Introduction
Pancreatic cancer is an aggressive disease with a
5-year survival rate of less than 5% and a median survival of 6
months after diagnosis, thereby exhibiting the poorest prognosis of
all solid tumors (1). It is
characterized by a high propensity for local invasion and distant
metastasis as well as a largely drug-resistant phenotype; however,
the molecular events underlying this remain to be elucidated.
Recent advances in genome analysis, including
microarray and massively parallel sequencing, have discovered
extensive transcription of large RNA transcripts that lack coding
protein function, termed long noncoding RNAs (lncRNAs). It is
becoming evident that lncRNAs may be an important class of
pervasive genes involved in tumorigenesis and metastasis (2). Metastasis-associated lung
adenocarcinoma transcript-1 (MALAT-1) is an evolutionarily highly
conserved and ubiquitously expressed lncRNA with a length of 8,700
nucleotides (3). Since its
discovery in non-small cell lung cancer (3), MALAT-1 has been linked to several
human tumor entities. In most cases, MALAT-1 is expressed higher in
tumor tissues; it may be closely related to clinical parameters and
may control tumor cell progression (4). However, little is known about the
expression pattern and biological function of MALAT-1 involved in
pancreatic cancer.
In the present study, we demonstrated that MALAT-1
expression levels were upregulated in pancreatic cancer tissue
compared with adjacent noncancerous controls. Consistently, higher
expression level of MALAT-1 was found in all seven pancreatic
cancer cell lines relative to the human pancreatic ductal
epithelial (HPDE) cells. Furthermore, our data revealed that
knockdown of MALAT-1 inhibited tumor cell growth via induction of
G2/M cell cycle arrest and apoptosis, and decreased cell
migration and invasion through regulation of epithelial-mesenchymal
transition (EMT) and stem-like cell marker expression.
Materials and methods
Human tissue samples and cell lines
Human tumor tissue samples and adjacent noncancerous
controls were obtained by surgical resection from six patients with
pancreatic cancer, at the Department of General Surgery, First
People’s Hospital, School of Medicine, Shanghai Jiao Tong
University, Shanghai, China. All samples were derived from patients
who had not received adjuvant treatment including radiotherapy or
chemotherapy prior to surgery. All samples were snap-frozen and
stored in liquid nitrogen after collection. Written informed
consent was obtained from all subjects, and the study was approved
and supervised by the Ethics Committee of the First People’s
Hospital, School of Medicine, Shanghai Jiao Tong University.
HPDE, pancreatic cancer cells BxPC-3, CFPAC-1,
CAPAN-1, SW1990, AsPC-1, PANC-1 and HS-766T were all obtained from
Chinese Academy of Sciences Cell Bank (Shanghai, China). PANC-1 and
HS-766T were grown in 5% CO2 saturated humidity, at
37°C, and cultured in DMEM supplemented with 2 mmol/l glutamine and
10% fetal bovine serum (FBS) (both from Gibco, USA) and subcultured
by harvesting with trypsin-EDTA. HPDE, BxPC-3, CAPAN-1, AsPC-1,
CFPAC-1 and SW1990 cells were cultured in RPMI-1640 (Gibco)
supplemented with 10% FBS.
Real-time-quantitative polymerase chain
reaction (RT-qPCR) analysis
Total RNA was isolated from the cultured cells and
tissue samples by using an RNA isolation kit (Takara Bio, Inc.)
according to the manufacturer’s instructions. Reverse transcription
and RT-qPCR kits (Takara Bio, Inc.) were applied to evaluate
expression of MALAT-1. Primers used were: MALAT-1 forward,
5′-GAATTGCGTCATTTAAAGCCTAGTT-3′ and reverse,
5′-GTTTCATCCTACCACTCCCAATTAAT-3′; GAPDH forward,
5′-ACAGTCAGCCGCATCTTCTT-3′ and reverse, 5′-GACAAGCTTCCCGTTCTCAG-3′.
The expression of GAPDH was detected as the endogenous control.
Relative mRNA expression of MALAT-1 was calculated with the
comparative threshold cycle (Ct) (2−ΔΔCt) method.
Western blot analysis
Cells were lysed in RIPA lysis buffer and the
protein concentration was determined (Beyotime). Total proteins
were fractionated using SDS-PAGE and transferred onto a
polyvinylidene fluoride membrane. The membranes were blocked in 3%
bovine serum albumin in TBST buffer containing 0.1% Tween-20 and
then incubated with the indicated primary antibodies at 4°C
overnight. Appropriate secondary antibodies were incubated at room
temperature for 1 h and detected using the enhanced
chemiluminescence detection system. The data was adjusted against
loading control using β-actin. The antibodies used for western blot
analyses were: mouse anti-N-cadherin, mouse anti-E-cadherin (BD
Biosciences, Bedford, MA, USA); mouse anti-vimentin, rabbit
anti-ALDH (Abcam); rabbit anti-Slug, rabbit anti-Snail, mouse
anti-CDC2, rabbit anti-matrix metalloproteinases-2 (MMP-2), rabbit
anti-MMP-9, rabbit anti-PCNA (Cell Signaling Technology); mouse
anti-p21, mouse anti-p53, mouse anti-CD44, rabbit anti-CD24, mouse
anti-β-actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA).
Establishment of pancreatic cancer cell
line with stable expression of MALAT-1 short hairpin RNA
(shRNA)
Based on principles of shRNA design and the human
MALAT-1 structure (NR_002819), three plasmid vectors encoding shRNA
directed against MALAT-1 mRNA were constructed. The three shRNA
targeting sequences were: shRNA-M1, 5′-GAGTAACTGGCATGTGAGCAA-3′;
shRNA-M2, 5′-CATGACGGAGGTTGAGATGAA-3′; shRNA-M3,
5′-AAGCCGAAATAAATGAGAGAT-3′. The scrambled sequence,
5′-TTC′TCCGAACGTGTCACGT-3′, was used as a negative control that
does not target any known human mRNA. The preparation of lentivirus
expressing human MALAT-1 shRNA was performed using the GV-248
lentiviral RNAi expression system (Genechem, Shanghai, China).
AsPC-1 and CFPAC-1 were infected with lentiviral particles
containing specific or negative control vectors, and the polyclonal
cells with puromycin resistance were selected for further
experiments.
Colony formation assay
Briefly, ~1,000 cells were added to each well of a
6-well culture plate. After 2 weeks of incubation, cell colonies
were washed twice with PBS, fixed with 4% paraformaldehyde for 15
min and then stained with crystal violet for 30 min. Individual
clones with >50 cells were counted.
Cell proliferation assay
A real-time imaging system (IncuCyte™) was used to
measure cell proliferation using non-label cell monolayer
confluence approach. IncuCyte provides the ability to acquire high
quality, phase-contrast images and an integrated confluence metric
as a surrogate for cell number (5).
We used a similar approach to determine the effect of MALAT-1 on
pancreatic cancer cell proliferation. Cell confluence was compared
between M-nc and M-si1 stable transfected cancer cells.
Wound healing assay
Cells were cultured and grown to 100% confluence.
Wounds were scratched in the monolayer with a 100-μl pipette tip.
Suspended cells and debris were washed with PBS buffer, then the
cells were incubated in RPMI-1640 medium containing 2% FBS at 37°C.
The migration of cells into the wounded areas was evaluated at the
indicated times using an inverted microscope, and then
photographed. Three different areas in each assay were selected to
measure the distance of the migrating cells to the origin of the
wound.
Boyden chamber and Transwell assay
The cell invasive and migratory potential were
evaluated using Boyden chamber and Transwell assay, respectively.
Briefly, Boyden chamber was conducted using specialized MilliCell
chambers, which included a 24-well tissue culture plate with 12
cell culture inserts (Millipore, Bedford, MA, USA). The inserts
contained an 8 μm pore size polycarbonate membrane with a
pre-coated thin layer of Matrigel (BD Biosciences). Ten percent
FBS-containing medium was placed in the lower chambers to act as a
chemo-attractant. Then, 1×105 AsPC-1 and CFPAC-1 in a
100 μl volume of serum-free medium were placed in the upper
chambers and incubated at 37°C for 48 h. Invasive cells on the
lower surface of the membrane, which had invaded the Matrigel and
had migrated through the polycarbonate membrane, were stained by
the staining solution, and counted under a microscope in five
randomly selected fields at a magnification of ×200. Transwell
assay was the same as the Boyden chamber with the exception that no
Matrigel was used and the permeating time for cells was 24 h.
Flow cytometry analysis of cell apoptosis
and cell cycle
For analysis of the cell apoptosis, cells were
harvested at 70–80% confluence and incubated with reagent
containing Annexin V-FITC and propidium iodide (BD Biosciences) for
15 min in darkness at room temperature. Apoptotic cells were
analyzed using FACSCalibur flow cytometer (BD Biosciences). For
cell cycle analysis, cells were fixed in 70% ethanol at 4°C
overnight and then treated with RNase A (50 μg/ml) and stained with
propidium iodide (25 μg/ml) for 30 min at 37°C. Distribution of
cell-cycle phases was determined using ModFit software (BD
Biosciences).
Statistical analysis
All statistical analyses were performed using SPSS
13.0. The data are presented as means ± SEM from at least three
separate experiments. The difference between two groups was
analyzed by the Student’s t-test (two-tailed). In vitro cell
growth assay was tested using factorial design one-way analysis of
variance (ANOVA). The P-value of <0.05 was considered to
indicate a statistically significant difference.
Results
MALAT-1 is upregulated in pancreatic
cancer cell lines and tumor tissue samples
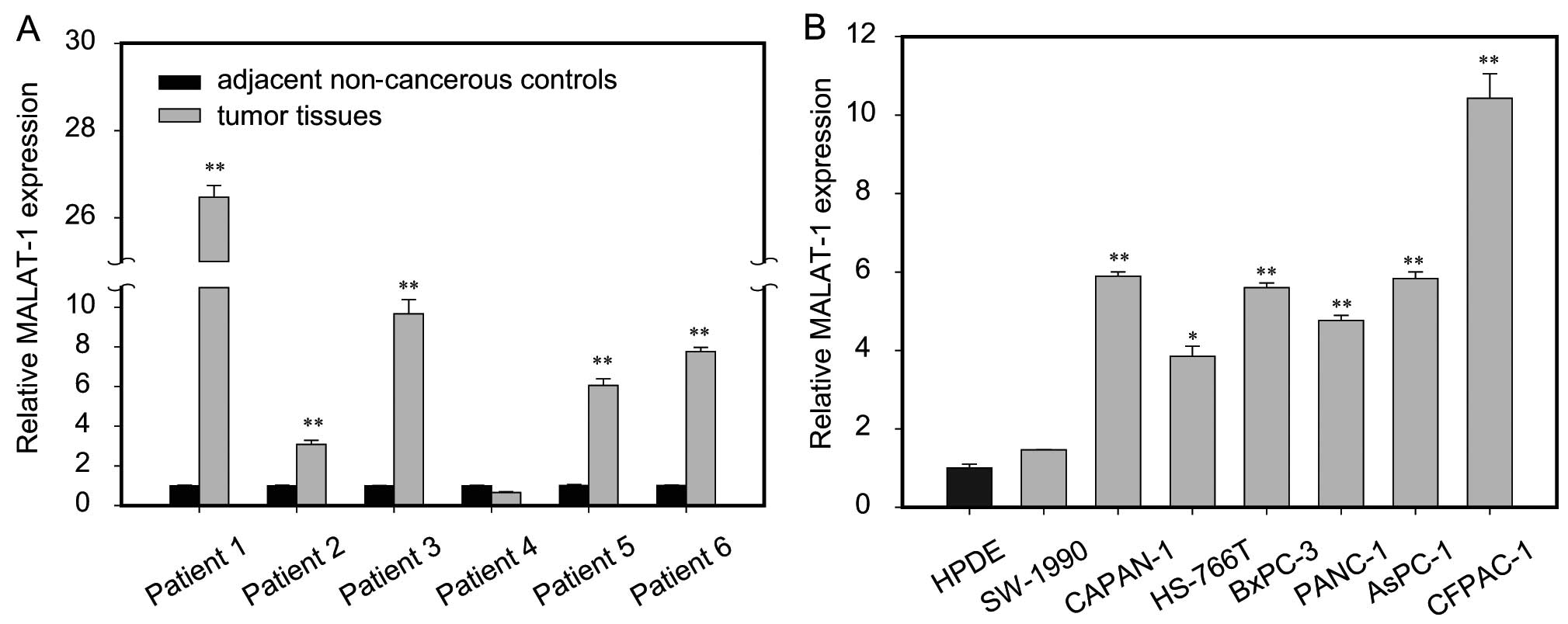
To assess the role of MALAT-1 in pancreatic cancer,
we first assayed MALAT-1 expression in tumor tissue samples and
adjacent non-cancerous pancreas tissues from six patients with
pancreatic cancer. The results showed that MALAT-1 expression was
significantly higher in five cases of tumor tissues than in matched
paracancerous controls (Fig. 1A).
Furthermore, we compared the expression of MALAT-1 between
pancreatic cancer cell lines and normal HPDE cells. Notably,
consistently higher expression level of MALAT-1 was found in all
seven pancreatic cancer cell lines relative to the HPDE, among
which five were upregulated >4-fold (Fig. 1B). Taken together, it is suggested
that upregulation of MALAT-1 is a frequent event in human
pancreatic cancer.
Stable downregulation of MALAT-1
expression inhibits cell proliferation in vitro
Among the pancreatic cancer cell lines, AsPC-1 and
CFPAC-1 had relatively higher expression levels of MALAT-1
(Fig. 1B), therefore these two cell
lines were chosen to study the functions of endogenous MALAT-1
through a loss-of-function approach. We used a lentiviral shRNA
vector to specifically and stably knock down the expression of
MALAT-1 in AsPC-1 and CFPAC-1. A knockdown effect was observed by
RT-qPCR, and we found that M-si1 achieved the greatest efficacy in
silencing MALAT-1 expression compared to the negative control M-nc
(Fig. 2A). Thus, stable
transfection cancer cell lines AsPC-1/M-nc and AsPC-1/M-si1, as
well as CFPAC-1/M-nc and CFPAC-1/M-si1 were used in the following
experiments.
Subsequently, we examined the effect of decreased
MALAT-1 expression on pancreatic cancer cell proliferation in
vitro by the IncuCyte real-time video imaging system. The
determined growth curves showed that suppressing MALAT-1
significantly reduced cell proliferation in comparison with M-nc
cells (Fig. 2B). The analysis of
plate clone formation assay in CFPAC-1 was concordant with the
above result (Fig. 2C). However,
for AsPC-1, the two groups had no cell clone formation.
Furthermore, western blot analysis showed that, compared to M-nc,
the level of proliferation marker PCNA was decreased in the M-si1
groups (Fig. 2D).
Silencing of MALAT-1 induces
G2/M cell cycle arrest and promotes cell apoptosis
Next, to investigate the mechanism involved in
growth suppression, we carried out flow cytometry analysis. The
cell cycle results showed that the G2/M-phase fraction
increased from 14.0±0.9% (AsPC-1/M-nc) to 23.9±0.7% (AsPC-1/M-si1),
1.8±0.2% (CFPAC-1/M-nc) to 4.6±0.7% (CFPAC-1/M-si1) respectively,
indicating G2/M cell cycle arrest after MALAT-1
knockdown (Fig. 3A). In addition,
we detected cell apoptosis after MALAT-1 downregulation. Compared
to the M-nc, significantly enhanced cell apoptosis was observed in
the M-si1 group (AsPC-1/M-si1 6.46% vs. AsPC-1/M-nc 3.45%;
CPFAC-1/M-si1 15.59% vs. CPFAC-1/M-nc 5.55%) (Fig. 3B).
The G2 checkpoint prevents cells from
entering mitosis when DNA is damaged, providing an opportunity for
repair and stopping the proliferation of damaged cells. CDC2, the
cyclin-dependent kinase that normally drives cells into mitosis, is
the ultimate target of pathways that mediate rapid arrest in
G2/M (6). A previous
study revealed that p53 plays an important role in maintaining
G2/M arrest and induces cell apoptosis (7). The contribution of p53 to
G2/M arrest and cell apoptosis involves some of its
transcriptional targets. p21, a well-known cell cycle regulator, is
a major target of p53 (7). Elevated
expression of p21 may result in increased association with CDKs and
inhibit their activity, blocking the entry of cells at the
G2-M-phase transition checkpoint, and induce apoptosis
(8). Our results revealed that p21
and p53 protein levels significantly increased following MALAT-1
downregulation, while CDC2 expression decreased (Fig. 3C). Taken together, these results
indicated an inhibiting effect of reduced MALAT-1 expression on
pancreatic cancer cell growth possibly through inducing
G2/M cell cycle arrest and promoting cell apoptosis.
Knockdown of MALAT-1 suppresses cell
migration and invasion in vitro
To examine the effect of MALAT-1 on pancreatic
cancer cell migration, we used a wound-healing assay and a
Transwell migration assay. According to the wound-healing assay,
the migratory areas of AsPC-1/M-si1 and CFPAC-1/M-si1 were
significantly smaller than those of control cells (Fig. 4A). Similarly, Transwell migration
assay showed that, after 24-h incubation, the number of migrated
cells in the M-si1 groups was significantly lower than that in the
M-nc cells (AsPC-1/M-si1 45±4 vs. AsPC-1/M-nc 107±8; CPFAC-1/M-si1
79±8 vs. CPFAC-1/M-nc 154±12), indicating a decreased migratory
ability following MALAT-1 downregulation (Fig. 4B). Next, using a Boyden chamber
pre-coated with Matrigel, we determined changes in cell
invasiveness after 48 h incubation. Compared with the M-nc cells,
M-si1 cells revealed significantly decreased invasiveness
(AsPC-1/M-si1 27±2 vs. AsPC-1/M-nc 82±5; CPFAC-1/M-si1 18±2 vs.
CPFAC-1/M-nc 90±3) (Fig. 4C). Based
on these results, knockdown of MALAT-1 clearly inhibited the
migration and invasion of pancreatic cancer cells in
vitro.
MALAT-1 facilitates tumor progression by
inducing EMT
It is believed that acquiring the migratory
characteristics of a mesenchymal-like state enhances the invasive
capabilities of cancer cells. The EMT switch is related to an
unfavorable prognosis in pancreatic ductal adenocarcinoma (9). During EMT of in situ tumor
cells, expression of proteins that promote cell-cell contact such
as E-cadherin can be lost, and mesenchymal markers such as
vimentin, N-cadherin and the metalloproteinases MMP-2 and MMP-9 can
be acquired, resulting in enhanced ability for cell migration and
invasion (10). We therefore
examined the expression of EMT-related genes in response to the
knockdown of MALAT-1. The results showed that the level of
E-cadherin was upregulated with the decrease in the level of
MALAT-1, while N-cadherin and vimentin decreased (Fig. 5A). In addition, EMT-related
transcriptional factors including Snail and Slug expression were
also reduced (Fig. 5A). Moreover,
we found that suppressing MALAT-1 expression decreased expression
of MMP-2 and MMP-9 (Fig. 5A).
Furthermore, CFPAC-1 cells were morphologically mixed populations
of epithelial and spindle-shaped mesenchymal type cells in nature
and led to typical epithelial morphology transition after MALAT-1
downregulation (Fig. 5B). However,
the change of morphological features for MALAT-1 knockdown in
AsPC-1 was not observed. Collectively, these findings suggest that
knockdown of MALAT-1 inhibits pancreatic cancer cell migration and
invasion possibly through suppression of EMT.
MALAT-1 knockdown decreases the
expression of cancer stem-like cell markers
Accumulating evidence suggests that cells can
acquire stem-like properties during induction of EMT (11). Pancreatic cancer stem cells (CSCs)
have been identified based on the expression of CD24, CD44, and
epithelial-specific antigen (ESA) (12). The expression of aldehyde
dehydrogenase (ALDH) was also used as special markers of pancreatic
CSCs (13). In order to gain
insight into the role of MALAT-1 involved in regulating pancreatic
CSCs, we performed western blot analysis to analyze the altered
expression of pancreatic CSC-associated markers following MALAT-1
downregulation, and the results showed that the levels of CD44,
CD24 and ALDH were significantly decreased in M-si1 compared with
the M-nc groups (Fig. 5A). The
above data suggest that MALAT-1 facilitates pancreatic cancer cell
progression possibly through acquiring cancer stem-like
properties.
Discussion
LncRNAs are broadly defined as transcribed RNA
molecules with a length greater than 200 nucleotides and lacking an
open reading frame of significant length (less than 100 amino
acids). Studies have begun to provide insight into the critical
roles played by lncRNA in a variety of cellular processes,
including differentiation, development and tumorigenesis (14). The importance of lncRNAs had opened
a new field of focus for cancer research.
MALAT-1, also known as nuclear enriched transcript-2
(NEAT-2), is a widely expressed lncRNA implicated in regulation of
alternative splicing or gene expression. Emerging evidence
indicates that MALAT-1 is overexpressed in many solid tumors, such
as hepatocellular carcinoma (15),
gastric (16) and gallbladder
cancer (17), and it plays a
significant role in the molecular events of neoplasia (4). Considering the observation that
MALAT-1 is implicated in multiple aspects of tumor biological
processes, we investigated the role of MALAT-1 in pancreatic
cancer. In the present study, we first compared the level of
MALAT-1 expression between six cases of fresh tumor tissue samples
and adjacent non-cancerous pancreas tissues, and the results
revealed higher MALAT-1 level in pancreatic cancer tissues.
Furthermore, all seven pancreatic cancer cell lines had relatively
higher level of MALAT-1 as compared to HPDE. A recent study showed
that MALAT-1 expression level correlated with tumor size, tumor
stage and depth of invasion in pancreatic cancer formalin-fixed,
paraffin embedded tissues (18).
The above results indicate that MALAT-1 overexpression may play an
important role in pancreatic cancer.
Next, to specifically determine the contributions of
MALAT-1 in the regulation of pancreatic cancer, we modulated its
expression in AsPC-1 and CFPAC-1 cell lines. The cell growth
results revealed that stably decreased expression of MALAT-1 could
lead to reduced cell proliferation and colony formation.
Furthermore, flow cytometry analyses and western blot results
showed that suppression of MALAT-1 expression could cause
G2/M cell cycle arrest and induce cell apoptosis. These
results suggested an inhibiting effect of MALAT-1 downregulation on
pancreatic cancer cell growth through inducing G2/M cell
cycle arrest and promoting cell apoptosis.
Moreover, wound healing assay, Transwell assays and
Boyden chamber revealed that silencing MALAT-1 could clearly
inhibit pancreatic cancer cell migratory and invasive ability in
vitro. Recent evidence suggests that EMT of pancreatic cancer
cells contributes to the development and increase in invasiveness
and metastasis (9). During EMT,
epithelial cells undergo profound phenotypic changes such as loss
of cell-cell adhesion, loss of cell polarity and acquisition of
migratory and invasive properties. It should be noted that EMT is
characterized by a downregulation of epithelial markers and an
upregulation of mesenchymal markers and EMT-related transcriptional
factors. In addition, relative research demonstrated that MMPs play
a key role in aberrant pancreatic cell growth and tumor formation,
since they provide space for the tumor to grow and release various
growth factors that drive tumor proliferation and progression
(19). In our studies, we found
that suppressing MALAT-1 expression decreased MMP-2, MMP-9 and
EMT-marker genes including Snail, Slug, N-cadherin, and vimentin,
while it upregulated the expression of E-cadherin significantly. In
addition, morphological change was observed in CFPAC-1 after
MALAT-1 downregulation.
Researchers recently demonstrated that the presence
of CSCs in pancreatic tumors contributes to the early metastasis
and chemotherapeutic drug resistance of pancreatic cancer (20). Accumulating evidence suggests that
EMT is important in cancer progression conceivably by inducing stem
cell properties to cancer cells (21,22).
Therefore, we speculated that MALAT-1 induced EMT and acquired
stem-like properties to facilitate tumor invasion and metastasis.
The protein expression of pancreatic CSC markers including CD44,
CD24 and ALDH were compared between M-nc and M-si1 groups. As
expected, the results showed that levels of all these markers
decreased after MALAT-1 knockdown. Taken together, these results
indicated that MALAT-1 facilitated pancreatic cancer progression
probably through inducing EMT and acquiring cancer stem-like
properties. However, the detailed molecular mechanisms of MALAT-1
regulating pancreatic CSCs and tumor progression require further
exploration.
In conclusion, our findings reveal strong expression
of MALAT-1 in patients with pancreatic cancer, and suggest that
MALAT-1 may serve as an oncogenic lncRNA that promotes pancreatic
cancer cell growth and progression. Since MALAT-1 is associated
with the malignancy phenotypes of pancreatic cancer, further study
is required to determine the potential roles of MALAT-1 as a
candidate therapeutic target in the clinic.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81101846, 81171887,
91229117 and 31101016), Program of Shanghai Subject Chief Scientist
(grant no. 12XD1404200), Shanghai International Science and
Technology Cooperation Project (grant no. 12410709000) and Shanghai
Science and Technology Committee (grant no. 11DZ1922002).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
He Y, Meng XM, Huang C, Wu BM, Zhang L, Lv
XW and Li J: Long noncoding RNAs: novel insights into hepatocelluar
carcinoma. Cancer Lett. 344:20–27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ji P, Diederichs S, Wang W, Böing S,
Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E,
Thomas M, Berdel WE, Serve H and Müller-Tidow C: MALAT-1, a novel
noncoding RNA, and thymosin beta4 predict metastasis and survival
in early-stage non-small cell lung cancer. Oncogene. 22:8031–8041.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gutschner T, Hämmerle M and Diederichs S:
MALAT1 - a paradigm for long noncoding RNA function in cancer. J
Mol Med (Berl). 91:791–801. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thon JN, Devine MT, Jurak Begonja A,
Tibbitts J and Italiano JE Jr: High-content live-cell imaging assay
used to establish mechanism of trastuzumab emtansine
(T-DM1)-mediated inhibition of platelet production. Blood.
120:1975–1984. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li W, Xie L, Chen Z, Zhu Y, Sun Y, Miao Y,
Xu Z and Han X: Cantharidin, a potent and selective PP2A inhibitor,
induces an oxidative stress-independent growth inhibition of
pancreatic cancer cells through G2/M cell-cycle arrest
and apoptosis. Cancer Sci. 101:1226–1233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stark GR and Taylor WR: Control of the
G2/M transition. Mol Biotechnol. 32:227–248. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ayyagari VN and Brard L: Bithionol
inhibits ovarian cancer cell growth in vitro - studies on
mechanism(s) of action. BMC Cancer. 14:612014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pan JJ and Yang MH: The role of
epithelial-mesenchymal transition in pancreatic cancer. J
Gastrointest Oncol. 2:151–156. 2011.PubMed/NCBI
|
|
10
|
Min C, Eddy SF, Sherr DH and Sonenshein
GE: NF-kappaB and epithelial to mesenchymal transition of cancer. J
Cell Biochem. 104:733–744. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell
LL, Polyak K, Brisken C, Yang J and Weinberg RA: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee CJ, Dosch J and Simeone DM: Pancreatic
cancer stem cells. J Clin Oncol. 26:2806–2812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rasheed ZA, Yang J, Wang Q, Kowalski J,
Freed I, Murter C, Hong SM, Koorstra JB, Rajeshkumar NV, He X,
Goggins M, Iacobuzio-Donahue C, Berman DM, Laheru D, Jimeno A,
Hidalgo M, Maitra A and Matsui W: Prognostic significance of
tumorigenic cells with mesenchymal features in pancreatic
adenocarcinoma. J Natl Cancer Inst. 102:340–351. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maruyama R and Suzuki H: Long noncoding
RNA involvement in cancer. BMB Rep. 45:604–611. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lai MC, Yang Z, Zhou L, Zhu QQ, Xie HY,
Zhang F, Wu LM, Chen LM and Zheng SS: Long non-coding RNA MALAT-1
overexpression predicts tumor recurrence of hepatocellular
carcinoma after liver transplantation. Med Oncol. 29:1810–1816.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Su L, Chen X, Li P, Cai Q, Yu B,
Liu B, Wu W and Zhu Z: MALAT1 promotes cell proliferation in
gastric cancer by recruiting SF2/ASF. Biomed Pharmacother.
68:557–564. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu XS, Wang XA, Wu WG, Hu YP, Li ML, Ding
Q, Weng H, Shu YJ, Liu TY, Jiang L, Cao Y, Bao RF, Mu JS, Tan ZJ,
Tao F and Liu YB: MALAT1 promotes the proliferation and metastasis
of gallbladder cancer cells by activating the ERK/MAPK pathway.
Cancer Biol Ther. 15:806–814. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu JH, Chen G, Dang YW, Li CJ and Luo DZ:
Expression and prognostic significance of lncRNA MALAT1 in
pancreatic cancer tissues. Asian Pac J Cancer Prev. 15:2971–2977.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Łukaszewicz M, Mroczko B and Szmitkowski
M: The role of metalloproteinases and their inhibitors in
pancreatic cancer. Postepy Hig Med Dosw (Online). 62:141–147.
2008.(In Polish).
|
|
20
|
Li Y, Kong D, Ahmad A, Bao B and Sarkar
FH: Pancreatic cancer stem cells: emerging target for designing
novel therapy. Cancer Lett. 338:94–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sarkar FH, Li Y, Wang Z and Kong D:
Pancreatic cancer stem cells and EMT in drug resistance and
metastasis. Minerva Chir. 64:489–500. 2009.PubMed/NCBI
|
|
22
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|