Introduction
Gallbladder cancer (GBC) is the most common cancer
of the bile duct system and is the fifth most lethal cancer of the
digestive system (1). The incidence
of GBC in China, Thailand, Chile and Northern India is higher when
compared with the United States and European countries (2). At present, radical surgical resection
is the most effective treatment of GBC. However, for the majority
of patients, surgery is not curative because of late detection
and/or early, regional or distant metastasis. Additionally, few
patients experience complete responses to chemotherapy, mainly due
to chemoresistance. Thus, identifying novel approaches to enhance
the antitumor effects of chemotherapeutic drugs and reduce
chemoresistance are imperative.
Maslinic acid (MA), a pentacyclic triterpene acid,
is widely present in dietary plants, especially in olive fruit
skins and hawthorn berries (3). The
compound has attracted much interest due to its proven
pharmacological safety and its many biological activities,
including anticancer such as anti-inflammation (2), anti-viral (4,5),
anti-oxidation (6),
anti-diabetogenic (7), anti-colonic
cancer (8,9) and anti-astrocytoma (10) activities. MA has been shown to
potentiate the anticancer activity of TNF-α in pancreatic cancer
cells through the inhibition of nuclear factor-κB (NF-κB) survival
signaling pathways (11).
NF-κB is a transcriptional activator that has been
extensively studied for its role in controlling the expression of
genes involved in immune and inflammatory processes. The classical
form of NF-κB is a ubiquitous heterodimeric complex composed
predominantly of IKKα/β and p65 subunits (12). In non-stimulated cells, NF-κB exists
in an inactive form in the cytoplasm bound to an inhibitor, IκBα.
Aberrant or constitutive activation of NF-κB has been shown to
stimulate cell growth and inhibit apoptosis in many human
malignancies (13). The
constitutive activation of NF-κB widely exists in many tumor types
and may play a role in oncogenesis by stimulating cell growth,
inhibiting apoptosis and promoting invasion (14,15).
Evidence suggests that NF-κB may also be involved in tumor cell
resistance to cancer chemotherapy and radiation (16). NF-κB can be activated in response to
treatment with anticancer drugs through a variety of mechanisms.
For example, in HeLa cells, the topoisomerase I inhibitor SN38 and
the topoisomerase II inhibitor doxorubicin induce NF-κB nuclear
translocation and activation of NF-κB target genes directly through
mobilization and stimulation of the IKK complex, leading to
increased cell survival (17). The
activation of NF-κB also leads to the transcription of genes that
regulate cell growth, apoptosis, and invasion. Cyclin D1, Bcl-2,
Bax, MMP-2 and MMP-9 are among the genes that are transcriptionally
regulated by NF-κB (18).
Gemcitabine (GEM) is one of the few chemotherapeutic
drugs used in the treatment of advanced and metastatic bile duct
cancer and GBC (19,20). However, GEM has been shown to induce
chemoresistance in many types of cancer, including pancreatic
(21), lung (22), ovarian (23), bladder (24) and biliary cancer (25).
It has been reported that MA significantly
potentiates the antitumor activities of antitumor agents (11,26).
In this study, we investigated the effects of MA alone and in
combination with GEM on human gallbladder carcinoma in vitro
and in vivo, and its underlying mechanisms.
Materials and methods
Cell culture and reagents
GBC cell lines were reserved by the Eastern
Hepatobiliary Surgery Institute. The gallbladder EH-GB1 (27), EH-GB2 (28), and GBC-SD human cancer cell lines
were maintained in DMEM (Invitrogen, Carlsbad, CA, USA). The cells
were regularly checked for mycoplasma by the PlasmoTest mycoplasma
detection kit (InvivoGen, San Diego, CA, USA) and found to be
negative. MA (>98%) was purchased from Cayman (Ann Arbor, MI,
USA) and was prepared as 20 mg/ml stock solutions in dimethyl
sulfoxide (DMSO; Sigma Chemical, St. Louis, MO, USA) and stored at
−20°C.
MTT and drug interaction analysis
For measurement of proliferation, the human GBC cell
lines, EH-GB1, EH-GB2 and GBC-SD, were placed into 96-well plates
at 1×104 cells/well. After 24 h, the cells were treated
with various concentrations of MA, GEM (Lilly, France) and MA +
GEM. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) assays were performed to evaluate cell growth and viability.
MTT solution was added to each well and incubated for 4 h at 37°C.
An extraction buffer (20% SDS and 50% dimethylformamide) was added,
and then cells were incubated overnight at 37°C. The absorbance of
the cell suspension was measured at 570 nm using a 96-well plate
reader (Seeuco Electronics Technology Co., Ltd., China). Each
experiment was repeated three times.
Drug interactions were quantified by median-dose
effect analysis (29), and
combination index values were derived using CompusSyn software
(CompuSyn, Inc., Paramus, NJ, USA). CI values of <1, =1 and
>1 indicated synergism, additivity, and antagonism,
respectively, between the drugs.
Measurement of apoptosis
An Annexin V kit (BD Pharmingen, San Diego, CA, USA)
was used to measure cell apoptosis. EH-GB1, EH-GB2 and GBC-SD cells
were treated as described above. Annexin V and propidium iodide
(PI) labeling followed, which was performed according to the
manufacturer’s instructions. The percentage of apoptosis in every
group was analyzed by flow cytometry (Becton-Dickinson, Mountain
View, CA, USA). Each experiment was repeated three times.
Migration assay
As previously described (11), EH-GB2 cells were allowed to grow
until full confluence in 6-well plates. Monolayer GBC cells were
wounded by scratching with a 1 ml pipette tip. DMSO solution, MA,
GEM, and MA + GEM were added respectively to plates at indicated
concentrations. Images were captured using a Olympus digital camera
after 10 h of incubation at 37°C and 5% CO2. The
migrated cells were quantified by manual counting, and percentage
inhibition was expressed using untreated wells at 100%.
Invasion assay
To test the effect of MA on cell invasion activity,
we performed Transwell invasion assays as previously described
(30). Briefly, starved cells
(1×105/well) were seeded in the top chambers of the
Transwell with an 8-μm pore polycarbonate filter insert coated with
0.1% gelatin (both from Corning, New York, NY, USA). The bottom
chambers were filled with DMEM with 10% FBS supplemented with or
without 0.1 nM GEM. The top and bottom chamber contained the same
concentration of MA. EH-GB2 cells were allowed to migrate for 12 h.
The cells were scraped on the top surface of the membrane and
stained. The cells on the bottom side of the membranes (migrated
cells) were counted using an Olympus inverted microscope.
Western blot analysis
Western blot analysis was performed to determine the
effect of MA and GEM on NF-κB nuclear translocation and various
NF-κB-regulated genes. Cell lysates (40 μg) were resolved by
SDS-PAGE. After electrophoresis, the proteins were
electro-transferred to nitrocellulose membranes (Bio-Rad
Laboratories, Hercules, CA, USA), blotted with the relevant
antibody, and detected by an enhanced chemiluminescence reagent
(Amersham, Piscataway, NJ, USA). Antibodies to phosho-IκBα, IκBα
and p65 (1:1,000 dilutions) were purchased from Thermo Scientific
(Rockford, IL, USA). Antibodies for β-actin, cyclin D1, Bcl-2, Bax,
MMP-9 and MMP-2 (1:1,000 dilutions) were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA).
Mouse xenograft model and tumor
treatment
Xenograft mouse models were performed as previously
described (31). Six-week-old
athymic nu/nu male mice were purchased from SIBS (Shanghai, China).
The experiments performed on the animals were approved by the
Animal Ethics Committee of the Second Military Medical University
prior to the study. EH-GB2 tumor cells were subcutaneously injected
into the mice (2×106 cells/mouse).
After the tumors were established (~100
mm3), the mice were randomized into the following
treatment groups (n=5): i) untreated control; ii) subcutaneously
injected with 30 mg/kg bodyweight of MA every 2 days for 30 days;
iii) GC 50 mg/kg of bodyweight intraperitoneal every 2 for 30 days;
and iv) MA and GEM following the schedule for the individual
treatments. The control mice were injected with DMSO. The mice body
weight and tumor sizes were recorded every other day, and the tumor
size was determined by vernier caliper measurements and calculated
as maximal diameter × minimal diameter2 ×0.5 (32). After 36 days, mice with tumors were
sacrificed. One section of the tissue was fixed in formalin and
another section was frozen in liquid nitrogen.
Histology and immunohistochemistry
(IHC)
After the tumors were removed, they were weighed,
fixed with 10% formalin, and embedded with paraffin. H&E and
IHC for Bcl-2 and Bax were performed on paraffin-embedded tissue
sections. The rabbit antibodies against Bcl-2 and Bax were
purchased from Santa Cruz Biotechnology, Inc. and applied for IHC
staining (1:1,000 dilution). PBS was used as a negative control.
After IHC was performed, images from each group were captured using
an Olympus BX60 upright microscope (Olympus, Tokyo, Japan).
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end-labelling (TUNEL) staining
Apoptotic cells in EH-GB2 tumor xenograft tissue
sections were detected by TUNEL using a commercially available kit
(EMD Millipore Corporation, Billerica, MA, USA). The tissue
sections were processed according to the manufacturer’s
instructions.
Statistical analysis
Data are presented as mean ± SD as indicated in the
vertical axis of figures. The statistical significance of
differential findings between experiments and controls was
determined by using the Student’s t-test or analysis of variance
(ANOVA). Statistical analyses were computed with SPSS 13.0 (SPSS
Inc., Chicago, IL, USA). Statistical significance was P<0.05
unless otherwise stated.
Results
MA inhibits proliferation, and
potentiates the apoptosis induced by GEM
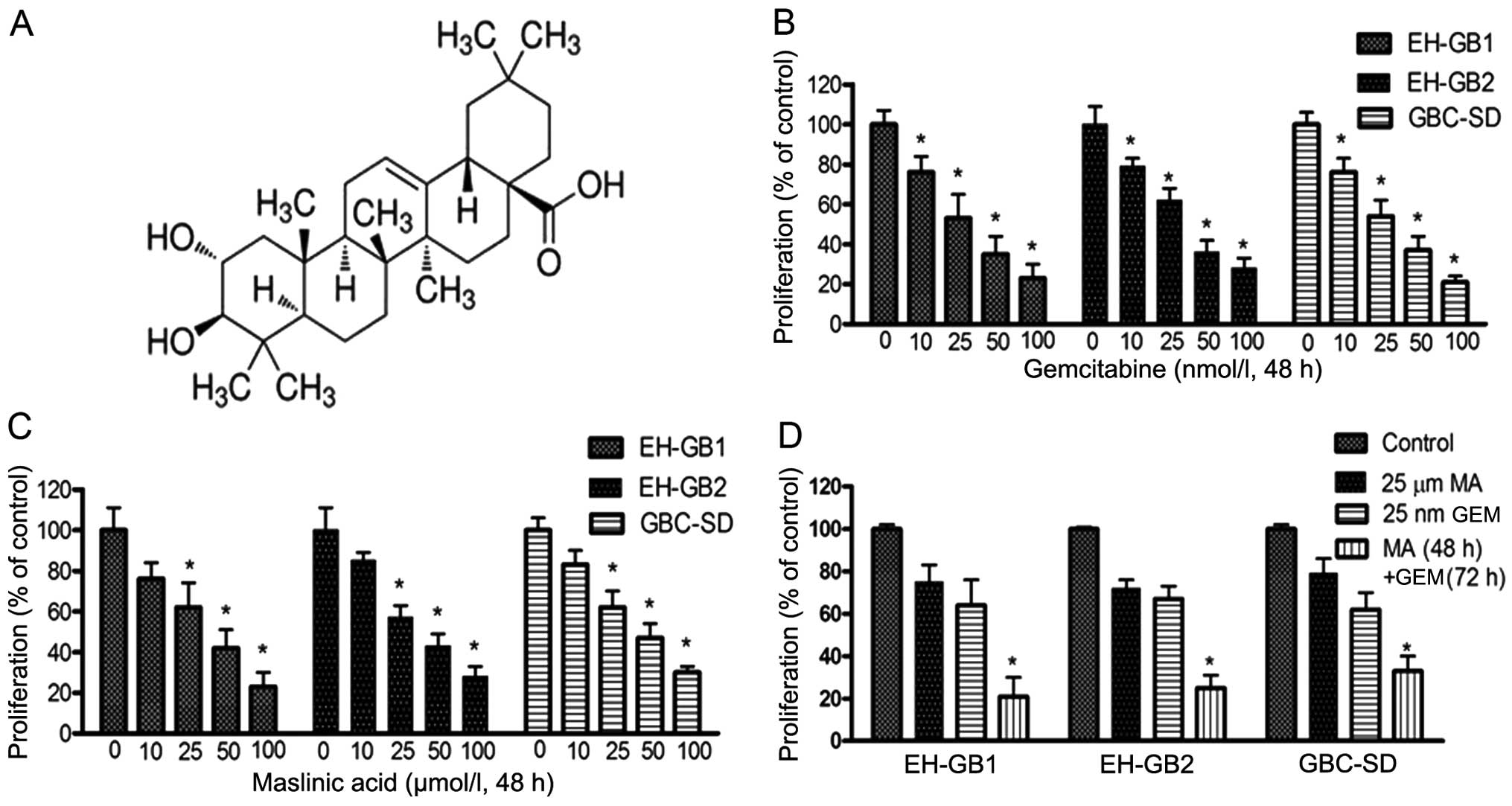
We first examined the effects of MA (Fig. 1A), GEM and MA + GEM on the
proliferation of human GBC cells (EH-GB1, EH-GB2 and GBC-SD) using
the MTT assay. The results showed that MA and GEM as single agents
both inhibited the proliferation of the GBC cell lines in a
dose-dependent manner (Fig. 1B and
C). MA and GEM combined significantly inhibited cell
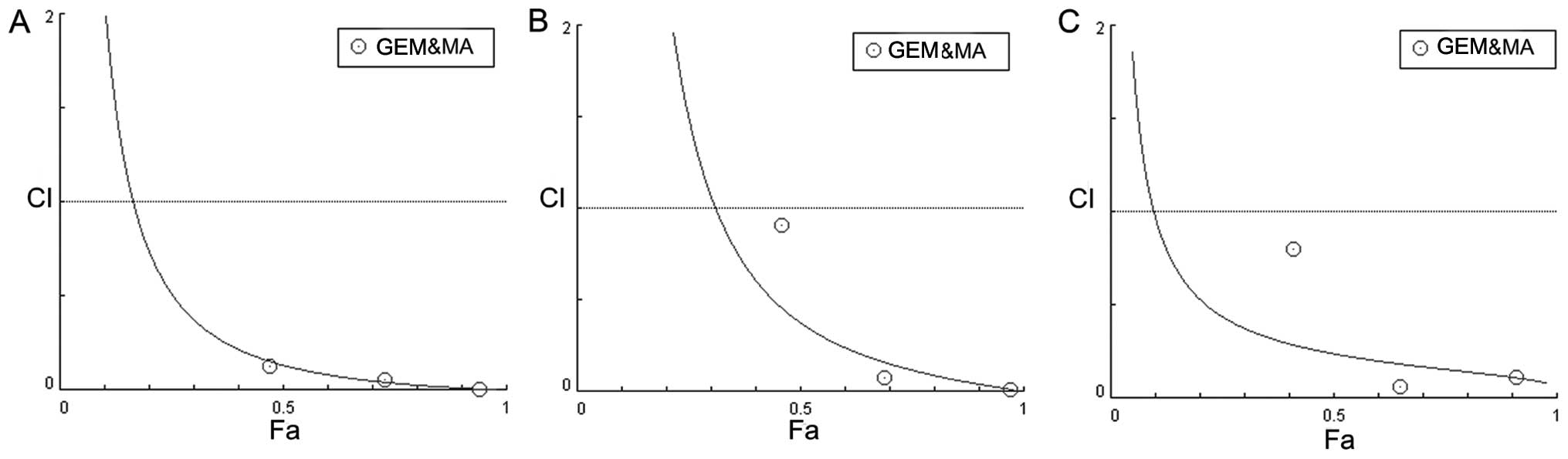
proliferation of all three GBC cell lines (Fig. 1D). A combination index (23) was calculated for all combinations of
GEM and MA examined in the three cell lines, indicating that the
interaction between the two drugs was synergistic (Fig. 2).
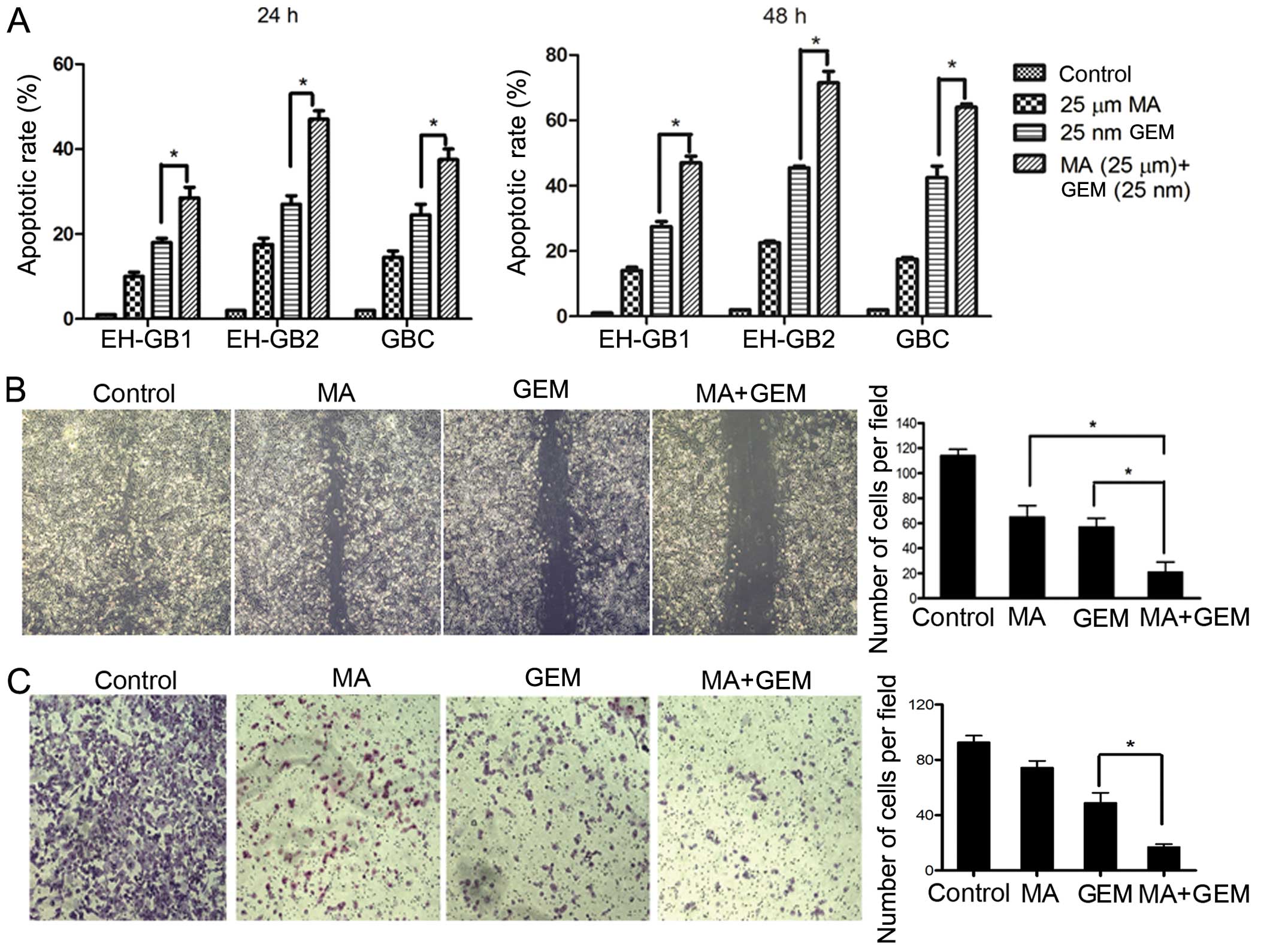
Subsequently, we examined whether the inhibition of
cell proliferation was due to enhanced apoptosis with the
combination of MA and GEM compared to single agents. Accordingly,
relative to the single agents, MA treatment (25 μmol/l) followed by
GEM treatment elicited significantly (P<0.05) higher apoptosis
in the investigated cancer cell lines, suggesting that the loss of
viable cells by MA + GEM was due to the induction of cell death
pathways (Fig. 3A).
MA enhances the inhibition of GEM in
cancer cell migration and invasion
Migration assays were performed to determine the
effects of MA on EH-GB2 migration. We found that the combination of
MA and GEM strongly inhibited the migration of EH-GB2 cells
(Fig. 3B). We performed Transwell
assays to evaluate the ability of EH-GB2 to filter through the
membrane barrier after treatment with MA, GEM and MA + GEM. MA, at
low concentrations of 10 μmol/l, significantly potentiated the
properties of GEM, inhibiting cancer cell invasion (Fig. 3C).
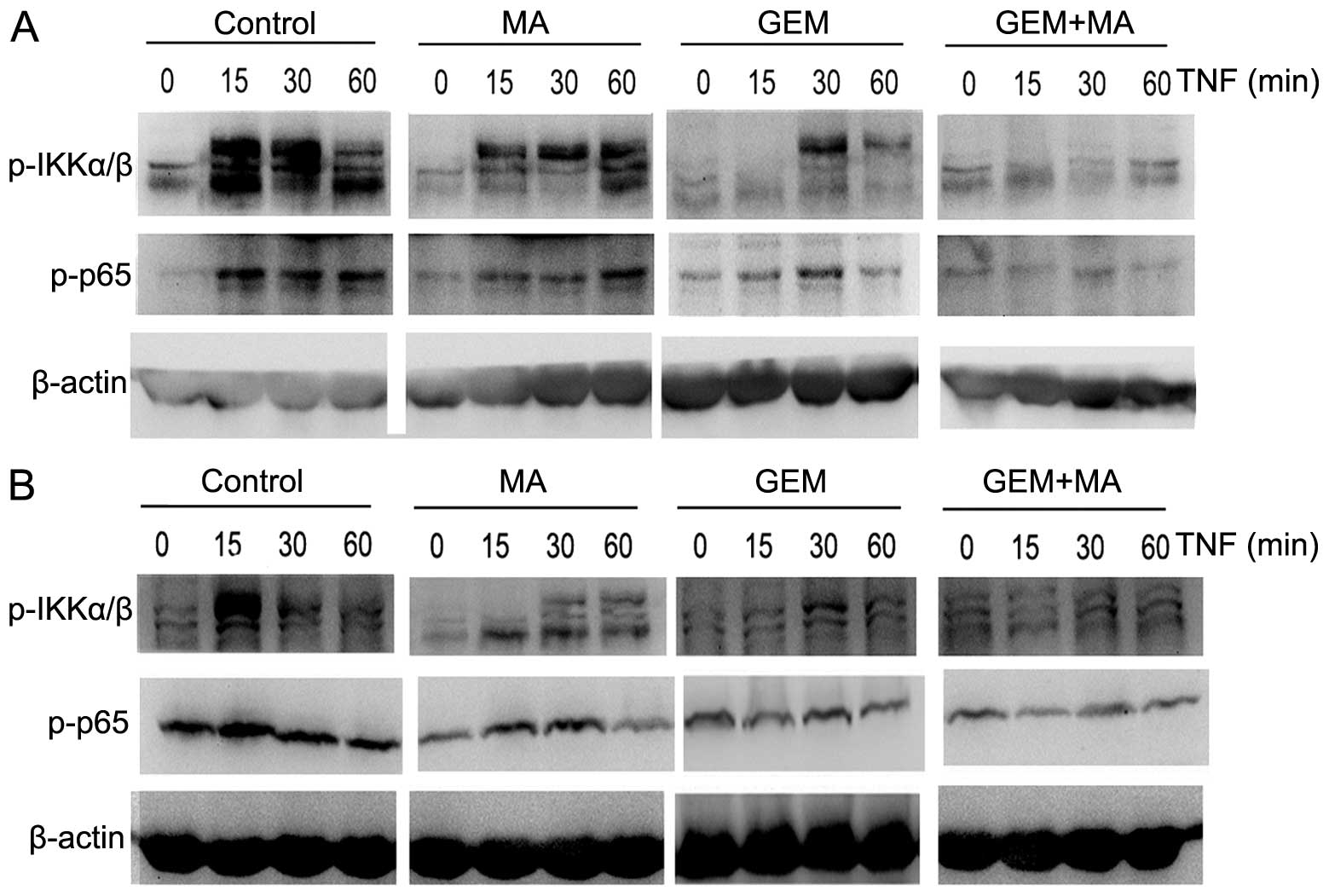
NF-κB activation is inhibited by the
combination of MA and GEM
We examined whether the combination of MA and GEM
was able to inhibit NF-κB activation. EH-GB1 and EH-GB2 cells were
incubated with suboptimal concentrations of MA and GEM alone and in
combination. IKKα/β and p65 activation were determined by western
blot analysis. MA potentiated the inhibitory effect of GEM on p65
nuclear translocation and phosphorylation in GBC cells (Fig. 4).
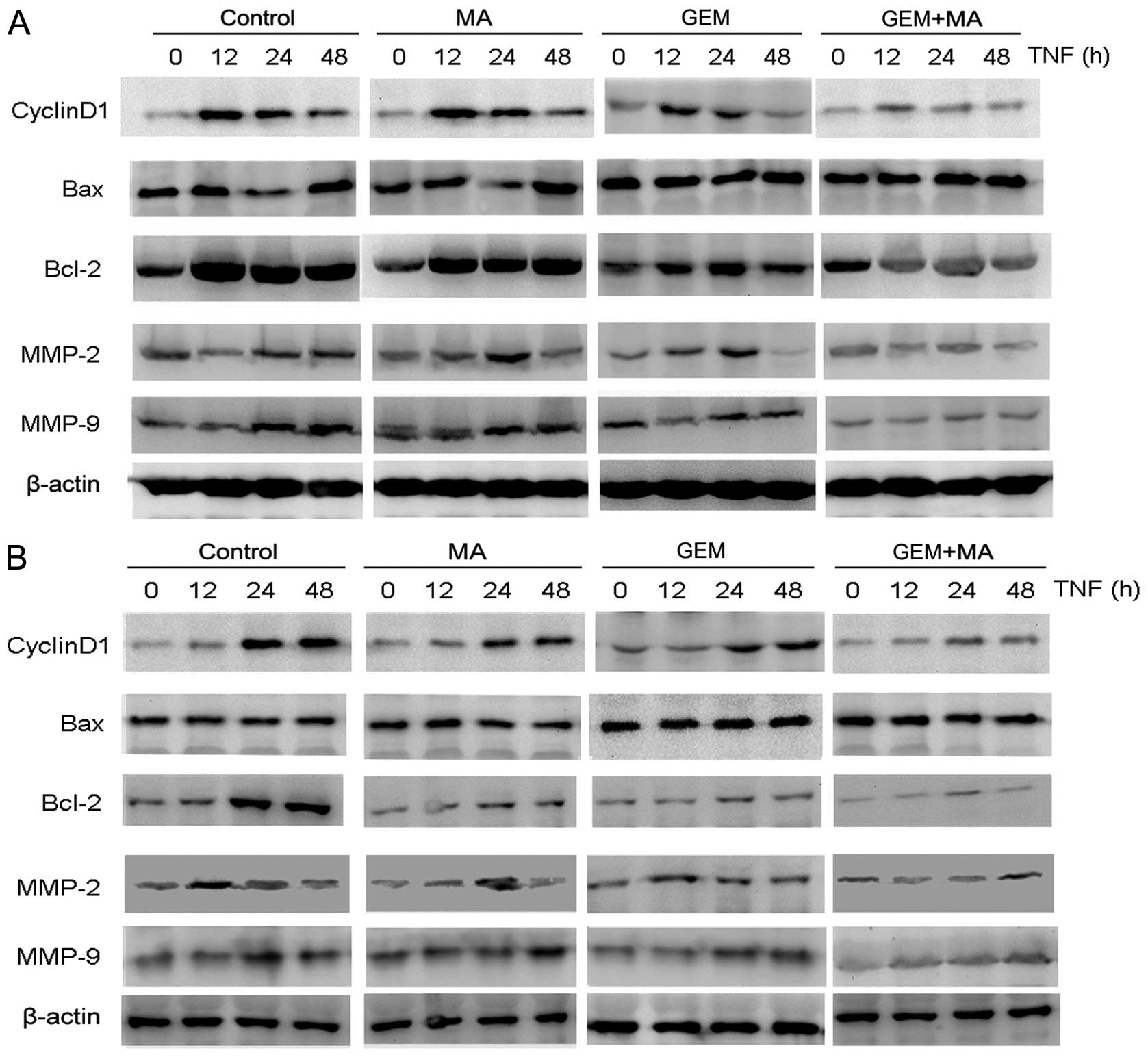
MA potentiates the effect of GEM in
downregulating the expression of NF-κB-regulated gene products in
vitro
We examined the effect of MA on the expression of
NF-κB-regulated gene products involved in cell proliferation
(cyclin D1), apoptosis (Bax and Bcl-2), and metastasis (MMP-2 and
MMP-9) (Fig. 5). The cells were
exposed to MA (25 μmol/l) for 48 h prior to the addition of GEM for
24 h. The results showed that the expression of cyclin D1 and Bcl-2
were significantly downregulated in the combination group compared
with the individual treatment groups. By contrast, Bax expression
was substantially increased after the combinatorial treatment when
compared to single agents. Furthermore, the activities of MMP-2 and
MMP-9 were significantly reduced by the treatment with MA +
GEM.
MA potentiates the antitumor effects of
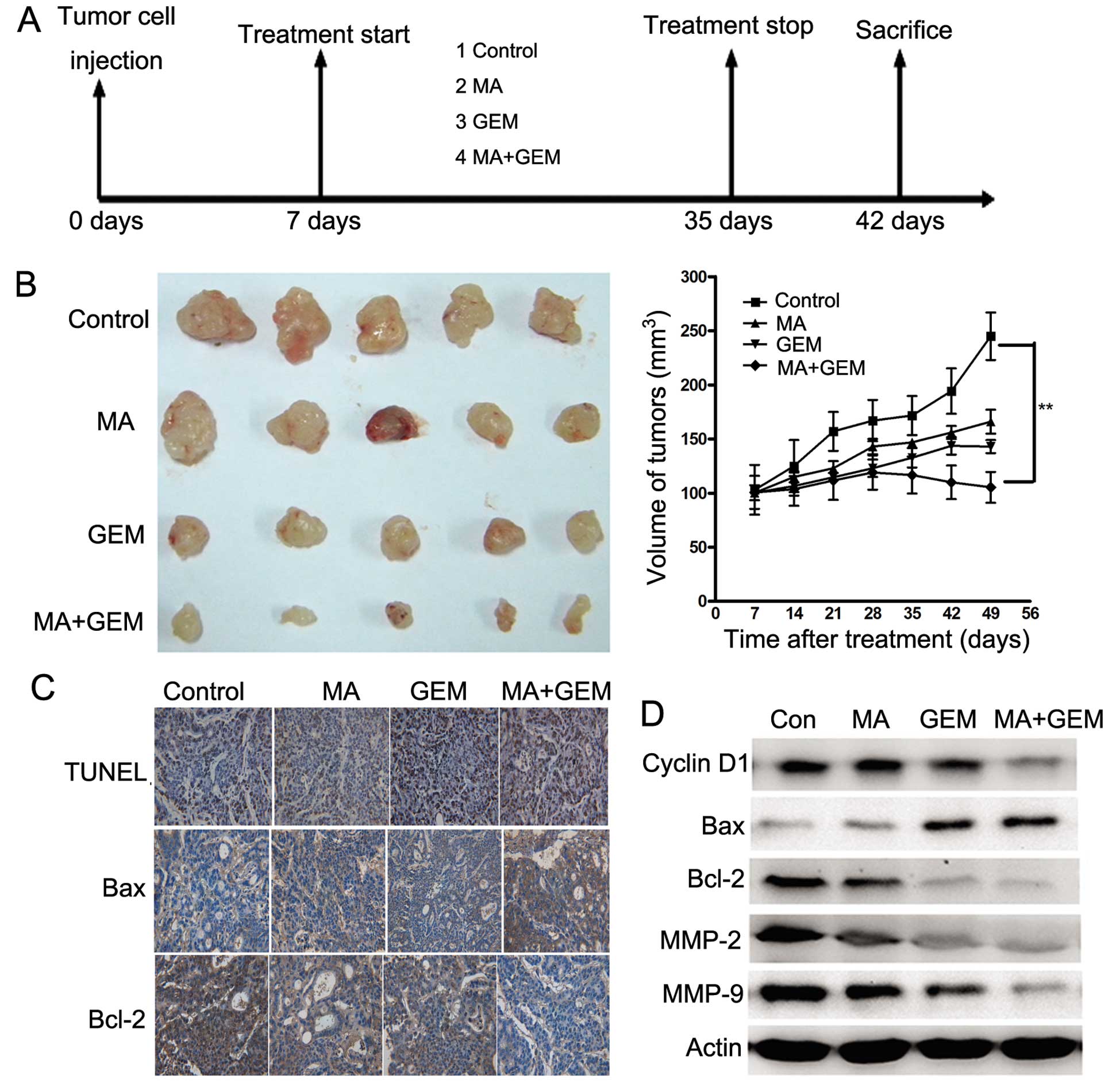
GEM in vivo
To determine whether MA enhances the antitumor
effects of GEM in GBC, we established a human GBC xenograft in nude
mice using EH-GB2 cells. Treatment was initiated 1 week after tumor
cell implantation and was continued up to 28 days (Fig. 6A). The tumor diameters were measured
at 1-week intervals. Animals were sacrificed 35 days after tumor
cell injection and 28 days after the treatment initiation date. At
this time the tumors were excised and the tumor diameters were
measured. We found that the tumor volume increased rapidly in the
control group compared with the other treatment groups (Fig. 6B). MA alone and GEM alone moderately
decreased the tumor volume. However, the tumor volume in the
combination group was significantly lower than that in the GEM and
MA alone groups at day 28 after treatment (P<0.05).
TUNEL staining of xenograft sections
To investigate whether MA potentiated GEM-induced
apoptosis in vivo, we stained the solid tumor sections with
an apoptosis staining kit. Significant differences in the
percentage of TUNEL-positive cells were noted in tumors derived
from the combination group relative to the single treatment groups
(P<0.05).
Immunohistochemical and western blot
analyses
Various mediators of apoptosis and survival
signaling were also examined in tumors collected from the xenograft
study. The expression level of Bcl-2 was significantly decreased,
while Bax was increased by IHC staining (Fig. 6C). The same trend of Bcl-2 and Bax
was identified in western blot assay (Fig. 6D). The expression of cyclin D1,
MMP-2 and MMP-9, which were involved in tumor progression, was
inhibited significantly by combining MA and GEM (Fig. 6D).
Discussion
MA, a natural triterpenoid that can be extracted
from olive skin, has been assessed for its antitumoral property in
colonic cancer (10), melanoma
(33,34), and astrocytoma (35) cells. GEM, which is a nucleoside
analog of deoxycytidine that inhibits DNA synthesis (36), has been increasingly prescribed for
GBC. Findings of recent studies have focused on improving the drug
efficacy by combining GEM with other agents (37,38).
We designed this study to determine whether MA can sensitize GBC to
GEM.
The constitutive activation of NF-κB is associated
with the growth and survival of cancer cells (39). In addition, studies have shown that
MA suppressed NF-κB activation (40). In the present study, we investigated
the mechanism of how MA enhanced the apoptotic effects of GEM in
cultured GBC cells. In vitro, we have demonstrated that MA
enhanced GEM-induced apoptosis and suppressed NF-κB activity. A
recent review by Nakanishi and Toi (41) details the activation of NF-κB by
chemotherapeutic agents as the major factor contributing to
chemoresistance. MA can inhibit IKKα/β degradation, block p65
nuclear translocation and phosphorylation, and downregulate the
expression levels of NF-κB-mediated genes/proteins involved in
proliferation, apoptosis and invasion (42). As previously reported, proteins
including cyclin D1, Bax, Bcl-2, MMP-2 and MMP-9, have been
associated with tumor growth, apoptosis, invasion, and metastasis.
In previous studies, it was shown that combining a NF-κB inhibitor
with a front-line anticancer drug enhances the overall antitumor
response (43,44). In this study, we have concluded that
the downregulation of NF-κB by MA can enhance the sensitivity of
GBC cells to GEM.
Our in vitro results were recapitulated in
vivo in a subcutaneous xenograft GBC model, wherein MA
significantly enhanced the antitumor efficacy of chemotherapeutics.
Although none of the mice from the combinatorial treatment group
were tumor-free, the therapeutic effect was significant compared
with single-drug treatment. These findings are concomitant with
increased TUNEL staining and reduced MA and GEM immunoreactivity
indicative of apoptosis and reduced cell proliferation within
tumors. These features are of significant value in predicting
improved therapeutic outcomes and require further
investigation.
Given the pharmacologic safety of MA (45), our studies suggest that this
compound has great potential as a chemopreventive and a
chemotherapeutic agent, especially when used in combination with
existing agents. Whether the concentrations of MA used in our
studies are achievable in the clinic remains to be determined. MA
metabolism in the cells is also unclear at present. Our results
also show that patient-derived GBC cells exhibit constitutive NF-κB
activation. This NF-κB activation was suppressed significantly by
GEM in combination with MA, thus leading to the inhibition of
proliferation, apoptosis and invasion of the cells.
In this study, MA decreased the protein expression
of cyclin D1, which is a cell cycle-positive regulator, indicating
a role for MA in GBC proliferation (46). Cyclin D1 adversely affects clinical
outcomes and serves as an independent marker in predicting
decreased survival for patients with GBC (47,48).
In the present study, the cells treated with GEM and
MA had a downregulated anti-apoptotic Bcl-2, but an increased
expression of pro-apoptotic Bax. The Bcl-2 family is important in
the regulation of apoptosis and comprises pro-apoptotic and
anti-apoptotic members (49). In
EH-GB1 and EH-GB2 cell lines, Bcl-2 was significantly downregulated
and Bax was upregulated in the combination group when compared with
the GEM treatment group and the non-treated control group.
MMPs are central mediators of tumor metastasis due
to their ability to degrade basement membrane and extracellular
matrix components (50). MMP-2 and
MMP-9 expression is positively associated with Nevin stage, distant
metastasis, and the degree of histological differentiation in GBC
(51). The results showed that MA
inhibited the invasion of GBC by reducing MMP-2 and MMP-9
activation through the suppression of NF-κB/p65 activation.
In conclusion, to the best of our knowledge, this is
the first study to demonstrate that MA can potentiate GEM activity
in vitro and in vivo in GBC models. The results
suggest that MA is a potentially valuable agent in the development
of a new class of drugs to assist in potentiating the anticancer
effects of conventional chemotherapeutics targeting specific
pathways for the treatment of human GBC. Future studies should be
conducted in the clinical setting to validate the biological
relevance of these results.
Acknowledgements
This study was supported by the General Program
(81172019) from the National Science Foundation of China.
References
|
1
|
Matsuba T, Qiu D, Kurosawa M, et al; JACC
Study Group. Overview of epidemiology of bile duct and gallbladder
cancer focusing on the JACC Study. J Epidemiol. 15(Suppl 2):
S150–S156. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Randi G, Malvezzi M, Levi F, et al:
Epidemiology of biliary tract cancers: an update. Ann Oncol.
20:146–159. 2009. View Article : Google Scholar
|
|
3
|
Tchivounda HP, Koudogbo B, Besace Y and
Casadevall E: Triterpene saponins from Cylicodiscus gabunensis.
Phytochemistry. 30:2711–2716. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu HX, Zeng FQ, Wan M and Sim KY: Anti-HIV
triterpene acids from Geum japonicum. J Nat Prod. 59:643–645. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Serra C, Lampis G, Pompei R and Pinza M:
Antiviral activity of new triterpenic derivatives. Pharmacol Res.
29:359–366. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Montilla MP, Agil A, Navarro MC, et al:
Antioxidant activity of maslinic acid, a triterpene derivative
obtained from Olea europaea. Planta Med. 69:472–474. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu J, Sun H, Duan W, Mu D and Zhang L:
Maslinic acid reduces blood glucose in KK-Ay mice. Biol Pharm Bull.
30:2075–2078. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reyes-Zurita FJ, Rufino-Palomares EE,
Lupianez JA and Cascante M: Maslinic acid, a natural triterpene
from Olea europaea L., induces apoptosis in HT29 human colon-cancer
cells via the mitochondrial apoptotic pathway. Cancer Lett.
273:44–54. 2009. View Article : Google Scholar
|
|
9
|
Juan ME, Planas JM, Ruiz-Gutierrez V,
Daniel H and Wenzel U: Antiproliferative and apoptosis-inducing
effects of maslinic and oleanolic acids, two pentacyclic
triterpenes from olives, on HT-29 colon cancer cells. Br J Nutr.
100:36–43. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martin R, Carvalho-Tavares J, Ibeas E,
Hernandez M, Ruiz-Gutierrez V and Nieto ML: Acidic triterpenes
compromise growth and survival of astrocytoma cell lines by
regulating reactive oxygen species accumulation. Cancer Res.
67:3741–3751. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li C, Yang Z, Zhai C, et al: Maslinic acid
potentiates the anti-tumor activity of tumor necrosis factor alpha
by inhibiting NF-kappaB signaling pathway. Mol Cancer. 9:732010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lafarge S, Hamzeh-Cognasse H, Richard Y,
et al: Complexes between nuclear factor-κB p65 and signal
transducer and activator of transcription 3 are key actors in
inducing activation-induced cytidine deaminase expression and
immunoglobulin A production in CD40L plus interleukin-10-treated
human blood B cells. Clin Exp Immunol. 166:171–183. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ozes ON, Mayo LD, Gustin JA, Pfeffer SR,
Pfeffer LM and Donner DB: NF-kappaB activation by tumour necrosis
factor requires the Akt serine-threonine kinase. Nature. 401:82–85.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mori N, Fujii M, Ikeda S, et al:
Constitutive activation of NF-kappaB in primary adult T-cell
leukemia cells. Blood. 93:2360–2368. 1999.PubMed/NCBI
|
|
15
|
Ma G, Tabanca N, Husnu Can Baser K, et al:
Inhibition of NF-kappaB-mediated transcription and induction of
apoptosis in human breast cancer cells by
epoxypseudoisoeugenol-2-methyl butyrate. Cancer Chemother
Pharmacol. 63:673–680. 2009. View Article : Google Scholar
|
|
16
|
Camp ER, Li J, Minnich DJ, et al:
Inducible nuclear factor-kappaB activation contributes to
chemotherapy resistance in gastric cancer. J Am Coll Surg.
199:249–258. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bottero V, Busuttil V, Loubat A, et al:
Activation of nuclear factor kappaB through the IKK complex by the
topoisomerase poisons SN38 and doxorubicin: a brake to apoptosis in
HeLa human carcinoma cells. Cancer Res. 61:7785–7791.
2001.PubMed/NCBI
|
|
18
|
Arlt A, Vorndamm J, Breitenbroich M, et
al: Inhibition of NF-kappaB sensitizes human pancreatic carcinoma
cells to apoptosis induced by etoposide (VP16) or doxorubicin.
Oncogene. 20:859–868. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Doval DC, Sekhon JS, Gupta SK, et al: A
phase II study of gemcitabine and cisplatin in chemotherapy-naive,
unresectable gall bladder cancer. Br J Cancer. 90:1516–1520. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Penz M, Kornek GV, Raderer M, et al: Phase
II trial of two-weekly gemcitabine in patients with advanced
biliary tract cancer. Ann Oncol. 12:183–186. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Desai S, Ben-Josef E, Griffith KA, et al:
Gemcitabine-based combination chemotherapy followed by radiation
with capecitabine as adjuvant therapy for resected pancreas cancer.
Int J Radiat Oncol Biol Phys. 75:1450–1455. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Scagliotti GV, Parikh P, von Pawel J, et
al: Phase III study comparing cisplatin plus gemcitabine with
cisplatin plus peme-trexed in chemotherapy-naive patients with
advanced-stage non-small-cell lung cancer. J Clin Oncol.
26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ferrandina G, Ludovisi M, Lorusso D, et
al: Phase III trial of gemcitabine compared with pegylated
liposomal doxorubicin in progressive or recurrent ovarian cancer. J
Clin Oncol. 26:890–896. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shelley M, Cleves A, Wilt TJ and Mason M:
Gemcitabine for unresectable, locally advanced or metastatic
bladder cancer. Cochrane Database Syst Rev. April 13–2011.(Epub
ahead of print). PubMed/NCBI
|
|
25
|
Knox JJ, Hedley D, Oza A, et al: Combining
gemcitabine and capecitabine in patients with advanced biliary
cancer: a phase II trial. J Clin Oncol. 23:2332–2338. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mooi LY, Wahab NA, Lajis NH and Ali AM:
Chemopreventive properties of phytosterols and maslinic acid
extracted from Coleus tuberosus in inhibiting the expression of EBV
early-antigen in Raji cells. Chem Biodivers. 7:1267–1275. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li LF, Hu HZ, Liu C, et al: Establishment
and characterization of a human gallbladder carcinoma cell line
EH-GB1 originated from a metastatic tumor. Zhonghua Zhong Liu Za
Zhi. 32:84–87. 2010.(In Chinese). PubMed/NCBI
|
|
28
|
Wang JH, Li LF, Yu Y, et al: Establishment
and characterization of a cell line, EH-GB2, derived from hepatic
metastasis of gall-bladder cancer. Oncol Rep. 27:775–782. 2012.
|
|
29
|
Chou TC: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Klein CA: Cancer. The metastasis cascade.
Science. 321:1785–1787. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carlsson G, Gullberg B and Hafström L:
Estimation of liver tumor volume using different formulas - an
experimental study in rats. J Cancer Res Clin Oncol. 105:20–23.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee CH, Jeon YT, Kim SH and Song YS:
NF-kappaB as a potential molecular target for cancer therapy.
Biofactors. 29:19–35. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li F and Sethi G: Targeting transcription
factor NF-kappaB to overcome chemoresistance and radioresistance in
cancer therapy. Biochim Biophys Acta. 1805:167–180. 2010.PubMed/NCBI
|
|
35
|
Ueno H, Kiyosawa K and Kaniwa N:
Pharmacogenomics of gemcitabine: can genetic studies lead to
tailor-made therapy? Br J Cancer. 97:145–151. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Knudsen KE, Diehl JA, Haiman CA and
Knudsen ES: Cyclin D1: polymorphism, aberrant splicing and cancer
risk. Oncogene. 25:1620–1628. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fan YZ, Fu JY, Zhao ZM and Chen CQ:
Inhibitory effect of norcantharidin on the growth of human
gallbladder carcinoma GBC-SD cells in vitro. Hepatobiliary Pancreat
Dis Int. 6:72–80. 2007.PubMed/NCBI
|
|
38
|
Ma HB, Hu HT, Di ZL, et al: Association of
cyclin D1, p16 and retinoblastoma protein expressions with
prognosis and metastasis of gallbladder carcinoma. World J
Gastroenterol. 11:744–747. 2005. View Article : Google Scholar
|
|
39
|
Reyes-Zurita FJ, Pachón-Peña G, Lizárraga
D, Rufino-Palomares EE, Cascante M and Lupianez JA: The natural
triterpene maslinic acid induces apoptosis in HT29 colon cancer
cells by a JNK-p53-dependent mechanism. BMC Cancer. 11:1542011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Parra A, Rivas F, Martin-Fonseca S,
Garcia-Granados A and Martinez A: Maslinic acid derivatives induce
significant apoptosis in b16f10 murine melanoma cells. Eur J Med
Chem. 46:5991–6001. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nakanishi C and Toi M: Nuclear
factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat
Rev Cancer. 5:297–309. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu AX, Hong TS, Hezel AF and Kooby DA:
Current management of gallbladder carcinoma. Oncologist.
15:168–181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bauer JA, Lupica JA, Schmidt H, et al:
Nitrosylcobalamin potentiates the anti-neoplastic effects of
chemotherapeutic agents via suppression of survival signaling. PLoS
One. 2:e13132007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chawla-Sarkar M, Bauer JA, Lupica JA, et
al: Suppression of NF-kappa B survival signaling by
nitrosylcobalamin sensitizes neoplasms to the anti-tumor effects of
Apo2L/TRAIL. J Biol Chem. 278:39461–39469. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Guan T, Qian Y, Tang X, et al: Maslinic
acid, a natural inhibitor of glycogen phosphorylase, reduces
cerebral ischemic injury in hyperglycemic rats by GLT-1
up-regulation. J Neurosci Res. 89:1829–1839. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chao DT and Korsmeyer SJ: BCL-2 family:
regulators of cell death. Annu Rev Immunol. 16:395–419. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Boise LH, Gottschalk AR, Quintans J and
Thompson CB: Bcl-2 and Bcl-2-related proteins in apoptosis
regulation. Curr Top Microbiol Immunol. 200:107–121.
1995.PubMed/NCBI
|
|
48
|
Fahy BN, Schlieman MG, Mortenson MM,
Virudachalam S and Bold RJ: Targeting BCL-2 overexpression in
various human malignancies through NF-kappaB inhibition by the
proteasome inhibitor bortezomib. Cancer Chemother Pharmacol.
56:46–54. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pulukuri SM and Rao JS: Matrix
metalloproteinase-1 promotes prostate tumor growth and metastasis.
Int J Oncol. 32:757–765. 2008.PubMed/NCBI
|
|
50
|
Wu W, Wang R, Liu H, et al: Prediction of
prognosis in gallbladder carcinoma by CD147 and MMP-2
immunohistochemistry. Med Oncol. 26:117–123. 2009. View Article : Google Scholar
|
|
51
|
Park SY, Nho CW, Kwon DY, Kang YH, Lee KW
and Park JH: Maslinic acid inhibits the metastatic capacity of
DU145 human prostate cancer cells: possible mediation via
hypoxia-inducible factor-1α signalling. Br J Nutr. 109:210–222.
2013. View Article : Google Scholar
|