Introduction
Non-small cell lung cancer (NSCLC) accounts for ~85%
of all lung cancers and is the leading cause of cancer-related
mortality worldwide (1). Surgery is
currently the most effective treatment for early NSCLC (2). However, most NSCLC patients are
inoperable due to advanced disease, and are managed with systemic
therapies (3,4). Cisplatin-based palliative chemotherapy
has been commonly used in patients with advanced NSCLC, yielding a
slight survival benefit (5,6). Development of drug resistance is
regarded as a major cause for chemotherapy failure in the treatment
of cancer (7). Therefore,
establishment of effective approaches to overcome cisplatin
chemoresistance is of paramount importance in the management of
unresectable NSCLC.
The anticancer activity of cisplatin involves the
generation of DNA lesions followed by the activation of the DNA
damage response and the induction of mitochondrial apoptosis.
Numerous mechanisms are responsible for the development of
cisplatin resistance, including reduced drug uptake, accelerated
drug inactivation, increased DNA damage repair and inhibition of
transmission of DNA damage recognition signals to the apoptotic
pathway (8). It has been documented
that oncogenic β-catenin signaling plays a critical role the
acquisition of cisplatin resistance in NSCLC cells (9). Glycogen synthase kinase 3β (GSK3β) is
a multifunctional serine/threonine protein kinase that acts as a
negative regulator of β-catenin signaling (10). GSK3β is activated upon
phosphorylation at Tyr216, which leads to phosphorylation and
degradation of β-catenin (11). In
contrast, phosphorylation of GSK3β at Ser9 inhibits its ability to
promote the degradation of β-catenin (12). Inactivation of GSK3β results in the
translocation of active β-catenin to the nucleus, where it
interacts with the transcription factor Tcf/Lef to activate
multiple pro-proliferative and survival genes such as c-Myc, cyclin
D1 and survivin (13). The
β-catenin pathway has been suggested as an attractive target
pathway for improving the susceptibility of cancer cells to
cisplatin (14,15).
Matrine is an alkaloid isolated from Sophora
flavescens and possesses multiple biological activities
including anti-inflammatory (16),
antiviral (17) and antitumor
(18) activities. Different
molecular pathways mediate the cytotoxic effects of matrine on
tumor cells (19,20). For instance, Niu et al
(19) reported that matrine induces
apoptosis of lung cancer cells through inhibition of Akt signaling.
Downregulation of the ERK-NF-κB pathway is causally linked to the
inhibition of human osteosarcoma cell invasion by matrine (20). In Hep3B hepatoma cells, matrine has
been shown to decrease β-catenin-dependent transcriptional activity
(21). Given the involvement of the
β-catenin pathway in cisplatin resistance in NSCLC cells, we
hypothesized that matrine could sensitizes NSCLC cells to cisplatin
through inactivation of β-catenin signaling.
Therefore, in the present study, we attempted to
explore the cytotoxic effects of matrine on cisplatin-resistant
NSCLC cells and to ascertain whether the anticancer activity of
matrine is mediated through modulation of β-catenin signaling.
Materials and methods
Cell lines
Two human NSCLC cell lines A549 and H460 were
purchased from the American Type Culture Collection (ATCC;
Rockville, MD, USA) and cultured in Dulbecco’s modified Eagle’s
medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100
U/ml penicillin and 100 μg/ml streptomycin (Invitrogen,
Carlsbad, CA, USA).
In accordance with previously described methods
(23), cisplatin (CDDP)-resistant
sublines A549/CDDP and H460/CDDP were established by continuous
exposure of the parental cells to increasing concentrations of
cisplatin, ranging from 2 nM to 4 μM for >6 months. The
drug-resistant cell lines were maintained in DMEM containing 4
μM cisplatin.
Matrine treatment
A549/CDDP and H460/CDDP and their parental cells
were seeded at 4-6×104 cells/well onto 12-well plates
and cultured overnight to allow attachment. The cells were exposed
to 1 or 2 g/l of matrine (Sigma, St. Louis, MO, USA) for 48 h.
After treatment, the cells were subjected to gene expression and
apoptosis analysis.
Plasmid transfection
Human survivin-expressing plasmid
(pcDNA3.1-survivin) was kindly provided by Dr Altieri (University
of Massachusetts, Worcester, MA, USA). Cells were seeded at a
density of 3×105 cells/well onto 6-well plates and
pre-transfected with empty vector or pcDNA3.1-survivin using
Lipofectamine 2000. The transfection efficiency was ~70%, which was
determined by transfection of a green fluorescent
protein-expressing plasmid (pGFP-N1; Clontech, Mountain View, CA,
USA). After incubation for 24 h, the transfected cells were exposed
to matrine for an additional 48 h before apoptosis analysis.
Cell viability assay
Cells were seeded onto 96-well plates at
3×103 cells/well and cultured overnight to allow
adherence. Different concentrations of cisplatin (i.e., 0.5, 1, 2,
4, 8, 16, 32 and 64 μM) were added to the cell culture.
After incubation for 48 h, cell viability was assessed using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. MTT solution (5 mg/ml) (Sigma) was added to each well and
incubated for 4 h. After removal of the MTT solution, formazan
crystals were dissolved in dimethyl sulfoxide. The absorbance was
measured at a wavelength of 570 nm. The 50% inhibitory
concentration (IC50) was calculated from the survival
curve.
Western blot analysis
Primary antibodies used in the present study are as
follows: anti-β-catenin (#9562), anti-phospho-β-catenin
(Ser33/37/Thr41) (#9561), anti-non-phospho (active) β-catenin
(Ser33/37/Thr41) (#4270), anti-GSK-3β (#9315), anti-phospho-GSK-3β
(Ser9) (#9323) (Cell Signaling Technology, Danvers, MA, USA),
anti-survivin (sc-10811) and anti-β-actin (sc-130301) (Santa Cruz
Biotechnology, Santa Cruz, CA, USA).
Following treatment, the cells were lysed in
radioimmuno-precipitation assay (RIPA) buffer [150 mM NaCl, 1%
NP40, 0.5% deoxycholic acid, 0.1% sodium dodecylsulfate (SDS), 50
mM Tris-HCl (pH 8.0)] supplemented with protease and phosphatase
inhibitors. The protein samples were separated on polyacrylamide
gels and then transferred to a nitrocellulose membrane. After
blocking for 1 h in a Tris-buffered solution (TBS) containing 5%
fat-free dried milk and 0.5% Tween-20, the membrane was incubated
with individual primary antibodies overnight at 4°C. The membrane
was washed three times and incubated for 1 h with horseradish
peroxidase-conjugated secondary antibodies (Santa Cruz
Biotechnology). The signals were visualized with an enhanced
chemiluminescence detection kit (Amersham Pharmacia Biotech,
Piscataway, NJ, USA). Densitometric analysis of the protein bands
was performed using the Quantity One software (Bio-Rad, Hercules,
CA, USA).
Luciferase reporter gene assay
A β-catenin/TCF firefly luciferase reporter
construct (pTopFlash) was purchased from Upstate Biotechnology
(Waltham, MA, USA). A control pRL-TK reporter plasmid encoding
Renilla luciferase was purchased from Promega (Madison, WI,
USA). Cells were seeded onto 12-well plates at 6×104
cells/well and transiently transfected with 0.2 μg of
pTopFlash along with 0.02 μg pRL-TK using Lipofectamine 2000
(Invitrogen) following the manufacturer’s protocol. Cells were
collected 24 h post-transfection or treated with matrine for 48 h
before collection. The cells were then lysed and centrifuged and
the supernatant was obtained for measurement of luciferase
activities using the Dual-Luciferase Assay System (Promega). The
firefly luciferase activity was normalized to Renilla
luciferase activity and expressed as relative luciferase
activity.
Apoptosis detection by Annexin V/PI
staining
After drug treatment, the cells were trypsinized and
centrifuged. The cell pellet was resuspended and incubated with 1
μl of fluorescein isothiocyanate (FITC)-conjugated Annexin V
and 5 μl of propidium iodide (PI) (Becton-Dickinson
Biosciences, San Diego, CA, USA) for 15 min at 4°C in the dark.
Apoptotic cells were analyzed on a FACSCalibur flow cytometer using
CellQuest software (Becton-Dickinson Biosciences).
Mitochondrial membrane potential (ΔΨm)
assay
ΔΨm was measured using the JC-1 mitochondrial
membrane potential assay kit (Biotium, Hayward, CA, USA). When ΔΨm
is relatively low, the cyanine dye JC-1
(5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimi-dazolylcarbocyanine
iodide) localizes in the cytoplasm in a green fluorescent monomeric
form. At a high ΔΨm, the JC-1 dye aggregates and yields red
fluorescence. A decrease in the ratio of the red/green fluorescence
indicates loss of ΔΨm. In brief, cells were harvested after drug
treatment and stained with 10 mM JC-1 at 37°C for 15 min in the
dark. Cells were washed and green/red fluorescence was analyzed by
flow cytometry.
Measurement of caspase-9 and -3
activities
Measurement of cellular caspase-9 and -3 activities
was carried out using the caspase-3 and -9 activity kits (Beyotime,
Haimen, Jiangsu, China) according to the manufacturer’s
instructions. Briefly, cells were collected after drug treatment,
washed and lysed in lysis buffer on ice for 10 min. Cell lysates
were incubated at 37°C for 4 h with 1X reaction buffer containing a
caspase-3 substrate (acetyl-Asp-Glu-Val-Asp-p-nitroanilide) or
caspase-9 substrate (acetyl-Leu-Glu-His-Asp-p-nitroanilide).
Caspase activities were measured by spectrofluorometry.
Statistical analysis
All data are expressed as mean ± standard deviation
(SD). Statistical significance was analyzed using the Student’s
t-test or one-way analysis of variance with Tukey’s post-hoc
test. A difference was defined as significant at P<0.05.
Results
Activation of β-catenin signaling is
associated with acquisition of cisplatin resistance
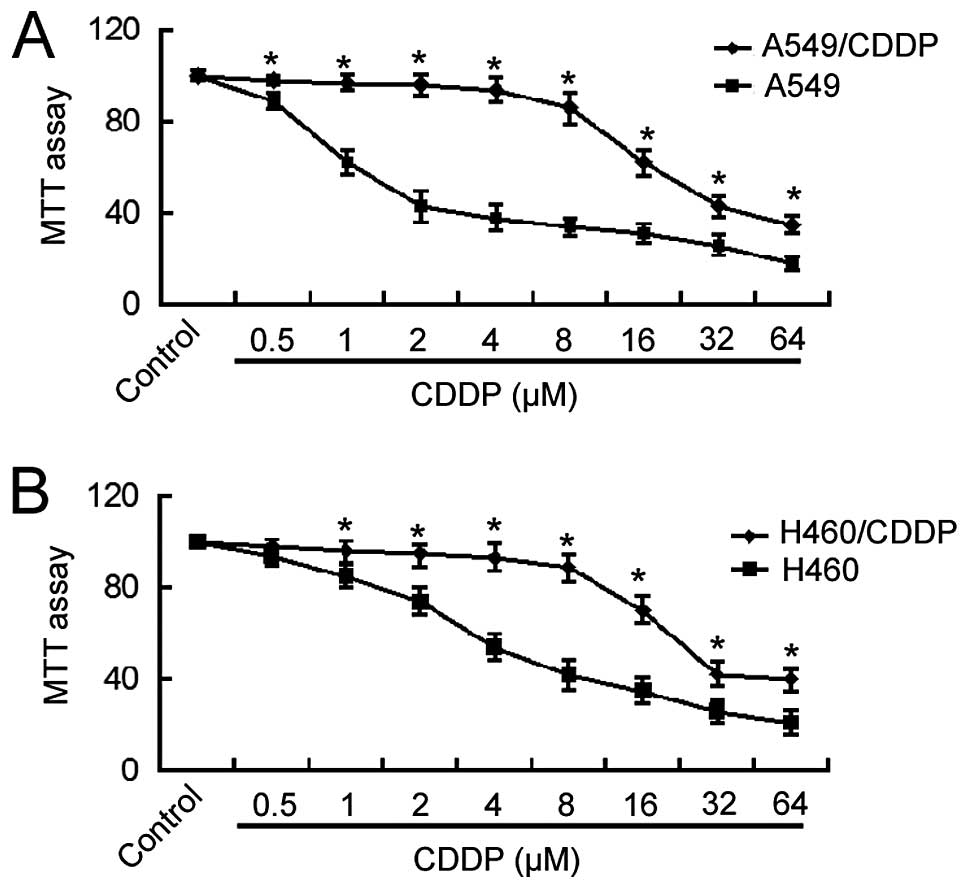
Cisplatin-resistant A549 cells were generated by
culturing cells in gradually increasing concentrations of
cisplatin. The MTT assay revealed that the IC50 value of
A549/CDDP cells for cisplatin was ~15-fold higher than that of the
parental A549 cells (24.7±1.5 vs. 1.6±0.2 μM; Fig. 1A). Similarly, H460/CDDP cells showed
an ~6-fold increase in the IC50 value for cisplatin
compared to the parental cells (28.3±1.2 vs. 4.6±0.4 μM;
Fig. 1B). These results indicate
the acquisition of cisplatin resistance in NSCLC cells following
long-term exposure to cisplatin.
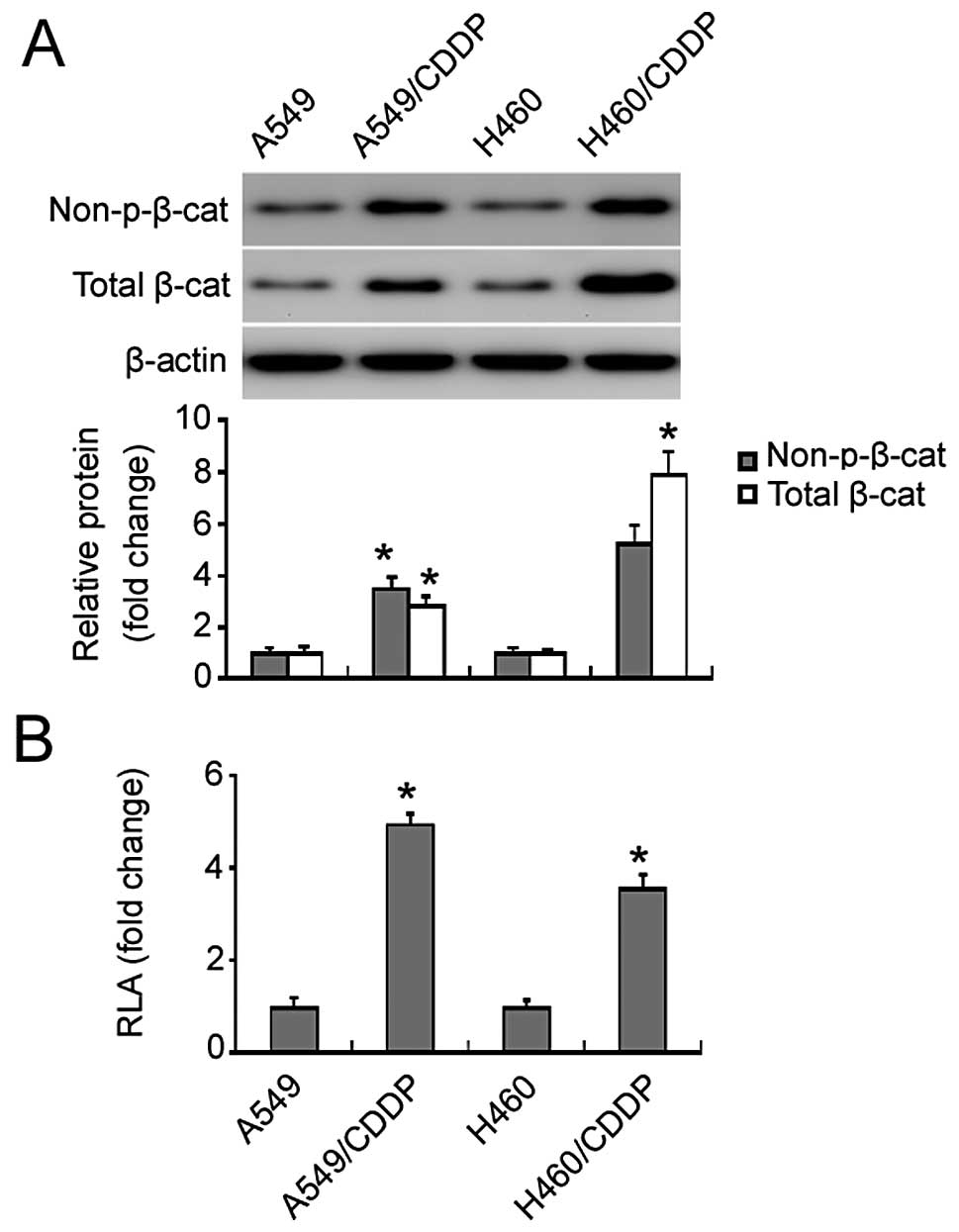
Western blot analysis identified a marked increase
in non-phospho (active) β-catenin (Ser33/37/Thr41) and total
β-catenin protein in the cisplatin-resistant NSCLC cells relative
to the parental cells (Fig. 2A). To
evaluate the changes in β-catenin transcriptional activity, cells
were transiently transfected with the β-catenin luciferase reporter
plasmid. We found that there was a 3.5- and 5-fold increase in the
β-catenin reporter activity in the cisplatin-resistant H460 and
A549 cells relative to the parental cells, respectively (Fig. 2B).
Matrine suppresses β-catenin signaling in
the cisplatin-resistant NSCLC cells
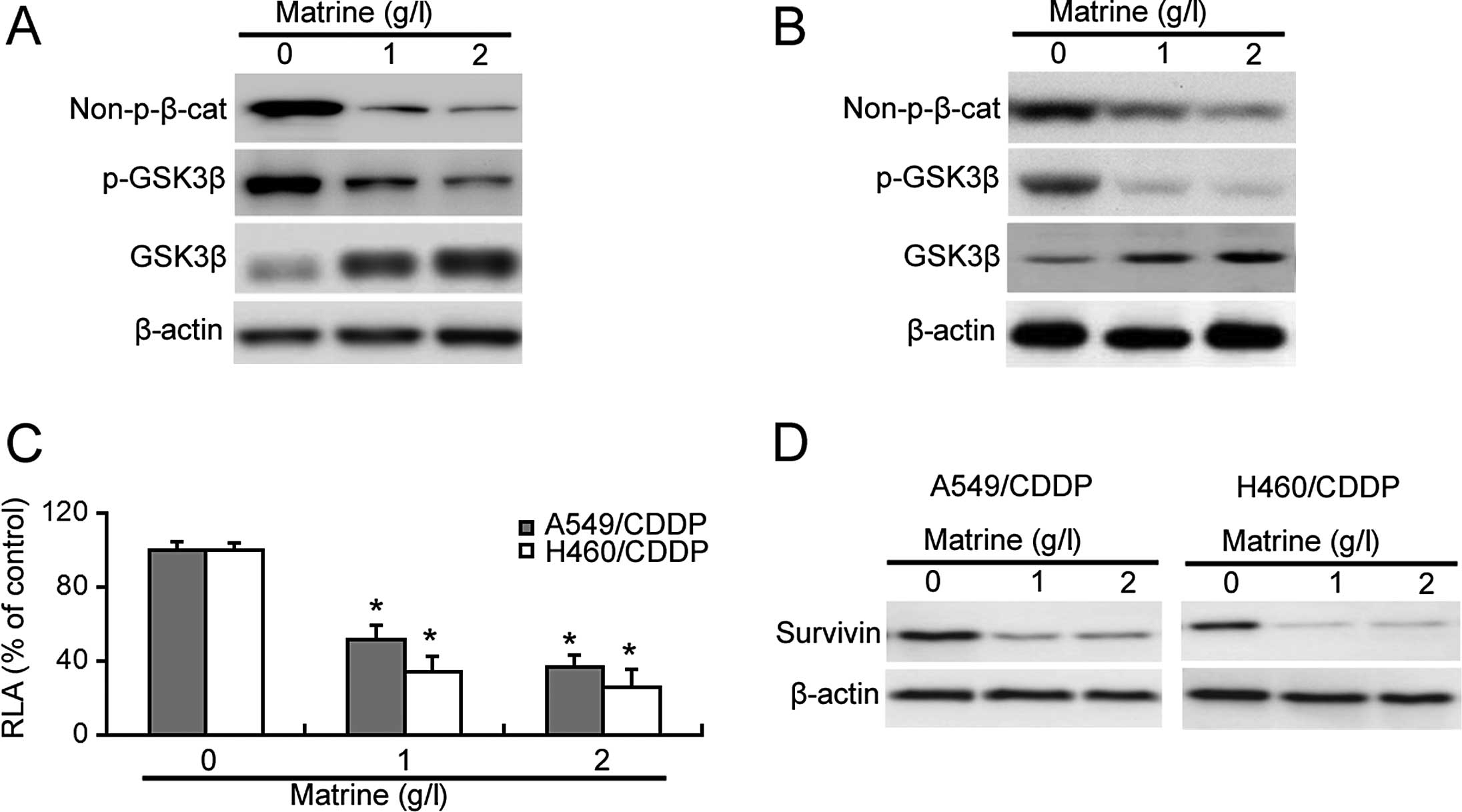
Next, we explored the effect of matrine on β-catenin
signaling in the cisplatin-resistant cells. As shown in Fig. 3A and B, matrine treatment reduced
the Ser33/37/Thr41-unphosphorylated active β-catenin protein level
in the cisplatin-resistant cells. Moreover, matrine exposure
resulted in a marked decrease in the phosphorylated level of GSK3β
(Ser9) and increase in the total level of GSK3β protein (Fig. 3A and B). The β-catenin luciferase
reporter assay confirmed a significant decrease in the
β-catenin-mediated transcriptional activity in matrine-treated
cisplatin-resistant cells (Fig.
3C). Additionally, survivin, a target gene of β-catenin, was
downregulated by matrine exposure (Fig.
3D).
Matrine induces apoptosis in
cisplatin-resistant cells via the mitochondrial death pathway
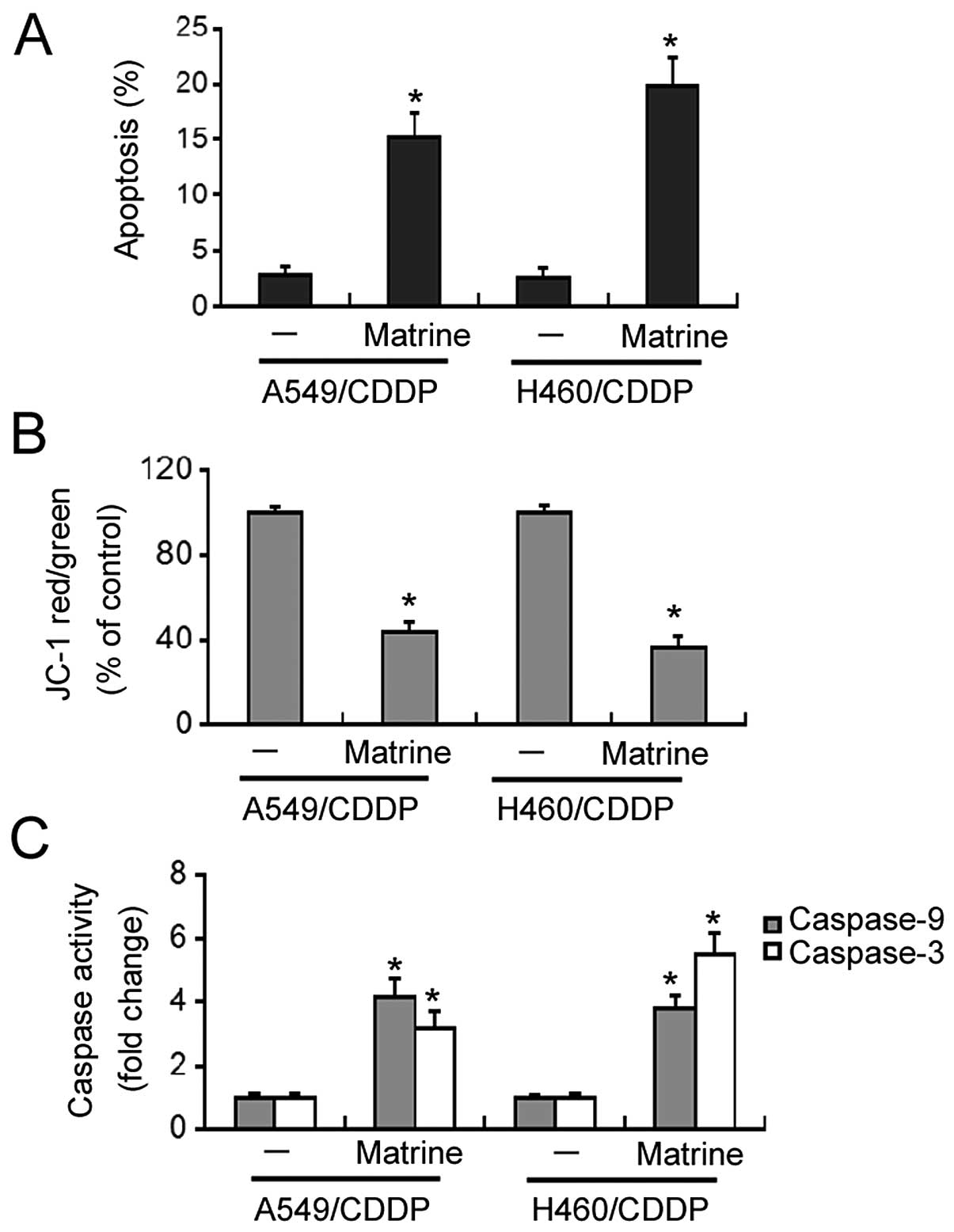
Flow cytometric analysis revealed that matrine at 2
g/l significantly induced apoptosis in the A549/CDDP and H460/CDDP
cells, with a 5-8-fold increase in the apoptosis rates relative to
the untreated cells (Fig. 4A).
Matrine-induced apoptosis was accompanied by a marked loss in ΔΨm
(Fig. 4B). Moreover, both caspase-9
and -3 activities were significantly increased in the
matrine-treated cisplatin-resistant cells, compared to the
untreated cells (Fig. 4C).
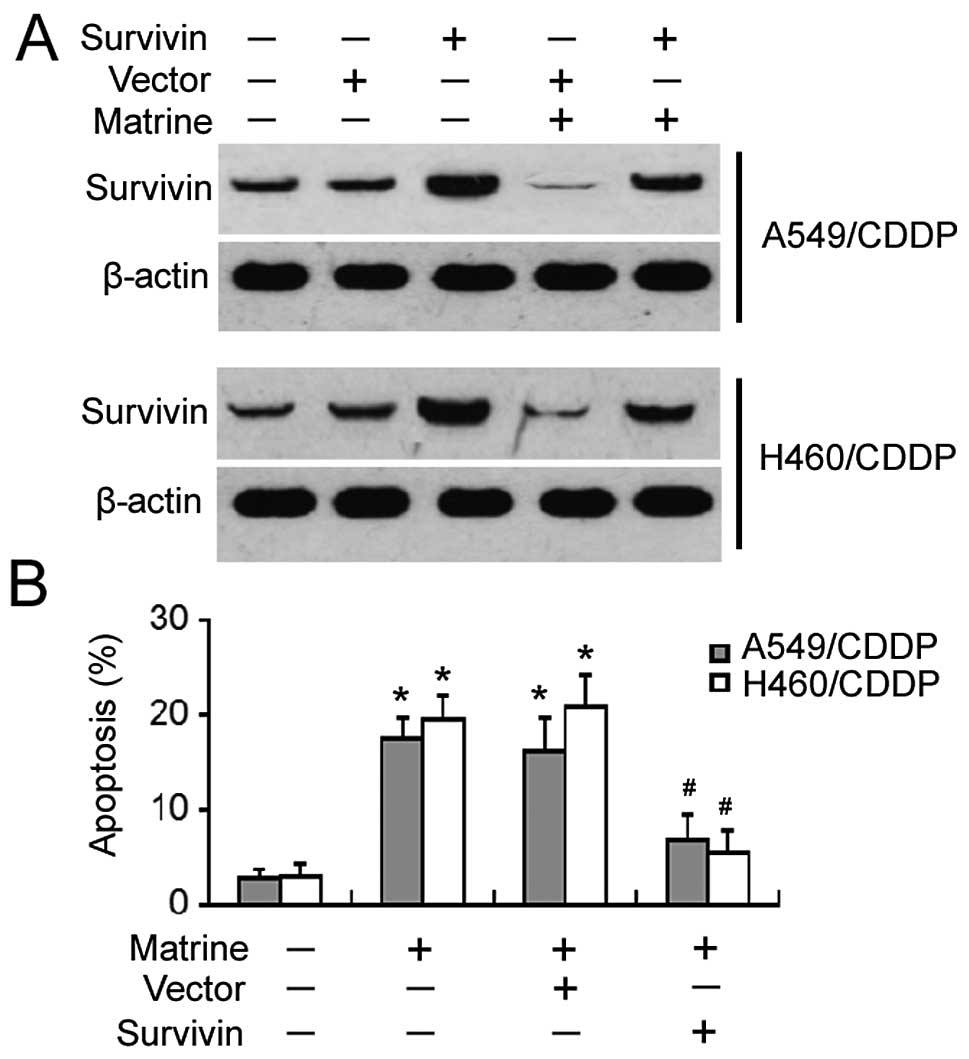
Ectopic expression of survivin protects
against matrine-induced apoptosis
To determine the role of survivin in matrine-induced
apoptosis of cisplatin-resistant NSCLC cells, the cells were
pre-transfected with the survivin-expressing plasmid before
exposure to matrine. Western blot analysis confirmed that the
expression level of survivin remained high after matrine treatment
in survivin-transfected cells, yet was markedly reduced in
vector-transfected cells (Fig. 5A).
After a 48-h incubation with matrine, apoptotic death was
significantly observed in the vector-transfected cells, yet not in
the survivin-transfected cells (Fig.
5B). These results indicate that survivin can rescue
cisplatin-resistant cells from matrine-induced apoptosis.
Discussion
Aberrant activation of β-catenin signaling is
causally linked to the development of resistance to anticancer
drugs (24). It has been reported
that miR-29a-induced resistance to gemcitabine in pancreatic cancer
cells is mediated through activation of the Wnt/β-catenin signaling
pathway (25). In contrast,
inhibition of Wnt/β-catenin signaling reverses multidrug resistance
in cholangiocarcinoma cells (26).
A previous study showed that inhibition of cytoplasmic GSK3β in
A549/CDDP cells leads to activation of Wnt/β-catenin signaling,
consequently increasing cisplatin resistance (9). In line with these studies, our data
demonstrated that the acquisition of cisplatin resistance in NSCLC
cells after chronic exposure to cisplatin was associated with
elevated β-catenin activity. Downregulation of β-catenin via RNA
interference technology reversed drug resistance in A549/CDDP cells
(27), confirming the essential
role for β-catenin activity in cisplatin resistance in NSCLC
cells.
Matrine has been shown to inhibit β-catenin
signaling in hepatoma cells (22).
However, in WB-F344 rat liver epithelial stem-like cells, matrine
has been found to induce β-catenin activation (21). Our data provide initial evidence
that matrine treatment led to impaired β-catenin activation in
cisplatin-resistant NSCLC cells. These findings suggest that the
regulatory effects of matrine on β-catenin signaling are cellular
context-dependent. GSK3β is a pivotal negative regulator of
β-catenin signaling and its phosphorylation at Ser9 decreases its
ability to promote β-catenin degradation (12). We found that matrine-treated cells
showed increased total GSK3β protein and reduced
Ser9-phosphorylated GSK3β protein, coupled with reduced
β-catenin-dependent transcriptional activity. These results
indicate that upregulation of GSK3β accounts for matrine-mediated
inactivation of β-catenin in cisplatin-resistant NSCLC cells.
Apoptosis induction is an important mechanism for
the action of anticancer agents. The proapoptotic activity of
matrine has been described in a variety of cancer cells, such as
lung cancer (19) and
hepatocellular carcinoma cells (28). Our data confirmed that matrine was
also able to induce apoptotic death in cisplatin-resistant NSCLC
cells. The mitochondrial pathway is an important pathway of
apoptosis (29), which involves
loss of ΔΨm and release of several proapoptotic proteins including
cytochrome c from the mitochondrial intermembrane space to
the cytosol, leading to activation of procaspase-9 and -3. Notably,
matrine treatment of cisplatin-resistant NSCLC cells resulted in
dramatic loss of ΔΨm and increased caspase-9 and -3 activities,
indicating activation of the mitochondrial death pathway. In
agreement with our findings, matrine also induced mitochondrial
apoptosis in human acute myeloid leukemia cells (30).
Having identified that matrine suppressed β-catenin
signaling and induced apoptosis in cisplatin-resistant NSCLC cells,
we next checked whether inactivation of β-catenin signaling is
causally linked to the proapoptotic activity of matrine. It has
been documented that activated β-catenin regulates the
transcription of several oncogenic target genes (13). Survivin is an important target gene
of β-catenin and its overexpression induces anticancer drug
resistance (31). In NSCLC,
survivin expression affects the susceptibility to drug-induced cell
apoptosis (32,33). Okamoto et al (32) reported that stable overexpression of
survivin attenuated apoptotic death induced by gefitinib, an
epidermal growth factor receptor-tyrosine kinase inhibitor.
Targeting survivin has been shown to enhance cisplatin sensitivity
in lung cancer xenografts (33).
Notably, we found that matrine treatment resulted in a significant
reduction in survivin expression. Moreover, restoration of survivin
counteracted matrine-induced apoptosis in cisplatin-resistant NSCLC
cells. These findings highlight an important role for survivin in
the regulation of NSCLC cell susceptibility to matrine.
Matrine-induced downregulation of survivin has also been described
in multiple myeloma cell lines (34). Despite the importance of survivin,
we cannot exclude the possibility that other target genes of
β-catenin may have an impact on the anticancer activity of matrine
in NSCLC cells.
In conclusion, matrine has the capacity to induce
mitochondrial apoptosis in cisplatin-resistant NSCLC cells, which
is associated with inactivation of β-catenin/survivin signaling.
Therefore, matrine represents a novel anticancer agent for
overcoming cisplatin resistance in NSCLC.
References
|
1
|
Herbst RS, Heymach JV and Lippman SM: Lung
cancer. N Engl J Med. 359:1367–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Howington JA, Blum MG, Chang AC, Balekian
AA and Murthy SC: Treatment of stage I and II non-small cell lung
cancer: diagnosis and management of lung cancer, 3rd ed: American
College of Chest Physicians evidence-based clinical practice
guidelines. Chest. 143(Suppl 5): e278S–e313S. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peters S, Zimmermann S and Adjei AA: Oral
epidermal growth factor receptor tyrosine kinase inhibitors for the
treatment of non-small cell lung cancer: comparative
pharmacokinetics and drug-drug interactions. Cancer Treat Rev.
40:917–926. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Krzakowski M, Lucas C and Gridelli C:
Fractionated scheme of oral vinorelbine as single-agent therapy or
in combination with cisplatin concomitantly with thoracic
radiotherapy in stage III non-small-cell lung cancer:
dose-escalation phase I trial. Clin Lung Cancer. 15:266–273. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ardizzoni A, Boni L, Tiseo M, et al:
Cisplatin-versus carboplatin-based chemotherapy in first-line
treatment of advanced non-small-cell lung cancer: an individual
patient data meta-analysis. J Natl Cancer Inst. 99:847–857. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
D’Addario G, Pintilie M, Leighl NB, Feld
R, Cerny T and Shepherd FA: Platinum-based versus
non-platinum-based chemotherapy in advanced non-small-cell lung
cancer: a meta-analysis of the published literature. J Clin Oncol.
23:2926–2936. 2005. View Article : Google Scholar
|
|
7
|
Rosell R, Lord RV, Taron M and Reguart N:
DNA repair and cisplatin resistance in non-small-cell lung cancer.
Lung Cancer. 38:217–227. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Galluzzi L, Senovilla L, Vitale I, Michels
J, Martins I, Kepp O, Castedo M and Kroemer G: Molecular mechanisms
of cisplatin resistance. Oncogene. 31:1869–1883. 2012. View Article : Google Scholar
|
|
9
|
Gao Y, Liu Z, Zhang X, He J, Pan Y, Hao F,
Xie L, Li Q, Qiu X and Wang E: Inhibition of cytoplasmic GSK-3β
increases cisplatin resistance through activation of Wnt/β-catenin
signaling in A549/DDP cells. Cancer Lett. 336:231–239. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Doble BW and Woodgett JR: GSK-3: tricks of
the trade for a multi-tasking kinase. J Cell Sci. 116:1175–1186.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Orford K, Crockett C, Jensen JP, Weissman
AM and Byers SW: Serine phosphorylation-regulated ubiquitination
and degradation of β-catenin. J Biol Chem. 272:24735–24738. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cohen P and Goedert M: GSK3 inhibitors:
development and therapeutic potential. Nat Rev Drug Discov.
3:479–487. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kolligs FT, Bommer G and Göke B:
Wnt/beta-catenin/tcf signaling: a critical pathway in
gastrointestinal tumorigenesis. Digestion. 66:131–144. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dvory-Sobol H, Sagiv E, Kazanov D,
Ben-Ze’ev A and Arber N: Targeting the active β-catenin pathway to
treat cancer cells. Mol Cancer Ther. 5:2861–2871. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei Y, Shen N, Wang Z, Yang G, Yi B, Yang
N, Qiu Y and Lu J: Sorafenib sensitizes hepatocellular carcinoma
cells to cisplatin via suppression of Wnt/β-catenin signaling. Mol
Cell Biochem. 381:139–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang WC, Chan CC, Wu SJ, Chen LC, Shen
JJ, Kuo ML, Chen MC and Liou CJ: Matrine attenuates allergic airway
inflammation and eosinophil infiltration by suppressing eotaxin and
Th2 cytokine production in asthmatic mice. J Ethnopharmacol.
151:470–477. 2014. View Article : Google Scholar
|
|
17
|
Yang Y, Xiu J, Zhang X, Zhang L, Yan K,
Qin C and Liu J: Antiviral effect of matrine against human
enterovirus 71. Molecules. 17:10370–10376. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Y, Xu Y, Ji W, Li X, Sun B, Gao Q and
Su C: Anti-tumor activities of matrine and oxymatrine: literature
review. Tumour Biol. 35:5111–5119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Niu H, Zhang Y, Wu B, Zhang Y, Jiang H and
He P: Matrine induces the apoptosis of lung cancer cells through
downregulation of inhibitor of apoptosis proteins and the Akt
signaling pathway. Oncol Rep. 32:1087–1093. 2014.PubMed/NCBI
|
|
20
|
Li Y, Zhang ZN, Zhao HM, Tong ZC, Yang J,
Wang H and Liang XJ: Matrine inhibits the invasive properties of
human osteosarcoma cells by downregulating the ERK-NF-κB pathway.
Anticancer Drugs. 25:1035–1043. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie BS, He XX, Ai ZL and Yao SK:
Involvement of β-catenin in matrine-induced autophagy and apoptosis
in WB-F344 cells. Mol Med Rep. 9:2547–2553. 2014.PubMed/NCBI
|
|
22
|
Guo D, Chen NN, Zhou P, Pan B and Hou LB:
Suppressive effect of matrine on cell growth and decreases
beta-catenin-dependent transcriptional activity in hepatoma cell
line Hep3B. Zhong Yao Cai. 33:778–781. 2010.In Chinese. PubMed/NCBI
|
|
23
|
Jiang Z, Yin J, Fu W, Mo Y, Pan Y, Dai L,
Huang H, Li S and Zhao J: miRNA 17 family regulates
cisplatin-resistant and metastasis by targeting TGFbetaR2 in NSCLC.
PLoS One. 9:e946392014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He K, Xu T, Xu Y, Ring A, Kahn M and
Goldkorn A: Cancer cells acquire a drug resistant, highly
tumorigenic, cancer stem-like phenotype through modulation of the
PI3K/Akt/β-catenin/CBP pathway. Int J Cancer. 134:43–54. 2014.
View Article : Google Scholar
|
|
25
|
Nagano H, Tomimaru Y, Eguchi H, Hama N,
Wada H, Kawamoto K, Kobayashi S, Mori M and Doki Y: MicroRNA-29a
induces resistance to gemcitabine through the Wnt/β-catenin
signaling pathway in pancreatic cancer cells. Int J Oncol.
43:1066–1072. 2013.PubMed/NCBI
|
|
26
|
Shen DY, Zhang W, Zeng X and Liu CQ:
Inhibition of Wnt/β-catenin signaling downregulates P-glycoprotein
and reverses multi-drug resistance of cholangiocarcinoma. Cancer
Sci. 104:1303–1308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Teng Y, Wang X, Wang Y and Ma D:
Wnt/β-catenin signaling regulates cancer stem cells in lung cancer
A549 cells. Biochem Biophys Res Commun. 392:373–379. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou H, Xu M, Gao Y, et al: Matrine
induces caspase-independent program cell death in hepatocellular
carcinoma through bid-mediated nuclear translocation of apoptosis
inducing factor. Mol Cancer. 13:592014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Green DR and Kroemer G: The
pathophysiology of mitochondrial cell death. Science. 305:626–629.
2014. View Article : Google Scholar
|
|
30
|
Zhang S, Zhang Y, Zhuang Y, Wang J, Ye J,
Zhang S, Wu J, Yu K and Han Y: Matrine induces apoptosis in human
acute myeloid leukemia cells via the mitochondrial pathway and Akt
inactivation. PLoS One. 7:e468532012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheung CH, Huang CC, Tsai FY, et al:
Survivin - biology and potential as a therapeutic target in
oncology. Onco Targets Ther. 6:1453–1462. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Okamoto K, Okamoto I, Okamoto W, Tanaka K,
Takezawa K, Kuwata K, Yamaguchi H, Nishio K and Nakagawa K: Role of
survivin in EGFR inhibitor-induced apoptosis in non-small cell lung
cancers positive for EGFR mutations. Cancer Res. 70:10402–10410.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tian H, Liu S, Zhang J, et al: Enhancement
of cisplatin sensitivity in lung cancer xenografts by
liposome-mediated delivery of the plasmid expressing small hairpin
RNA targeting Survivin. J Biomed Nanotechnol. 8:633–641. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu Q, Chen B, Zhang X, Qian W, Ye B and
Zhou Y: Arsenic trioxide-enhanced, matrine-induced apoptosis in
multiple myeloma cell lines. Planta Med. 79:775–781. 2013.
View Article : Google Scholar : PubMed/NCBI
|