Introduction
The disease frequency of neuroendocrine tumors has
been growing throughout the world. Pancreatic neuroendocrine tumors
(PNETs) are one type of neuroendocrine tumors, and their frequency
has also been increasing (1). PNETs
account for ~2% of all pancreatic neoplasms (1). Although some variants of PNETs exist
and often induce hormone hypersecretion and symptoms, PNETs are
characteristically slow-growing tumors compared with pancreatic
carcinoma. Owing to the multimodal treatment strategy for PNETs,
including surgical resection, transcatheter arterial
chemoembolization (TACE) and molecular targeting inhibitors, the
estimated 5- and 10-year overall survival (OS) after surgical
resection have been comparatively favorable, reported as ~65 and
45%, respectively (2).
However, hepatic recurrence is a factor that affects
patient prognosis (3,4). Bloomston et al reported that
the liver is the most common site of recurrence, with liver
recurrence occurring in 16.7% of patients following resection of
the primary site of PNETs (5). It
is important to predict asynchronous hepatic metastasis or
recurrence based on clinicopathological characteristics to allow
earlier treatment with adjuvant therapy after primary site
resection.
Some investigators have analyzed tumor-infiltrating
lymphocytes (TILs) in many types of tumor (such as colorectal
cancer and hepatocellular carcinoma), and have suggested a
correlation between TILs and disease-free survival (DFS) and/or OS
(6,7). Therefore, analyzing the local immune
response at the primary site of PNETs may predict the risk for
hepatic recurrence following curative R0 resection. Jiao et
al investigated exome sequencing in sporadic PNETs for other
biomarkers, and identified the presence of somatic mutations in the
following genes: multiple endocrine neoplasia-1 (MEN1),
death domain-associated protein (DAXX), α thalassemia/mental
retardation X-linked (ATRX) and phosphatase and tensin
homolog (PTEN). Moreover, patients who had somatic mutations
in MEN1 and/or DAXX/ATRX had better prognosis than
patients who had wild-type alleles (8). Mammalian target of rapamycin (mTOR) is
another key molecule for predicting patient prognosis. Some
investigators have analyzed the expression of mTOR in PNETs. mTOR
is involved with the highly conserved 3-kinase-related protein
kinase and plays a key role in cellular growth, survival,
metabolism and development (9,10);
mTOR is activated by phosphorylation. Everolimus, which is one of
the molecular targeted inhibitors of mTOR for anti-PNET therapy,
improved progression-free survival in patients with advanced PNETs
as compared with a placebo (11).
The aim of the present study was to investigate
tumor biomarkers at the primary disease site that could be used to
predict postoperative hepatic recurrence (PHR) of PNETs. Our
investigation used immunohistochemical analysis for TILs (CD3, CD8
and CD45RO), human leukocyte antigen (HLA) class I, ATRX, DAXX,
mTOR and phospho-mTOR (p-mTOR). The other aim was to analyze the
correlations between the clinicopathological features and PNET
patient prognosis.
Materials and methods
Patients
We retrospectively reviewed 24 patients with PNETs
who underwent radical surgery at the Department of
Gastroenterological Surgery II, Hokkaido University Hospital
between 2003 and 2010. We excluded patients who had the following
conditions: presence of distant metastasis upon preoperative
examination, such as computer tomography (CT), ultrasonography
(US), and magnetic resonance imaging (MRI); previous treatment
(surgery, chemotherapy or radiation therapy) for PNETs; and/or 9
month or less follow-up period. The reason for this period was that
the median period of appearance of PHR was 10 months after surgery
at our department. After surgical treatment, the patients underwent
follow-up examinations to detect hepatic recurrence including CT
and/or US every 6 months.
Clinical tumor typing was determined according to
the 2010 World Health Organization (WHO) classification (12). All specimens were fixed in 10%
formalin and embedded in paraffin wax. The thickest part of each
tumor was selected for evaluation. Serial 4-μm-thick sections were
obtained and examined by immunohistochemistry. Informed patient
consent for immunohistochemical staining was obtained in accordance
with the guidelines of the Hokkaido University Institutional Review
Board authorization for the present study.
Antibodies
The mouse monoclonal primary antibody used was
EMR8-5, which recognizes the heavy chains of HLA-A, HLA-B and HLA-C
(clone EMR8-5; Hokudo, Sapporo, Japan) at a 1:1,000 dilution in
phosphate-buffered saline (PBS); human CD3-specific monoclonal
antibody (mAb) and human CD8-specific mAb (Histofine®
CD3 mouse IgG1 mAb and CD8, mouse IgG1j mAb; Nichirei, Tokyo,
Japan) at non-dilution; and anti-CD45RO antibody (UCH-L1; Abcam,
Cambridge, UK) at a 1:2,000 dilution were used.
The rabbit monoclonal primary antibody used was mTOR
rabbit mAb (#2983) at a 1:50 dilution, and phospho-mTOR rabbit mAb
(#2475) (both from Cell Signaling Technology, Boston, MA, USA) at a
1:100 dilution.
The rabbit polyclonal antibody used was anti-ATRX
(HPA001906) at a 1:200 dilution in PBS; and anti-DAXX (HPA008736)
(both from Atlas Antibodies AB, Stockholm, Sweden) at a 1:200
dilution in PBS.
Immunohistochemical staining
Immunohistochemical reactions were carried out using
the streptavidin-biotin-peroxidase method. As positive controls,
normal adenoid tissue was used for CD3, CD8 and CD45RO. Normal
mucosal tissues were used for HLA class I heavy chains as internal
positive controls. Lung cancers were used for mTOR and p-mTOR,
normal pancreas specimens for ATRX, and urinal bladder specimens
for DAXX as positive controls. Sections were deparaffinized in
xylene, washed in PBS (pH 7.4), and rehydrated in a graded series
of ethanol solutions. Antigen retrieval was performed by heating
citrate buffer (pH 6.0) at 120°C for 15 min. Endogenous peroxidase
activity was blocked by 10-min incubation with 3% hydrogen peroxide
in methanol. After washing in PBS, the specimens were saturated
with 10% normal goat serum (Histofine® SAB-PO kit;
Nichirei, Tokyo, Japan) for 5 min and stained with the primary
antibody at 4°C overnight. After washing in PBS, a biotinylated
goat anti-mouse immunoglobulin antibody (Histofine®
SAB-PO kit) was applied for 30 min at room temperature.
Immunohistochemical reactions were visualized with freshly prepared
3,3′-diaminobenzidine tetrahydrochloride (Histofine®
SAB-PO kit). Slides were counterstained with hematoxylin and
mounted on coverslips. All the specimens were evaluated by two
investigators blinded to the patient clinical information under the
instruction of a pathologist.
Scoring of HLA class I expression
status
HLA class I heavy chain expression in the tumor
cells was scored using a scale of negative, heterogeneous or
positive, when the percentage of stained tumor cells was <25,
25–75 or >75%, respectively.
Scoring of CD3+ T cells and
CD8+ T cells, and CD45RO+ infiltration
Immunohistochemistry testing and the evaluation of
immune cells were performed according to a previous report
(13). Briefly, the degree of
immune cell infiltration was analyzed in >10 independent
high-power (x200) microscopic fields for each tissue sample. The
numbers of CD3+ T cells, CD8+ T cells and
CD45RO+ cells were counted in the tumor cell nests.
Scoring of ATRX, DAXX, mTOR and
p-mTOR
For both ATRX and DAXX scoring, nuclear protein
staining was only considered positive in at least 5% of tumor cells
with nuclear labeling, as in a past report (14). Tumor cells were scored as negative
if the pattern was that of cytoplasmic accumulation with nuclear
clearing, as long as adequate internal controls (for example,
nuclear labeling of adjacent endothelial cells, lymphocytes and/or
islets of Langerhans) were present (14). For mTOR and p-mTOR scoring, the
tissues with >10% cytoplasmic- and/or membranous-stained tumor
cells were considered positive and the others were negative.
Statistical analysis
All statistical analyses were performed using
StatFlex 6.0 software (Artech Company, Osaka, Japan). The
Mann-Whitney U test, the Chi-square and Fisher’s exact probably
tests were applied for comparisons. The durations of DFS and OS
were calculated from the date of diagnosis until recurrence or
death or until the date of the last follow-up visit for patients
still alive. Survival was estimated according to the Kaplan-Meier
product limit method. Survival curves were compared using the
log-rank test. Results were considered statistically significant
when P<0.05 was obtained.
Results
Background characteristics
The 16 of the 24 patients who underwent radical
surgery were included in the present study. (Among the patients
excluded, 5 had distant metastasis, 1 had received previous
treatment and 2 had been followed up for only two months,
postoperatively). The characteristics of the 16 study patients are
summarized in Table I. Of the 16
patients who were reviewed, 5 patients had PHR during the follow-up
period (PHR-positive), and 11 did not (PHR-negative). There were no
statistically significant differences in terms of the preoperative
background characteristics between the PHR-positive and the
PHR-negative group. The median follow-up period was 39 months
ranging 11–86 months in the PHR-positive group, and 54 months
ranging 17–96 months in the PHR-negative group (P=0.50). There was
a statistically significant difference between groups in terms of
death (P=0.017). Only the PHR-positive group included deaths; one
patient died of interstitial pneumonia and two died of PNETs. There
was no significant difference between the two groups in terms of
the grade of WHO classification (PHR-positive vs. -negative;
P=0.105).
 | Table IBackground characteristics of the 16
study patients. |
Table I
Background characteristics of the 16
study patients.
| Postoperative hepatic
recurrence | |
|---|
|
| |
|---|
| Clinical feature | Positive
n=5 | Negative
n=11 | P-value |
|---|
| Gender |
| Male/female | 2/3 | 4/7 | 1.00a |
| Age, years
(range) | 61 (35–75) | 61 (31–72) | 0.82b |
| Tumor size (mm,
range) | 35 (3.5–70) | 13 (7–40) | 0.23b |
| Hormonal
function | | | 0.77a |
| Insulinoma | 0 | 2 | |
| Glucagonoma | 0 | 1 | |
| PPoma | 0 | 1 | |
| Nonfunctional
tumor | 5 | 7 | |
| WHO grade 1/2,3 | 1/4 | 8/3 | 0.105a |
| Surgery | | | 1.00a |
| PD | 2 | 3 | |
| DP | 3 | 5 | |
| DPPHR | 0 | 2 | |
| Other | 0 | 1 | |
| Follow-up period
(months, range) | 39 (11–86) | 54 (17–96) | 0.50b |
| Death | | | 0.017a |
| Yes | 3 | 0 | |
| No | 2 | 11 | |
Immunohistochemistry for immune-related
cells
CD4+ T cells, CD8+ T cells and
CD45RO+ T cells were detected within cancer cell nests.
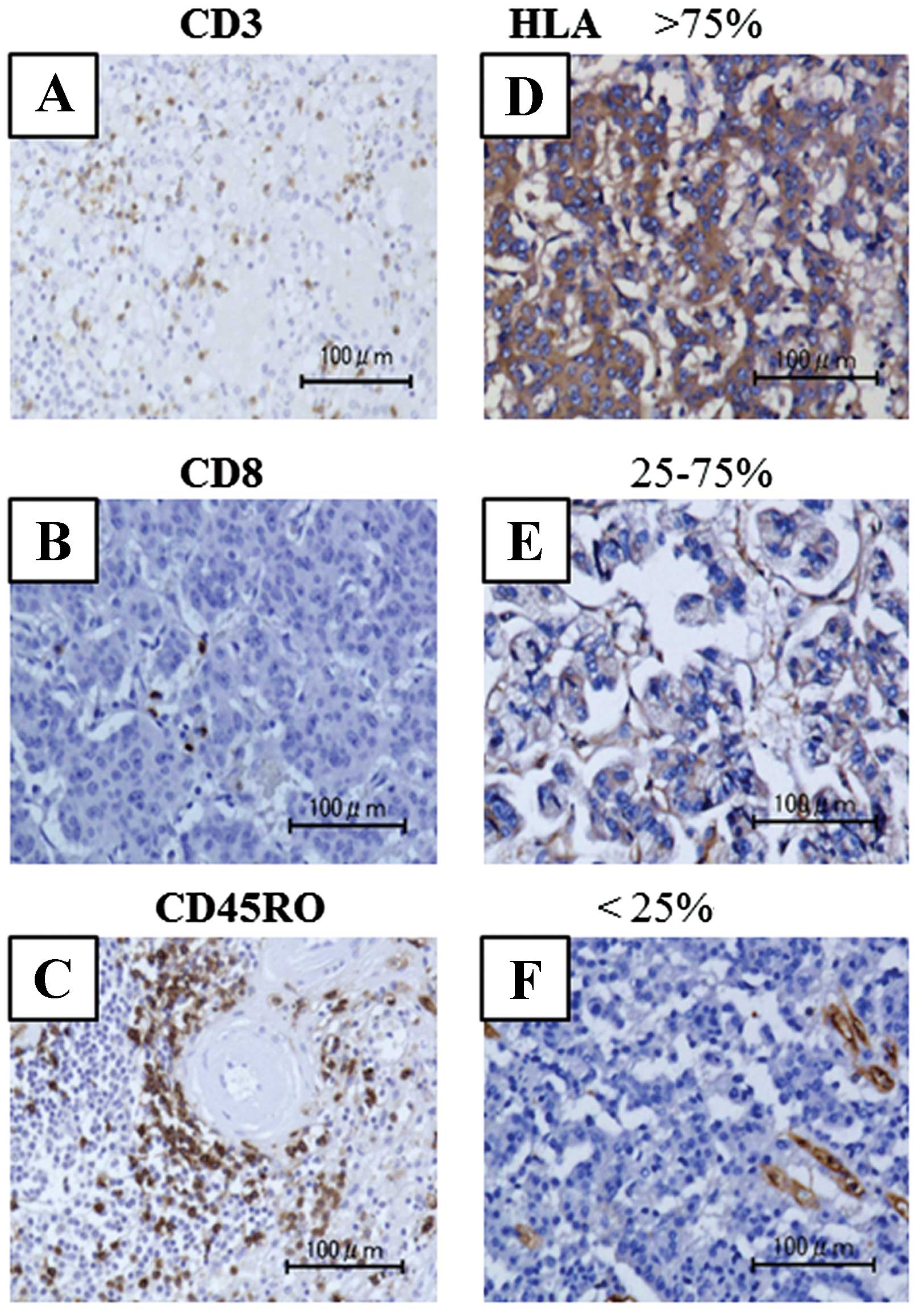
Representative images of immunohistochemical staining of CD4, CD8
and CD45RO are shown in Fig. 1A–C.
The HLA class I expression pattern was classified into three
groups; <25, 25–75 and >75% (Fig.
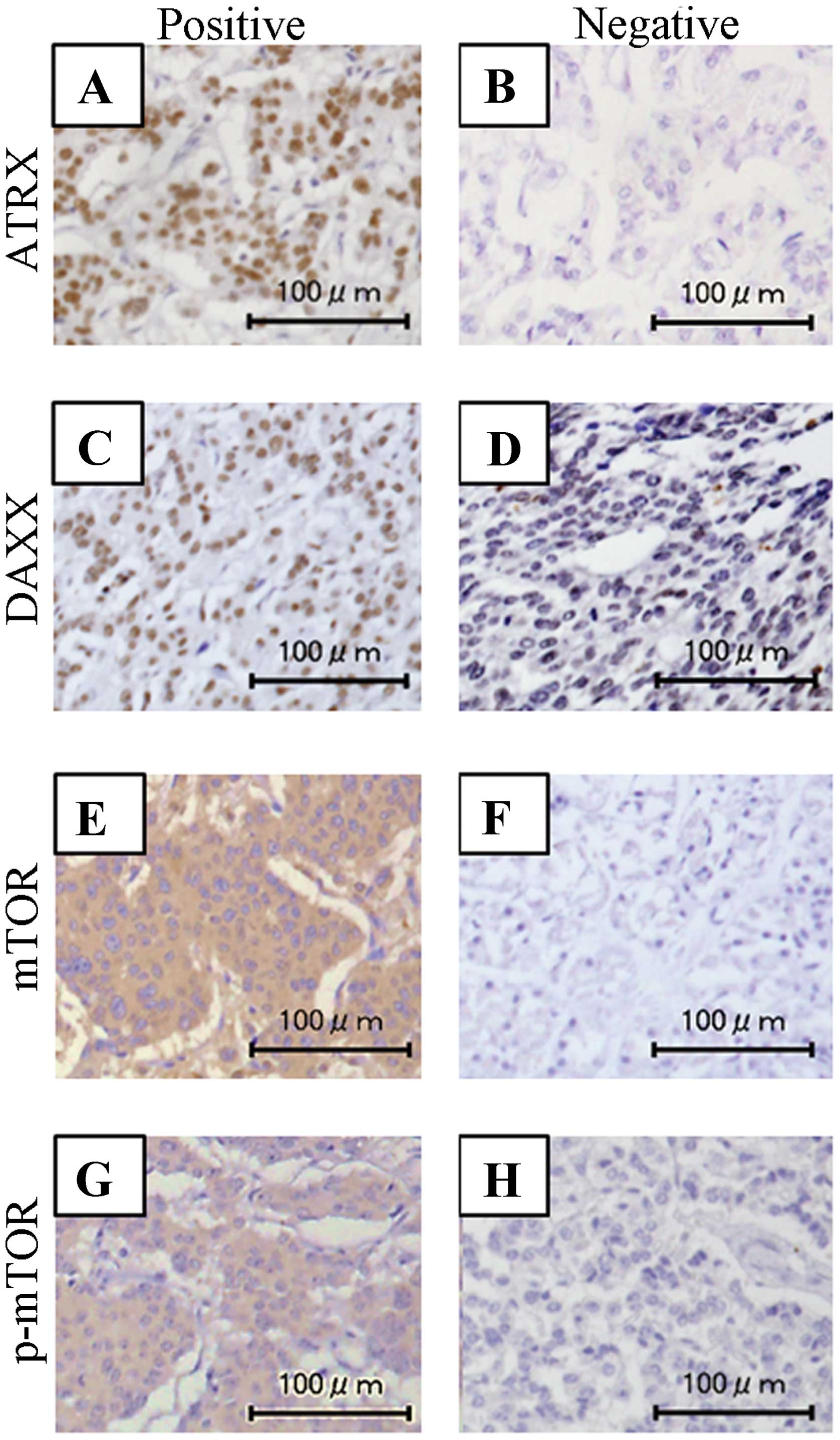
1D–F). Expression patterns of ATRX, DAXX, mTOR and p-mTOR are
shown in Fig. 2.
Degree of immune cell infiltration in the
tumors and correlation with PHR
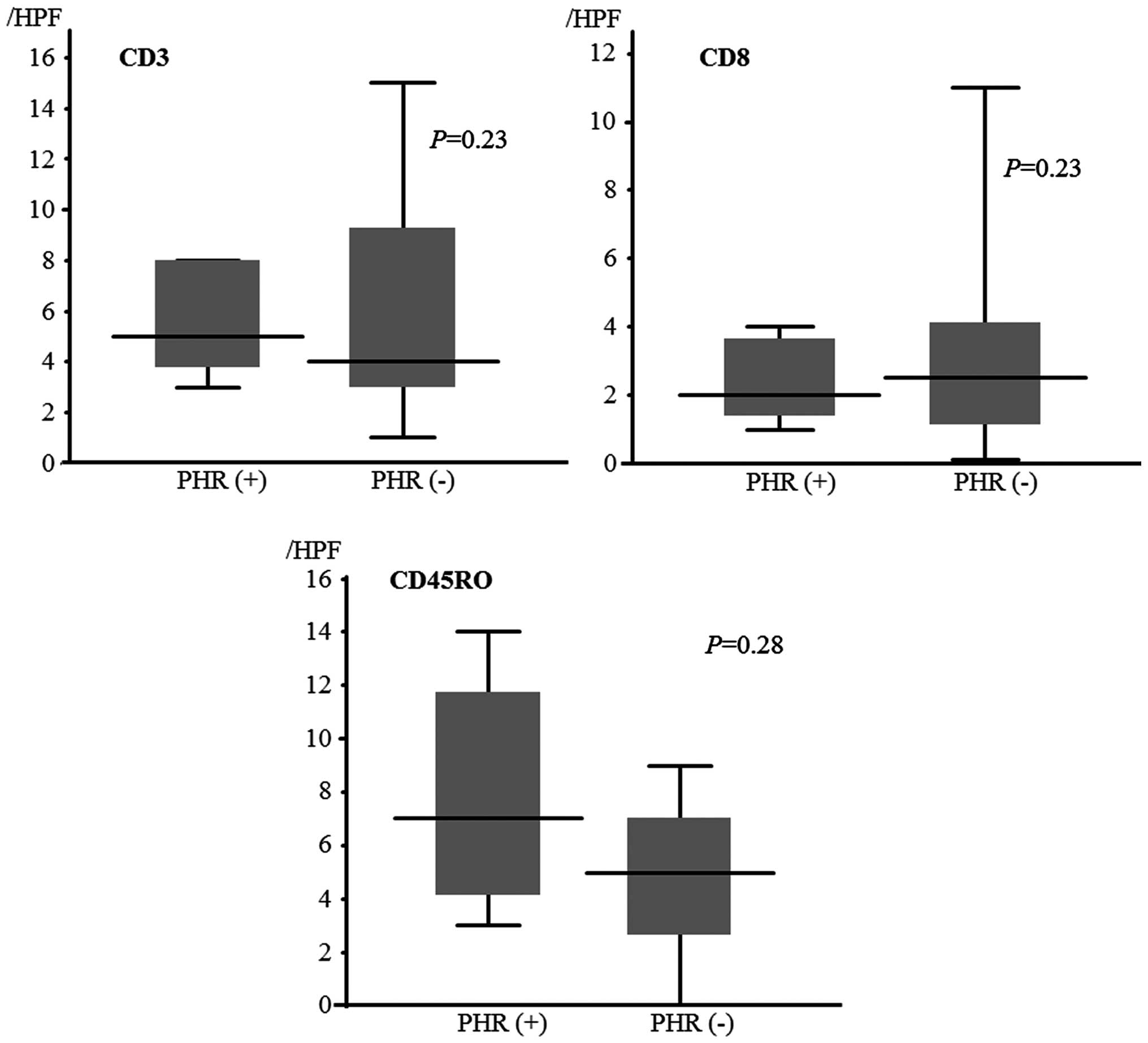
We analyzed the infiltration status of CD4, CD8 and
CD45RO (Fig. 3) between the
PHR-positive and -negative group, and there were no statistically
significant differences (P=0.23, 0.23 and 0.28, respectively). In
the HLA class I expression pattern, there was no correlation with
TILs (data not shown).
Expression of biomarkers and correlation
with PHR
Among all PNETs, the positive rates for ATRX, DAXX,
mTOR and p-mTOR were 75.0% (12/16), 52.5% (10/16), 52.5% (10/16)
and 37.5% (6/16), respectively. The correlations were analyzed
between the expression levels of the biomarkers (ATRX, DAXX, mTOR
and p-mTOR) and PHR (Table II).
There were correlations between DAXX-negative and p-mTOR-positive
statuses and PHR-positive patients (P=0.036 and P=0.036,
respectively).
 | Table IIComparison of ATRX, DAXX, m-TOR,
p-mTOR and HLA class I expression patterns between the PHR-positive
and PHR-negative group. |
Table II
Comparison of ATRX, DAXX, m-TOR,
p-mTOR and HLA class I expression patterns between the PHR-positive
and PHR-negative group.
| Postoperative hepatic
recurrence | |
|---|
|
| |
|---|
| Expression
patterns | Positive
n=5 | Negative
n=11 | P-value |
|---|
| ATRX nuclear
labeling | | | 0.063a |
| Positive | 2 | 10 | |
| Negative | 3 | 1 | |
| DAXX nuclear
labeling | | | 0.036a |
| Positive | 1 | 9 | |
| Negative | 4 | 2 | |
| mTOR
expression | | | 0.59a |
| Positive | 4 | 6 | |
| Negative | 1 | 5 | |
| p-mTOR
expression | | | 0.036a |
| Positive | 4 | 2 | |
| Negative | 1 | 9 | |
| HLA class I
expression pattern (%) | | | 0.097b |
| >75 | 1 | 3 | |
| 25–75 | 2 | 4 | |
| <25 | 2 | 4 | |
Correlations between TILs and expression
levels of the biomarkers, and prognosis
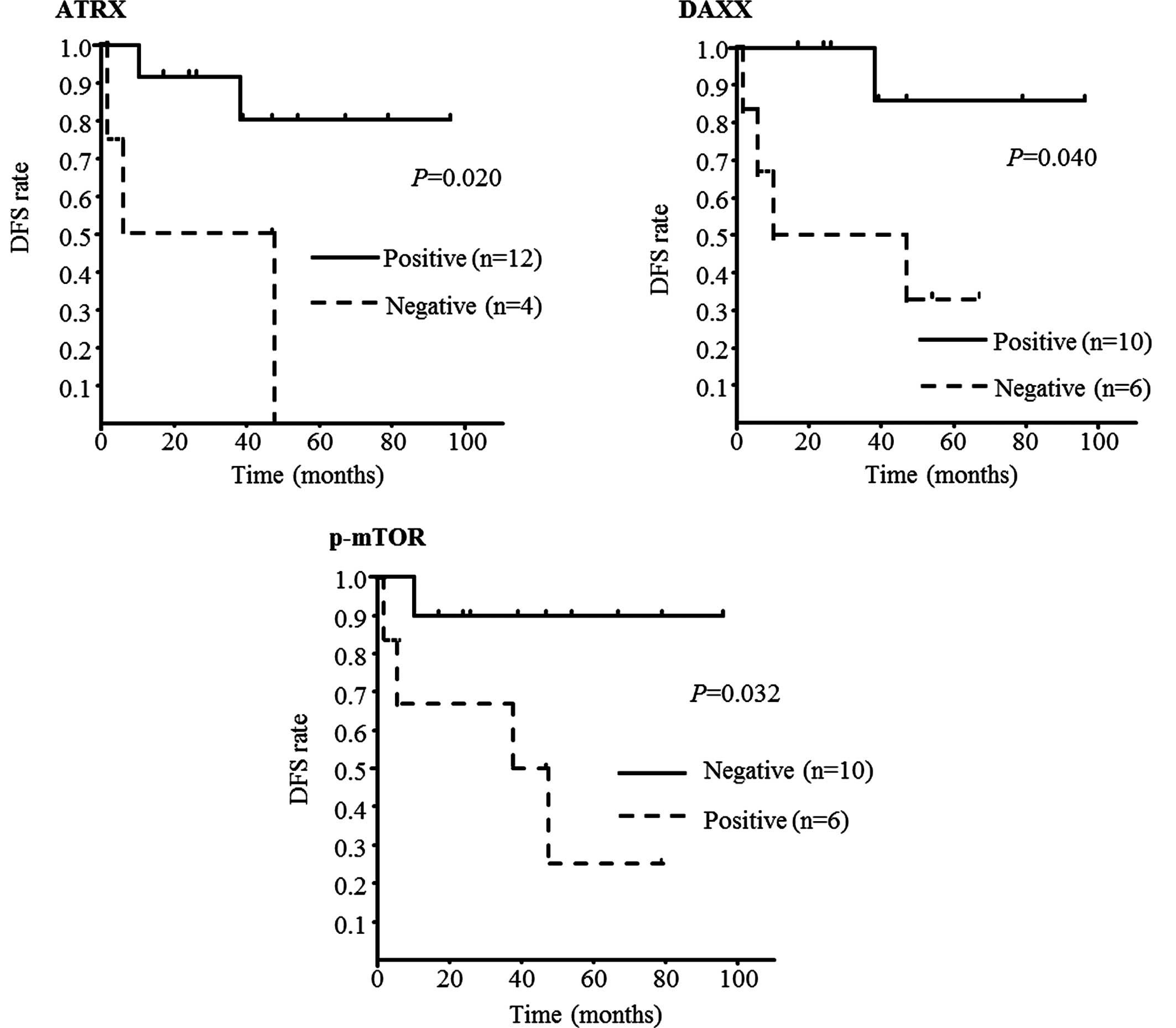
In DFS, there was no correlation with TILs (data not
shown). However, there were correlations with three biomarkers
(ATRX, DAXX and p-mTOR; P=0.020, 0.040 and 0.032, respectively);
patients with ATRX-negative, DAXX-negative, or p-mTOR-positive
PNETs experienced worse prognosis (Fig.
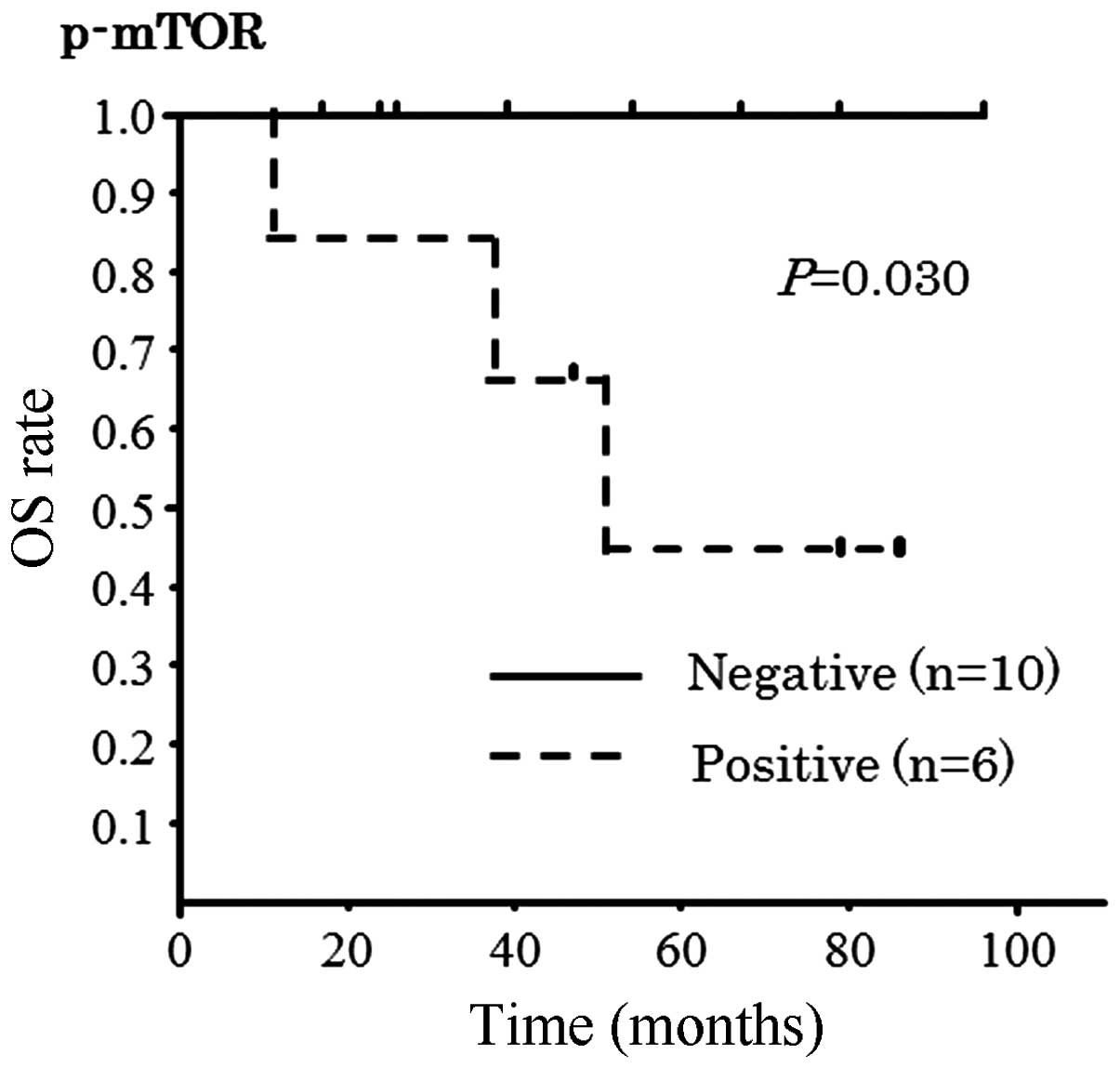
4). In OS, there was a correlation with p-mTOR only (P=0.029,
Fig. 5).
Discussion
Several studies have been conducted to investigate
the prognosis of patients suffering from PNETs. These studies have
demonstrated useful clinical indicators such as tumor size, WHO
grade, TNM stage and lymph node metastasis (15,16).
Recently, clinicopathological and molecular biomarkers such as
TILs, PTEN and p-mTOR have been found useful for estimating the
prognosis of patients with PNETs (17,18).
However, the primary disease sites of these studies were not unique
with respect to the various origins of the neuroendocrine tumors
with distant metastasis. In the present study, only patients
undergoing surgical resection without residual tumor were included
in the investigation of correlations between TILs or other
biomarkers and occurrence of PHR.
The present study found no correlation between the
number of TILs at the primary disease site and PHR, DFS or OS. Katz
et al reported that the degree of infiltration by
FoxP3-positive TILs could predict OS following treatment of the
neuroendocrine tumor liver metastasis nest, while CD3+,
CD4+ and CD8+ TILs could not (17). Yet, the primary sites of the
metastasis were not only the pancreas but also the small bowel and
other in the present study. In pancreatic ductal cancer,
tumor-infiltrating CD4+, CD8+, regulatory T
cells (Treg) and tumor-infiltrating macrophages (type 1 and 2) have
been reported as independent prognosticators (19). The HLA expression pattern of PNETs
in the present study was lower compared with that of pancreatic
cancer; this was reported in our previous study (20). Given the lower expression pattern of
HLA class I in PNETs compared with pancreatic carcinoma, it may be
speculated that the extent of TILs in PNETs is not sufficiently
adequate to serve as an independent prognosticator.
In terms of molecular biomarkers such as ATRX, DAXX,
mTOR and p-mTOR, there were statistically significant correlations
between DAXX, p-mTOR and PHR in the present study. In addition,
lower ATRX expression, lower DAXX expression and higher p-mTOR
expression were found to be significant prognostic factors for DFS.
de Wilde et al suggested that loss of nuclear expression of
ATRX and/or DAXX, occurring in MEN1 neuroendocrine tumors, is
correlated with the existence of the alternative lengthening of
telomeres (ALT) phenotype. The present study also showed that loss
of nuclear expression of ATRX and/or DAXX and the presence of the
ALT phenotype occurred only in larger (≥3 cm) PNETs, and these
changes are late events in PNETs (14). Marinoni et al showed that
loss of DAXX or ARTX protein and ALT are associated with
chromosomal instability in PNETs. In addition, loss of DAXX or ARTX
was found to be correlated with tumor stage and metastasis, DFS and
OS (21). In terms of p-mTOR, Han
et al suggested that high expression of p-mTOR and low
expression of the PTEN group portended worse prognosis than low
expression of p-mTOR and high expression of PTEN, but did not
estimate worse prognosis in high expression of p-mTOR only
(18). In the present study, we
found that the expression patterns of ATRX, DAXX and p-mTOR were
correlated with DFS, while only p-mTOR was found to correlate with
OS.
In other malignancies, earlier introduction of
postoperative adjuvant therapy has been proven to improve patient
prognosis based on such clinical risk factors as node metastasis,
human epidermal growth factor receptor 2 (HER2) status, and
epidermal growth factor receptor (EGFR) expression level (22,23).
Collectively, this evidence, along with the data from the present
study, supports a strategy to introduce cytostatic drugs such as
mTOR, sunitinib or ocreotide immediately following curative
resection of the primary tumor site, based on the risk factors for
disease recurrence identified by the expression patterns of the
various molecular markers investigated in the present study.
The present investigation was limited by its
retrospective study design and the small number of study patients.
Further exploratory studies concerning the prediction of the
recurrence of PNETs are warranted.
In conclusion, ATRX, DAXX and p-mTOR are useful
molecular biomarkers for predicting PHR in patients who undergo
radical surgery for PNETs. Use of these biomarkers will enable
earlier decisions on which patients may benefit from adjuvant
therapy.
Acknowledgements
The authors thank Hiraku Shida for his technical
assistance in the immunohistochemical analysis.
References
|
1
|
Metz DC and Jensen RT: Gastrointestinal
neuroendocrine tumors: pancreatic endocrine tumors.
Gastroenterology. 135:1469–1492. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Wilde RF, Edil BH, Hruban RH and Maitra
A: Well-differentiated pancreatic neuroendocrine tumors: from
genetics to therapy. Nat Rev Gastroenterol Hepatol. 9:199–208.
2012.PubMed/NCBI
|
|
3
|
Panzuto F, Nasoni S, Falconi M, et al:
Prognostic factors and survival in endocrine tumor patients:
comparison between gastrointestinal and pancreatic localization.
Endocr Relat Cancer. 12:1083–1092. 2005. View Article : Google Scholar
|
|
4
|
Yao JC, Hassan M, Phan A, et al: One
hundred years after ‘carcinoid’: epidemiology of and prognostic
factors for neuroendocrine tumors in 35,825 cases in the United
States. J Clin Oncol. 26:3063–3072. 2008.
|
|
5
|
Bloomston M, Muscarella P, Shah MH, et al:
Cytoreduction results in high perioperative mortality and decreased
survival in patients undergoing pancreatectomy for neuroendocrine
tumors of the pancreas. J Gastrointest Surg. 10:1361–1370. 2006.
View Article : Google Scholar
|
|
6
|
Kobayashi N, Hiraoka N, Yamagami W, et al:
FOXP3+ regulatory T cells affect the development and
progression of hepatocarcinogenesis. Clin Cancer Res. 13:902–911.
2007.
|
|
7
|
Galon J, Costes A, Sanchez-Cabo F, et al:
Type, density, and location of immune cells within human colorectal
tumors predict clinical outcome. Science. 313:1960–1964. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiao Y, Shi C, Edil BH, et al:
DAXX/ATRX, MEN1, and mTOR pathway genes are
frequently altered in pancreatic neuroendocrine tumors. Science.
331:1199–1203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nave BT, Ouwens M, Withers DJ, Alessi DR
and Shepherd PR: Mammalian target of rapamycin is a direct target
for protein kinase B: identification of a convergence point for
opposing effects of insulin and amino-acid deficiency on protein
translation. Biochem J. 344:427–431. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wullschleger S, Loewith R and Hall MN: TOR
signaling in growth and metabolism. Cell. 124:471–484. 2006.
View Article : Google Scholar
|
|
11
|
Yao JC, Shah MH, Ito T, et al: Everolimus
for advanced pancreatic neuroendocrine tumors. N Engl J Med.
364:514–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Crippa S, Partelli S, Boninsegna L and
Falconi M: Implications of the new histological classification (WHO
2010) for pancreatic neuroendocrine neoplasms. Ann Oncol.
23:19282012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cho Y, Miyamoto M, Kato K, et al:
CD4+ and CD8+ T cells cooperate to improve
prognosis of patients with esophageal squamous cell carcinoma.
Cancer Res. 63:1555–1559. 2003.
|
|
14
|
de Wilde RF, Heaphy CM, Maitra A, et al:
Loss of ATRX or DAXX expression and concomitant acquisition of the
alternative lengthening of telomeres phenotype are late events in a
small subset of MEN-1 syndrome pancreatic neuroendocrine tumors.
Mod Pathol. 25:1033–1039. 2012.
|
|
15
|
La Rosa S, Klersy C, Uccella S, et al:
Improved histologic and clinicopathologic criteria for prognostic
evaluation of pancreatic endocrine tumors. Hum Pathol. 40:30–40.
2009.PubMed/NCBI
|
|
16
|
Scarpa A, Mantovani W, Capelli P, et al:
Pancreatic endocrine tumors: improved TNM staging and
histopathological grading permit a clinically efficient prognostic
stratification of patients. Mod Pathol. 23:824–833. 2010.
View Article : Google Scholar
|
|
17
|
Katz SC, Donkor C, Glasgow K, et al: T
cell infiltrate and outcome following resection of
intermediate-grade primary neuroendocrine tumours and liver
metastases. HPB. 12:674–683. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han X, Ji Y, Zhao J, Xu X and Lou W:
Expression of PTEN and mTOR in pancreatic neuroendocrine tumors.
Tumour Biol. 34:2871–2879. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ino Y, Yamazaki-Itoh R, Shimada K, et al:
Immune cell infiltration as an indicator of the immune
microenvironment of pancreatic cancer. Br J Cancer. 108:914–923.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsuchikawa T, Hirano S, Tanaka E, et al:
Novel aspects of preoperative chemoradiation therapy improving
anti-tumor immunity in pancreatic cancer. Cancer Sci. 104:531–535.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marinoni I, Kurrer AS, Vassella E, et al:
Loss of DAXX and ATRX are associated with chromosome instability
and reduced survival of patients with pancreatic neuroendocrine
tumors. Gastroenterology. 146:453–460.e5. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Inada S, Koto T, Futami K, Arima S and
Iwashita A: Evaluation of malignancy and the prognosis of
esophageal cancer based on an immunohistochemical study (p53,
E-cadherin, epidermal growth factor receptor). Surg Today.
29:493–503. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Garcia I, Vizoso F, Martin A, et al:
Clinical significance of the epidermal growth factor receptor and
HER2 receptor in resectable gastric cancer. Ann Surg Oncol.
10:234–241. 2003. View Article : Google Scholar : PubMed/NCBI
|