parental cells.
In the present study, our data also showed that the
pattern of cell division in cisplatin-resistant NSCLC cells was
significantly different from the pattern of cell division in the
parental cells. Traditionally, there have been two basic models of
stem cell division; asymmetric and symmetric (21). We found that symmetric and
asymmetric division co-exist in cisplatin-resistant NSCLC and
parental cells, but in different proportions; in particular,
cisplatin-resistant NSCLC cells mainly divide symmetrically,
whereas the parental cells mainly divide asymmetrically. According
to an asymmetric cell division model, two daughter cells with
divergent fates are generated, with one daughter cell capable of
self-renewal and the other daughter cell committed to
differentiation. According to a symmetric division model, two
identical daughter cells are formed, which indicates that the cells
retain stem cell properties or become committed cells early in the
developmental process (22). The
asymmetric model has the advantage of keeping the stem cell
population level stable; however, an obvious disadvantage is an
inability to replenish the stem cell pool in case of injury. This
problem is naturally solved by the symmetric model (23). Several studies have shown that
progenitor/stem cells divide asymmetrically during physiologic
tissue homeostasis; however, these progenitor/stem cells favor
symmetric division and rapid proliferation after tissue injury
(21,24). In addition, previous studies have
shown that asymmetric division functions as a mechanism of tumor
suppression in Drosophila neuroblasts. Loss-of-function
mutations of cell polarity and cell fate determinants induce
neuroblasts to divide symmetrically, leading to an increase in
number, tissue overgrowth and ultimately transplantable tumors that
resemble mammalian cancers (9).
Recent evidence has revealed that cisplatin-resistant
subpopulations of NSCLC cells have a putative stem-like character,
including increased invasive ability and tumorigenic ability
(25). Thus, our findings are
consistent with previous studies, which showed an increased
proportion of symmetric division in cisplatin-resistant NSCLC cells
compared to the parental cells.
The most notable finding of the present study was
the potential relationship between the level of ABCG2 expression
and the pattern of NSCLC cell division. To better explain the
molecular mechanisms involved in ABCG2-mediated cell division, we
established stable ABCG2-overexpressing and ABCG2-knockdown cell
lines and assessed cell division by PKH-26 staining. We found that
self-renewing division of ABCG2-overexpressing cells was more prone
to divide symmetrically than the parental cells, and knockdown of
ABCG2 expression could decrease the proportion of symmetric
division in cisplatin-resistant A549 cells. These data provide
evidence that ABCG2 can regulate the pattern of cell division in
cisplatin-resistant NSCLC cell lines. These results were consistent
with a recent study, which demonstrated that ABCG2 can directly
regulate the switch between symmetric and asymmetric cell division
in a cardiac side population (7).
Although our data showed that ABCG2 can regulate
cell division in cisplatin-resistant NSCLC cells, the specific
pathway is at present unknown. Given that ABCG2 is capable of
transporting a diverse array of substrates, ABCG2 could participate
in regulating cell division via the transport of exogenous or
endogenous signaling molecules, which in turn may promote cell
symmetric division. This hypothesis has been confirmed by several
previous studies and some potential regulating pathways have been
reported. For example, Susanto et al (26) showed that ABCG2 can regulate
embryonic stem cell self-renewal through maintenance of porphyrin
homeostasis. Cicalese et al (27) suggested that p53 regulated polarity
of cell division in mammary cancer stem cells and loss of p53
favored symmetric divisions of cancer cells, thus contributing to
tumor growth. Additionally, an increased expression of p53 has been
reported in ABCG2 knockout mice (7). Further studies are needed to clarify
the mechanism by which ABCG2 regulates cancer cell division.
Moreover, the present study may provide a new
strategy for the clinical treatment of NSCLC. Chemotherapy is
typically administered in cycles with 3-week intervals to recover
the hematopoietic system and other normal cells. However, previous
studies have reported that tumor cells can aggressively repopulate
during these intervals and implant into the pre-treated location
(28). We hypothesized that
conventional chemotherapy can increase the level of expression of
ABCG2, which further increases the proportion of symmetric division
in NSCLC cells and promotes rapid cancer proliferation and restores
the tumor to its pre-treatment size. This hypothesis has been
supported by several recent studies. For example, Chen et al
(10) reported that ABCG2 is
involved in the proliferation of cancer cells. Cicalese et
al (27) demonstrated that loss
of p53 promotes symmetric division and contributes to tumor rapid
growth, and pharmacologic reactivation of p53 is correlated with
restoration of asymmetric divisions and tumor growth reduction.
Collectively, our findings together with previous reports, suggest
that therapies aimed to increase asymmetric division inhibit the
rapid proliferation of NSCLC cells, and potentially improve
long-term outcomes for lung cancer patients.
The decreased accumulation of antitumor drugs
mediated by ABCG2 is considered to be one of the cellular
mechanisms involved in drug resistance of cancer cells. In the
present study, we found that ABCG2 also contributes to symmetric
division in cancer cells, particularly in drug-resistant cancer
cells. Therefore, ABCG2 is involved in the modulation of drug
resistance through regulation of the pattern of cell division and
drug effluence. Our findings provide a better understanding of the
function of ABCG2 in cancer chemoresistance; however, additional
research is required to study the new function of this transporter
and to explain the interactions between ABCG2 and pathways that
regulate the cell division of stem and cancer cells.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81201684).
References
|
1
|
Bunn PA Jr and Kelly K: New
chemotherapeutic agents prolong survival and improve quality of
life in non-small cell lung cancer: a review of the literature and
future directions. Clin Cancer Res. 4:1087–1100. 1998.PubMed/NCBI
|
|
2
|
Giaccone G, Splinter TA, Debruyne C, et
al: Randomized study of paclitaxel-cisplatin versus
cisplatin-teniposide in patients with advanced non-small-cell lung
cancer. The European Organization for Research and Treatment of
Cancer Lung Cancer Cooperative Group. J Clin Oncol. 16:2133–2141.
1998.PubMed/NCBI
|
|
3
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Robey RW, To KK, Polgar O, et al: ABCG2: a
perspective. Adv Drug Deliv Rev. 61:3–13. 2009. View Article : Google Scholar
|
|
5
|
Nakamura Y, Oka M, Soda H, et al:
Gefitinib (‘Iressa’, ZD1839), an epidermal growth factor receptor
tyrosine kinase inhibitor, reverses breast cancer resistance
protein/ABCG2-mediated drug resistance. Cancer Res. 65:1541–1546.
2005.
|
|
6
|
Yoh K, Ishii G, Yokose T, et al: Breast
cancer resistance protein impacts clinical outcome in
platinum-based chemotherapy for advanced non-small cell lung
cancer. Clin Cancer Res. 10:1691–1697. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sereti KI, Oikonomopoulos A, Unno K, Cao
X, Qiu Y and Liao R: ATP-binding cassette G-subfamily transporter 2
regulates cell cycle progression and asymmetric division in mouse
cardiac side population progenitor cells. Circ Res. 112:27–34.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lathia JD, Hitomi M, Gallagher J, et al:
Distribution of CD133 reveals glioma stem cells self-renew through
symmetric and asymmetric cell divisions. Cell Death Dis.
2:e2002011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gonzalez C: Spindle orientation,
asymmetric division and tumour suppression in Drosophila
stem cells. Nat Rev Genet. 8:462–472. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Z, Liu F, Ren Q, et al: Suppression
of ABCG2 inhibits cancer cell proliferation. Int J Cancer.
126:841–851. 2010.PubMed/NCBI
|
|
11
|
To KK, Robey RW, Knutsen T, Zhan Z, Ried T
and Bates SE: Escape from hsa-miR-519c enables drug-resistant cells
to maintain high expression of ABCG2. Mol Cancer Ther. 8:2959–2968.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Litman T, Brangi M, Hudson E, et al: The
multidrug-resistant phenotype associated with overexpression of the
new ABC half-transporter, MXR (ABCG2). J Cell Sci. 113:2011–2021.
2000.PubMed/NCBI
|
|
13
|
Lanzkron SM, Collector MI and Sharkis SJ:
Hematopoietic stem cell tracking in vivo: a comparison of
short-term and long-term repopulating cells. Blood. 93:1916–1921.
1999.PubMed/NCBI
|
|
14
|
Doyle L and Ross DD: Multidrug resistance
mediated by the breast cancer resistance protein BCRP (ABCG2).
Oncogene. 22:7340–7358. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ueda T, Brenner S, Malech HL, et al:
Cloning and functional analysis of the rhesus macaque ABCG2 gene.
Forced expression confers an SP phenotype among hematopoietic stem
cell progeny in vivo. J Biol Chem. 280:991–998. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rosell R, Lord RV, Taron M and Reguart N:
DNA repair and cisplatin resistance in non-small-cell lung cancer.
Lung Cancer. 38:217–227. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ikuta K, Takemura K, Sasaki K, et al:
Expression of multidrug resistance proteins and accumulation of
cisplatin in human non-small cell lung cancer cells. Biol Pharm
Bull. 28:707–712. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu J, Peng H and Zhang JT: Human multidrug
transporter ABCG2, a target for sensitizing drug resistance in
cancer chemotherapy. Curr Med Chem. 14:689–701. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Doyle LA, Yang W, Abruzzo LV, et al: A
multidrug resistance transporter from human MCF-7 breast cancer
cells. Proc Natl Acad Sci USA. 95:15665–15670. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ota S, Ishii G, Goto K, et al:
Immunohistochemical expression of BCRP and ERCC1 in biopsy specimen
predicts survival in advanced non-small-cell lung cancer treated
with cisplatin-based chemotherapy. Lung Cancer. 64:98–104. 2009.
View Article : Google Scholar
|
|
21
|
Morrison SJ and Kimble J: Asymmetric and
symmetric stem-cell divisions in development and cancer. Nature.
441:1068–1074. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Knoblich JA: Mechanisms of asymmetric stem
cell division. Cell. 132:583–597. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shahriyari L and Komarova NL: Symmetric
vs. asymmetric stem cell divisions: an adaptation against cancer?
PLoS One. 8:e761952013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang R, Zhang Z, Zhang C, et al: Stroke
transiently increases subventricular zone cell division from
asymmetric to symmetric and increases neuronal differentiation in
the adult rat. J Neurosci. 24:5810–5815. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barr MP, Gray SG, Hoffmann AC, et al:
Generation and characterisation of cisplatin-resistant non-small
cell lung cancer cell lines displaying a stem-like signature. PLoS
One. 8:e541932013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Susanto J, Lin YH, Chen YN, et al:
Porphyrin homeostasis maintained by ABCG2 regulates self-renewal of
embryonic stem cells. PLoS One. 3:e40232008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cicalese A, Bonizzi G, Pasi CE, et al: The
tumor suppressor p53 regulates polarity of self-renewing
divisions in mammary stem cells. Cell. 138:1083–1095. 2009.
|
|
28
|
Kim JJ and Tannock IF: Repopulation of
cancer cells during therapy: an important cause of treatment
failure. Nature Rev Cancer. 5:516–525. 2005. View Article : Google Scholar : PubMed/NCBI
|