Introduction
A specific subpopulation of cancer cells, called
cancer stem cells (CSCs), was recently proposed to be responsible
for tumorigenesis and heterogeneity in primary cancers. This
behavior was attributed to the extensive self-renewal activities
and multipotent differentiation abilities of the CSCs (1). Furthermore, CSCs have enhanced ability
to invade surrounding tissues by undergoing
epithelial-to-mesenchymal transition (EMT), which results in local
and distant metastasis (2). CSCs
are also regarded as the cause of failure of chemotherapy and
radiotherapy, leading to the recurrence of tumors (3,4).
Therefore, the isolation and identification of CSCs in vitro
are important and basic processes for the development of novel
strategies to treat cancer in vivo by targeting CSCs.
Specifically, CSCs express a series of proteins
including the sex-determining region Y-box 2 (SOX-2),
octamer-binding transcription factor 4 (OCT-4), CD133 (also known
as prom-inin-1, PROM1) and nestin (5–10).
CSCs also display enhanced ability for EMT and some transcription
factors, such as TWIST, regulate EMT progress involved in
metastasis (11,12). CD44 and CD133 surface marker
expression have been widely used to isolate CSCs in vitro
using specific antibodies and fluorescence-activated cell sorting
(FACS) (4,13). However, the specificity and
efficiency of CD133 in the enrichment of CSCs concerning stemness
and self-renewal are complex and controversial (10). For example, neither the expression
of stemness genes nor the self-renewal capacities of
CD133+ and CD133− cells were significantly
different between freshly isolated glioma and glioma sphere
cultures (14). It has been
demonstrated that CSCs can proliferate under serum-free culture
conditions in a non-adherent manner to form sphere-like structures
(15–22). Thus, the culture of cancer cells
under non-adherent conditions to allow sphere formation and/or
evaluation of the expression of CSCs markers are the simplest and
most convenient ways for enriching and identifying CSCs.
To induce anchorage-independent growth in
vitro, various culture plate surface conditions have been
applied with several supplemental factors, including epidermal
growth factor (EGF) and basic fibroblast growth factor (bFGF)
(23). The surface of standard
cell/tissue culture plates is negatively charged and hydrophilic,
and is widely used with diverse cell cultures, while poly
(2-hydroxyethyl methacrylate) (poly-HEMA)-coated, agar-coated, or
ultra-low attachment plates have been used in in vitro
studies to investigate anchorage-independent growth of cells
including CSCs (15–22). Agar plates are also used for
anoikis, a form of programmed cell death that is induced by
detachment of anchorage-dependent cells from the surrounding
extracellular matrix (ECM) (24–27).
The ultra-low attachment surface has a neutral, hydrophilic
hydrogel coating that reduces binding of attachment proteins. This
surface is useful to maintain cells in a suspended, unattached
state, thereby preventing stem cells from attachment-mediated
differentiation and preventing anchorage-dependent cells from
dividing (28). Cells are usually
grown as monolayers on normal culture plates where CSCs can
proliferate and form spheres during long-term culture (~2–3 weeks)
under the three conditions noted above.
The malignant clinical outcome of glioblastoma is
based on the aggressive invasion ability of cancer cells into the
adjacent normal brain parenchyma, which eventually affects normal
brain function despite standard therapies, such as radiotherapy
(21,29). Therefore, defining these
characteristics of CSCs of glioblastoma that survive or proliferate
even under non-adherent conditions is critical in strategies for
the treatment of glioblastoma. Silginer et al (30) noted that poly-HEMA-mediated
detachment promoted cell death of glioblastoma cells in a manner
similar to integrin inhibition. Although Hong et al
(31) reported that the spherical
characteristic of CSCs was observed for long-term cultured
glioblastoma cells in medium with or without serum, the combined
effects of different surface and media compositions with or without
serum on the expression of genes involved in stemness and EMT are
not well understood.
The present study explored how A172 glioblastoma
cells behave under different culture conditions, i.e., serum
deprivation and on plates with different surface types. We examined
whether these culture conditions affected gene expression in
relation to stemness- and EMT-related genes together with
viability, cell cycle and ROS generation.
Materials and methods
Cell culture
The human glioblastoma cell line A172 was purchased
from American Type Culture Collection (ATCC; Manassas, VA, USA) and
maintained in Dulbecco’s modified Eagle’s medium (DMEM; HyClone,
Logan, UT, USA) containing 10% heat-inactivated fetal bovine serum
(FBS), 100 U/ml penicillin and 100 mg/ml streptomycin at 37°C in a
5% CO2 atmosphere. A172 cells were maintained in the
above medium (Nm) and grown as monolayers on polystyrene-treated
tissue culture plates (NP). The cells (1×105 cells) were
cultured under the following conditions for 72 h: i) Nm or
serum-free glioblastoma sphere medium (GBM) containing EGF (10
ng/ml) and bFGF (10 ng/ml) (both from R&D Systems, Minneapolis,
MN, USA) as previously described (10,23),
and ii) NP, ultra-low attachment plates (UP; Corning, Tewksbury,
MA, USA), 20 mg/ml poly-HEMA-coated plates (HP) and 1% agar-coated
plates (AP). The morphology of cells was examined using an inverted
phase contrast microscope (x200, magnification). To observe the
effects of ROS scavengers on the induction of SOX-2, cells were
treated for 72 h with 10 mM N-acetyl-l-cysteine (NAC;
Sigma-Aldrich, St. Louis, MO, USA).
Plate preparation
Non-coated 6-well plates were coated with 1 ml of
poly-HEMA (20 mg/ml; Sigma-Aldrich), dried overnight at room
temperature, and washed twice with phosphate-buffered saline (PBS)
before use. For the agar plates, 1% agar (Sigma-Aldrich) solution
in PBS was added to 6-well plates and allowed to solidify for 1 h.
The ultra-low attachment plates were purchased from Corning
(Corning, NY, USA) and the polystyrene tissue culture plates were
from SPL Life Sciences (Pocheon, Gyeonggi-do, korea).
Cell viability assay
Cell proliferation was assessed as a function of
metabolic activity using an EZ-Cytox Cell Viability Assay kit
(ItsBio, Seoul, korea). The assay is based on reduction of
tetrazolium chloride to the water-soluble formazan by
succinate-tetrazolium reductase, which forms part of the
mitochondrial respiratory chain. After treatment with 20
µl/well, cells were incubated for 2 h at 37°C in a 5%
CO2 atmosphere. Absorbance was measured on a microplate
reader (Bio-Rad 680; Bio-Rad, Hercules, CA, USA) at 450 nm. After
subtraction of the background, the viability was determined as the
ratio relative to the control and reported as the mean ± standard
error (SE).
Quantitative real-time polymerase chain
reaction (qRT-PCR)
Total RNA was isolated using AccuZol (Bioneer,
Daejeon, korea) and first-strand cDNA was synthesized by reverse
transcription with 1 µg of total RNA using a ReverTra Ace
qPCR kit (Toyobo, Osaka, Japan). Quantitative real-time PCR was
performed to validate the expression level using SYBR®
Premix Ex Taq™ (Takara Bio, Otsu, Shiga, Japan) with specific
primers (Table I) on an Applied
Biosystems 7300 machine (Applied Biosystems, Carlsbad, CA, USA).
The relative values for specific mRNA were calculated after
normalization to the Ct value of β-actin in the same sample using
the 2−ΔΔCt method.
 | Table IPrimers for quantitative real-time
PCR. |
Table I
Primers for quantitative real-time
PCR.
| Gene | Sequence
(5′-3′) | Product (bp) |
|---|
| SOX-2 | Forward:
5′-TACCTCTTCCTCCCACTCCA-3′
Reverse: 5′-ACTCTCCTCTTTTGCACCCC-3′ | 268 |
| Nestin | Forward:
5′-CGGTGGCTCCAAGACTTCC-3′
Reverse: 5′-GGCACAGGTGTCTCAAGGGTA-3′ | 156 |
| OCT-4 | Forward:
5′-CAGCGACTATGCACAACGAGA-3′
Reverse: 5′-GCCCAGAGTGGTGACGGA-3′ | 196 |
| CD133 | Forward:
5′-ACCCAACATCATCCCTGTTCTT-3′
Reverse: 5′-AGCTCTTCAAGGTGCTGTTCATG-3′ | 100 |
| SNAIL | Forward:
5′-CCTCCCTGTCAGATGAGGAC-3′
Reverse: 5′-CCAGGCTGAGGTATTCCTTG-3′ | 235 |
| ZEB1 | Forward:
5′-GCCAATAAGCAAACGATTCTG-3′
Reverse: 5′-TTTGGCTGGATCACTTTCAAG-3′ | 100 |
| ZEB2 | Forward:
5′-TTCCTGGGCTACGACCATAC-3′
Reverse: 5′-TGTGCTCCATCAAGCAATTC-3′ | 159 |
| TWIST | Forward:
5′-GGAGTCCGCAGTCTTACGAG-3′
Reverse: 5′-TCTGGAGGACCTGGTAGAGG-3′ | 200 |
| SLUG | Forward:
5′-GGGGAGAAGCCTTTTTCTTG-3′
Reverse: 5′-TCCTCATGTTTGTGCAGGAG-3′ | 157 |
| N-cadherin | Forward:
5′-ACAGTGGCCACCTACAAAGG-3′
Reverse: 5′-CCGAGATGGGGTTGATAATG-3′ | 200 |
| Vimentin | Forward:
5′-GAGAACTTTGCCGTTGAAGC-3′
Reverse: 5′-GCTTCCTGTAGGTGGCAATC-3′ | 162 |
| β-actin | Forward:
5′-AGTACTCCGTGTGGATCGGC-3′
Reverse: 5′-GCTGATCCACATCTGCTGGA-3′ | 67 |
Cell cycle analysis
Spheres were trypsinized for 5 min at 37°C in a 5%
CO2 atmosphere, neutralized with complete medium
containing FBS, and centrifuged at 3,000 × g for 5 min. Single
cells were then fixed with 70% ethanol at −20°C overnight and
incubated with a solution containing 5 µg/ml RNase A and 10
µg/ml propidium iodide (PI) (both from Sigma-Aldrich) for 30
min at room temperature. After washing twice with cold PBS, the
cells were analyzed using a FACSCalibur (Becton-Dickinson, Bedford,
MA, USA). Mean fluorescence intensities were obtained using
CellQuest software (Becton-Dickinson).
Reactive oxygen species (ROS)
measurement
Intracellular ROS levels in cells were measured
using 5-(and-6)-car-boxy-2′,7′-dichlorodihydrofluorescein diacetate
(H2DCF-DA; Invitrogen, Carlsbad, CA, USA). Briefly, the
cells were washed in PBS, trypsinized, and then neutralized with
PBS-containing FBS. After two washes with PBS and further
centrifugation, the cells were resuspended in PBS with 20µM
DCF-DA and incubated for 20 min in the dark at 37°C. After the
cells were resuspended in PBS, the fluorescence was measured using
a FACSCalibur (excitation at 488 nm and emission at 515–545 nm).
The mean fluorescence intensity for 10,000 cells/sample was
determined using CellQuest software.
Western blot analysis
Cells were washed twice with PBS and lysed with RIPA
buffer (150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1%
sodium dodecyl sulfate, 50 mM Tris-HCl and pH 8.0) with protease
inhibitor (Roche Diagnostics, Penzberg, Germany) on ice for 30 min.
Protein concentration was determined using a BCA Protein Assay kit
(Pierce Biotechnology, Rockford, IL, USA). Equal amounts of protein
were separated by 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred onto nitrocellulose
membranes (Millipore, Billerica, MA, USA). The membranes were
incubated for 1 h with 5% dried skim milk in TBST (20 mM Tris, 137
mM NaCl and 0.1% Tween 20) buffer and then incubated with primary
antibodies to SOX-2 (Santa Cruz Biotechnology, Santa Cruz, CA,
USA), SOD1 (Enzo Life Sciences, Farmingdale, NY, USA) and SOD2
(Abcam, Cambridge Science Park, Cambridge, UK). Anti-β-actin
antibody (Sigma-Aldrich) was used as an internal control. After
incubation with horseradish peroxidase-conjugated secondary IgG
(Santa Cruz Biotechnology), the immunoreactive bands were
visualized on an enhanced chemiluminescence substrate (Thermo
Fisher Scientific, Waltham, MA, USA). For quantification of the
relative protein levels, densitometric analysis was carried out
using Image J software (National Institutes of Health, Bethesda,
MD, USA). The expression ratio relative to the control was
calculated after normalization with the intensity of β-actin from
each group.
Statistics
Student’s t-test was used for comparison of
differences between two groups. Each experiment was repeated at
least three times. In all analyses, P<0.05 was taken to indicate
statistical significance.
Results
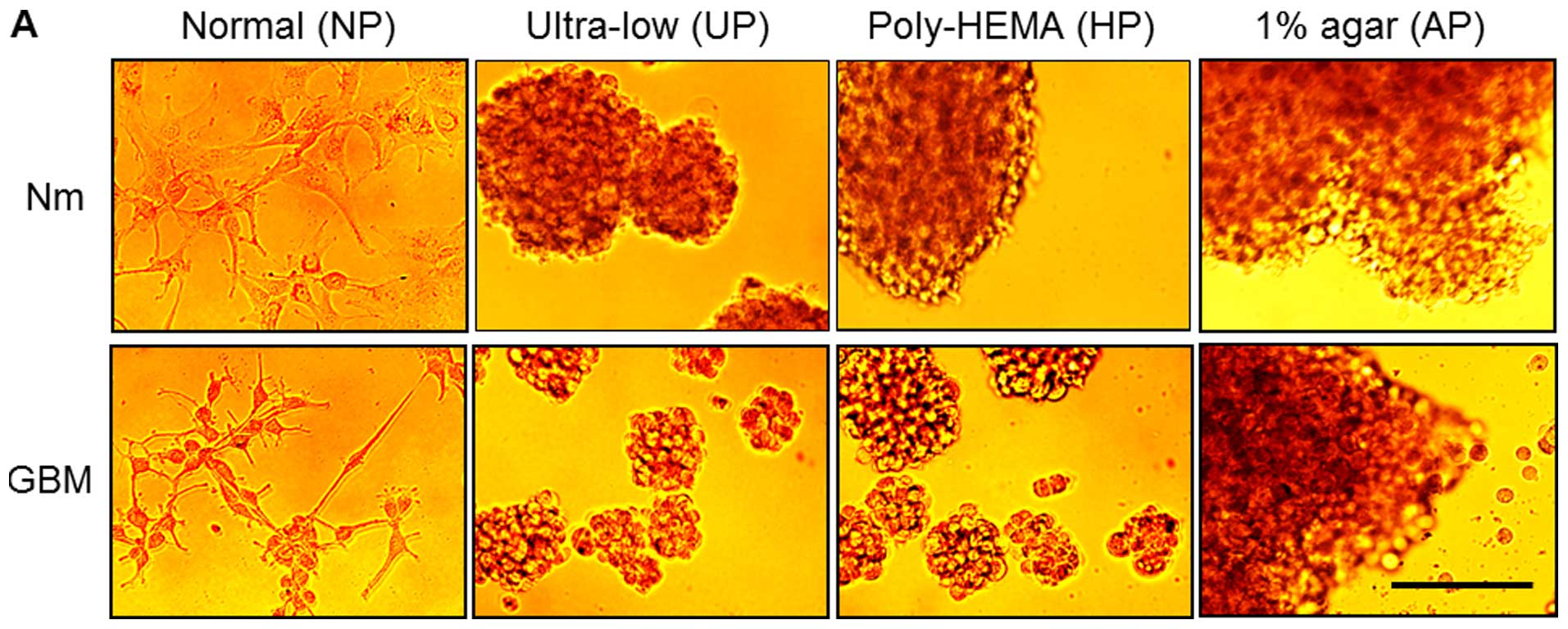
Cells have distinct morphologies and
adherence patterns under various cell culture conditions
A172 cells were cultured for 72 h in various culture
environments differing in plate and media conditions, as described
in the Materials and methods. The morphologies of cells from each
group were examined under an inverted microscope. Fig. 1A shows that the A172 cells in Nm on
NP (designated as controls) grew as a monolayer, while those in
serum-free medium supplemented with growth factors (GBM, 10 ng/ml
bFGF and EGF) on NP appeared loosely attached on the plates with
some cells showing stellate projections. In contrast, cells
cultured on the other plates (UP, HP and AP) in the Nm condition
formed floating aggregates, while those cultured on UP and HP
formed sphere-like colonies in GBM. To examine whether they could
re-adhere to NP, cells or colonies from each group were dissociated
using trypsin and seeded onto NP under the same media conditions.
After 4 h, most cells cultured in Nm on NP, UP, HP and AP showed
stable adherence to NP, whereas those cultured in GBM showed
delayed adherence to NP irrespective of the previous plate
conditions (Fig. 1B). After 24 h,
most cells in Nm or GBM on the four surface types adhered to NP,
but those cultured in GBM showed increased populations with
elongated and fibroblast-like morphologies (Fig. 1C).
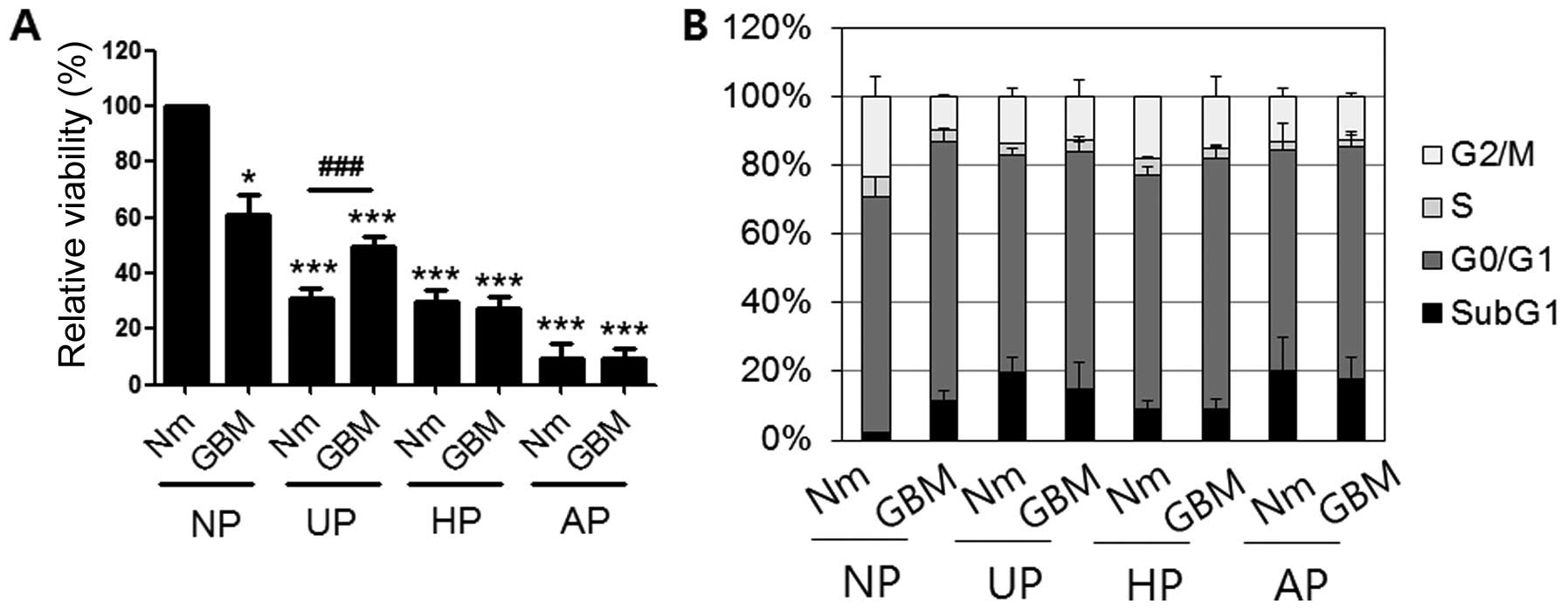
Cell viability decreased and subG1
proportion increased under conditions of serum-deprivation and
non-adhesion
To examine cellular viability under the various
conditions, equal numbers of cells from each condition were seeded
on plates (1×104/well) and incubated with tetrazolium
chloride to monitor mitochondrial reductase activity. Fig. 2A shows that the relative viability
of cells in GBM on NP (61±6.9%) was ~40% lower than that of the
control. Moreover, cells in Nm and GBM on three types of plates
(UP, HP and AP) had significantly lower activities than those in
GBM on NP. Specifically, cellular activity on AP was the lowest
among the conditions examined (9.3±5.32% in Nm and 9.39±3.88% in
GBM). We also performed cell cycle analysis by flow cytometry after
staining the cells with PI (Fig.
2B). Compared with control cells (2.05±0.03%), there was a ~10%
increase in the subG1 proportion of cells under GMB culture
conditions (GBM/NP, 11.4±3.2%) together with decreased G2/M phase.
However, on the other plates, incubation in GBM did not increase
the subG1 proportion (Nm/UP, 19.88±3.96%; GBM/UP, 14.64±8.12%;
Nm/HP, 9.16±2.22%; GBM/HP, 8.82±3.33%; Nm/AP, 20.13±9.83%; and
GBM/AP, 17.76±6.58%).
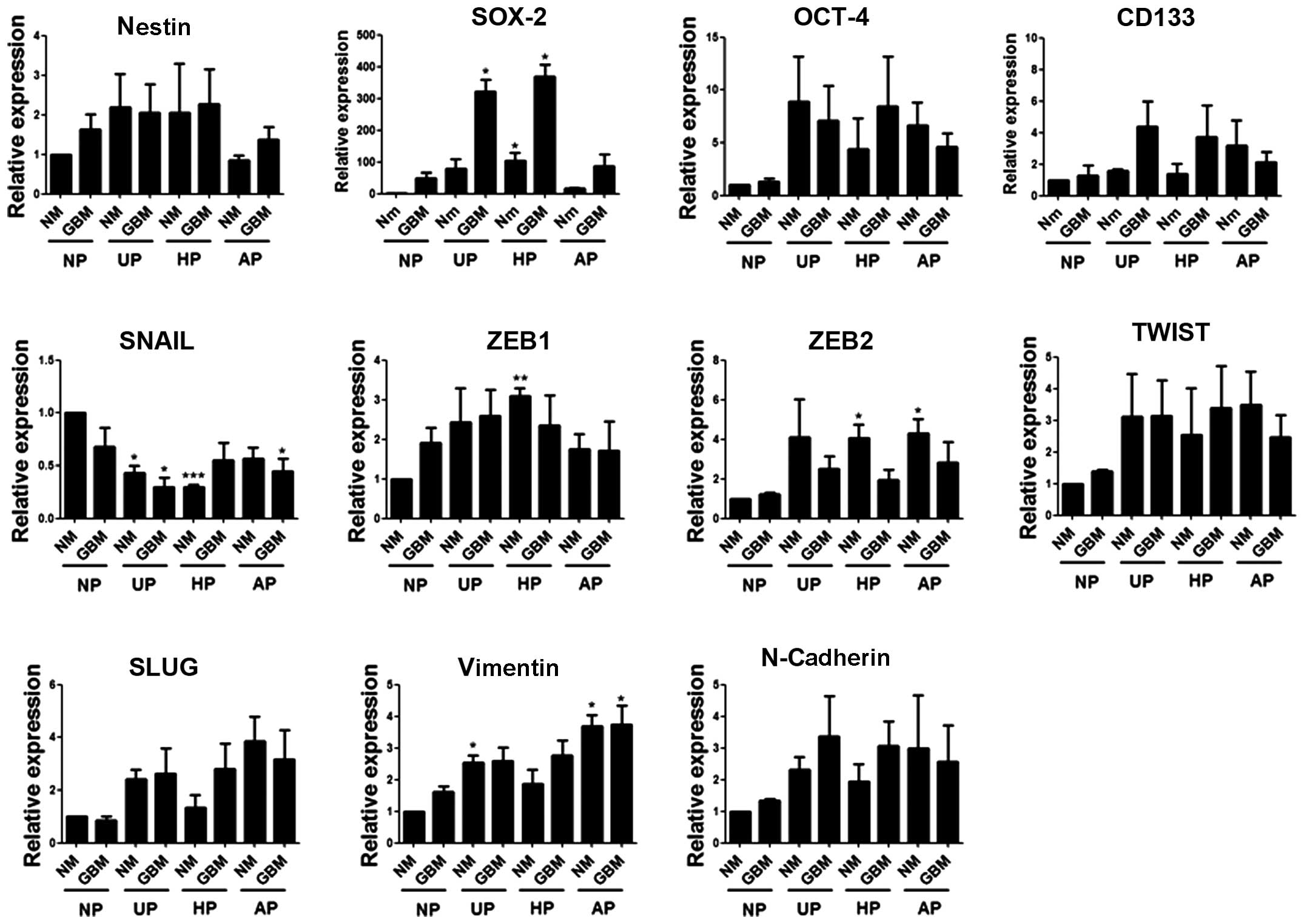
SOX-2 mRNA levels increased markedly in
GBM on UP and HP
To determine whether combined culture conditions
affected the self-renewal and EMT abilities of these cells, the
expression levels of four stem cell markers and seven EMT-related
markers were examined by real-time PCR using specific primers
(Table I). Compared with the
control, SOX-2 mRNA levels showed the greatest increase in GBM on
both UP and HP (fold-change: 321 and 369, respectively). CD133
expression also increased in GBM on UP and HP but to a lesser
extent, which was not statistically significant. Among the EMT
markers, SNAIL expression decreased on three plate types (UP, HP
and AP). The mRNA levels of the other EMT markers appeared to be
dependent on the plate type rather than the medium type (Fig. 3).
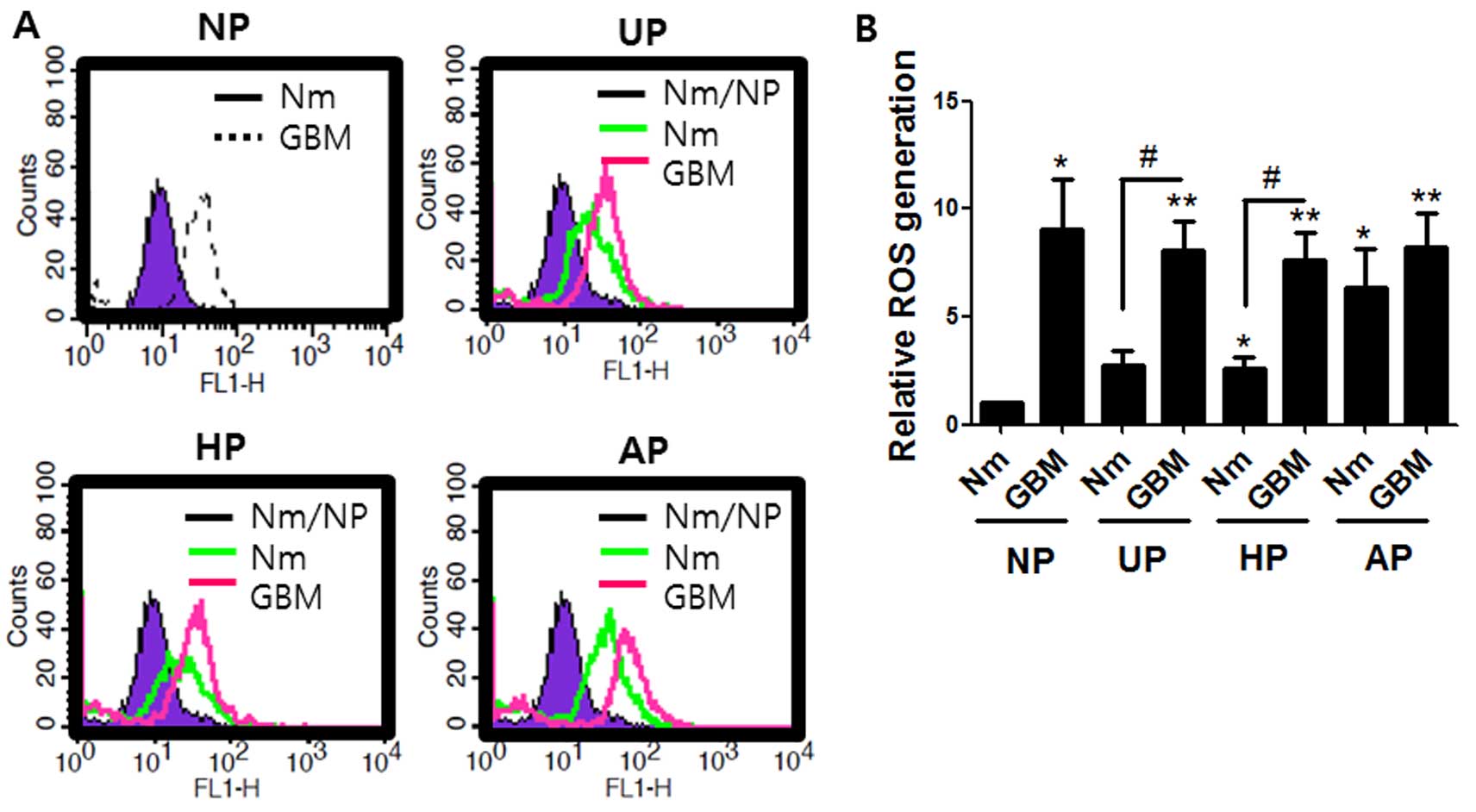
Both serum-deprivation and non-adherent
conditions enhanced ROS accumulation
The effects of different culture conditions on ROS
levels were investigated because ROS may be an important factor in
regulation of stem cells and the chemoresistance of cancer stem
cells (32–34). Performance was assessed by the
conversion of non-fluorescent H2DCF to the fluorescent
form (35). Fig. 4 shows that the level of ROS
generation in the cells cultured on UP or HP in normal medium was
about double that in those cultured on normal plates. Serum
deprivation GBM on UP or HP further increased ROS generation by
8-fold. Interestingly, ROS levels from cells cultured in both Nm
and GBM on AP were 6- and 8-fold higher, respectively, than the
control. These data indicate that the effect of serum deprivation
on ROS accumulation is greater than that of the non-adherent
condition.
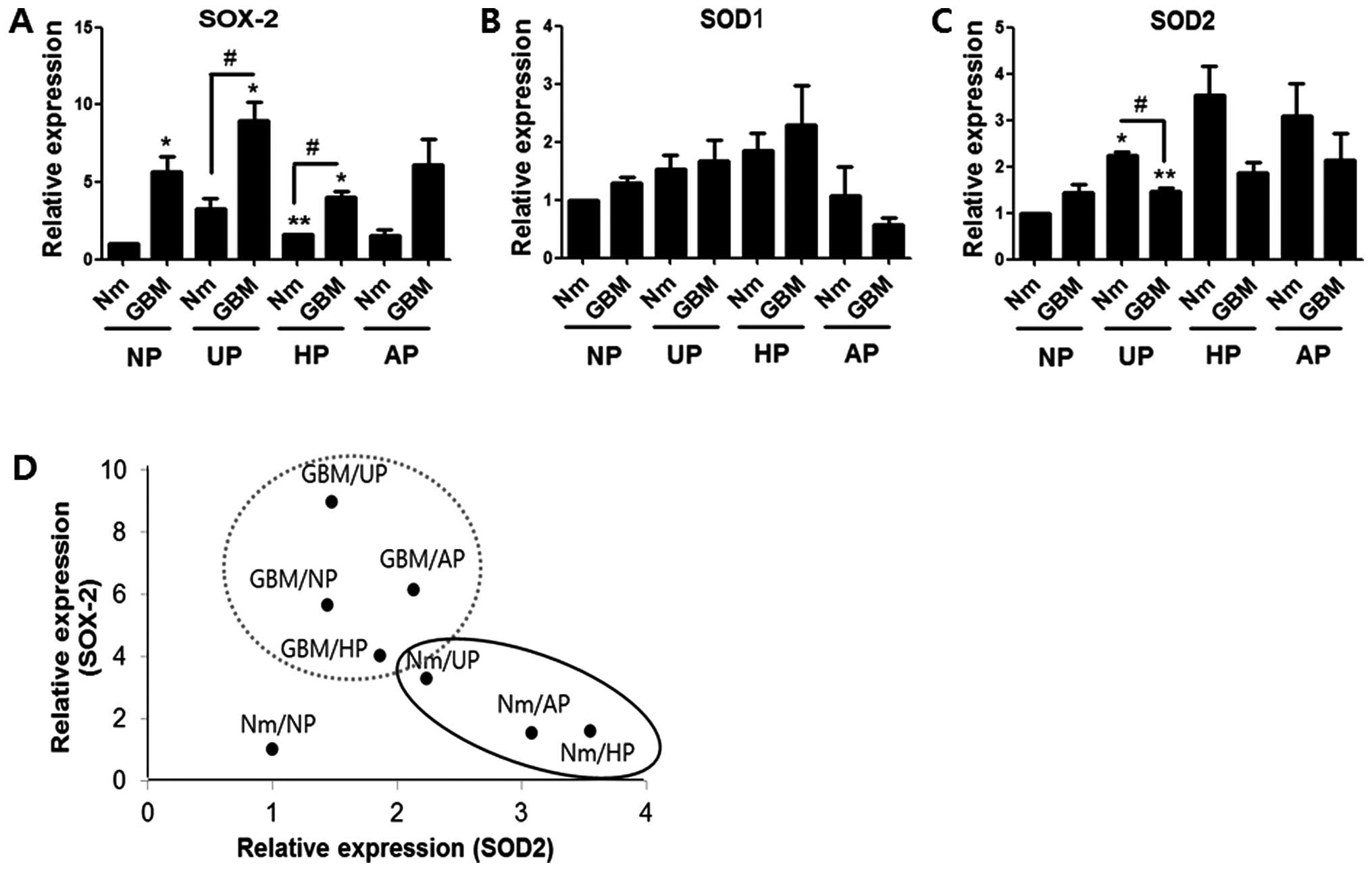
SOD2 expression was induced under
non-adherent conditions
As ROS generation was increased by serum
deprivation, which was also effective for SOX-2 induction, we
examined whether alterations in SOD1 and SOD2 expression were
responsible for ROS accumulation and SOX-2 induction under serum
deprivation or non-adherent conditions. Western blotting indicated
that the level of SOX-2 expression was higher in the GBM condition
than in normal medium on all four plate types, which was similar to
the mRNA profile (Fig. 5A). While
SOD1 expression showed no prominent pattern with respect to serum
deprivation or plate condition, the SOD2 expression profile
exhibited an inverse relationship with that of SOX-2 (Fig. 5B and C). However, with the exception
of values from UP in Nm or GBM, the correlations were not
statistically significant. Dot plot analysis was performed using
the mean values of the SOX-2 and SOD2 protein levels. Fig. 5D shows that the two protein values
from each group could be classified into a significantly larger
SOX-2-expressing group (gray-dotted circle; GBM on three plate
types) and a SOD2-expressing group (black ellipse; Nm on UP, HP and
AP). SOX-2 expression was shown to be dependent on the GBM
condition, while SOD2 expression was dependent on the non-adherent
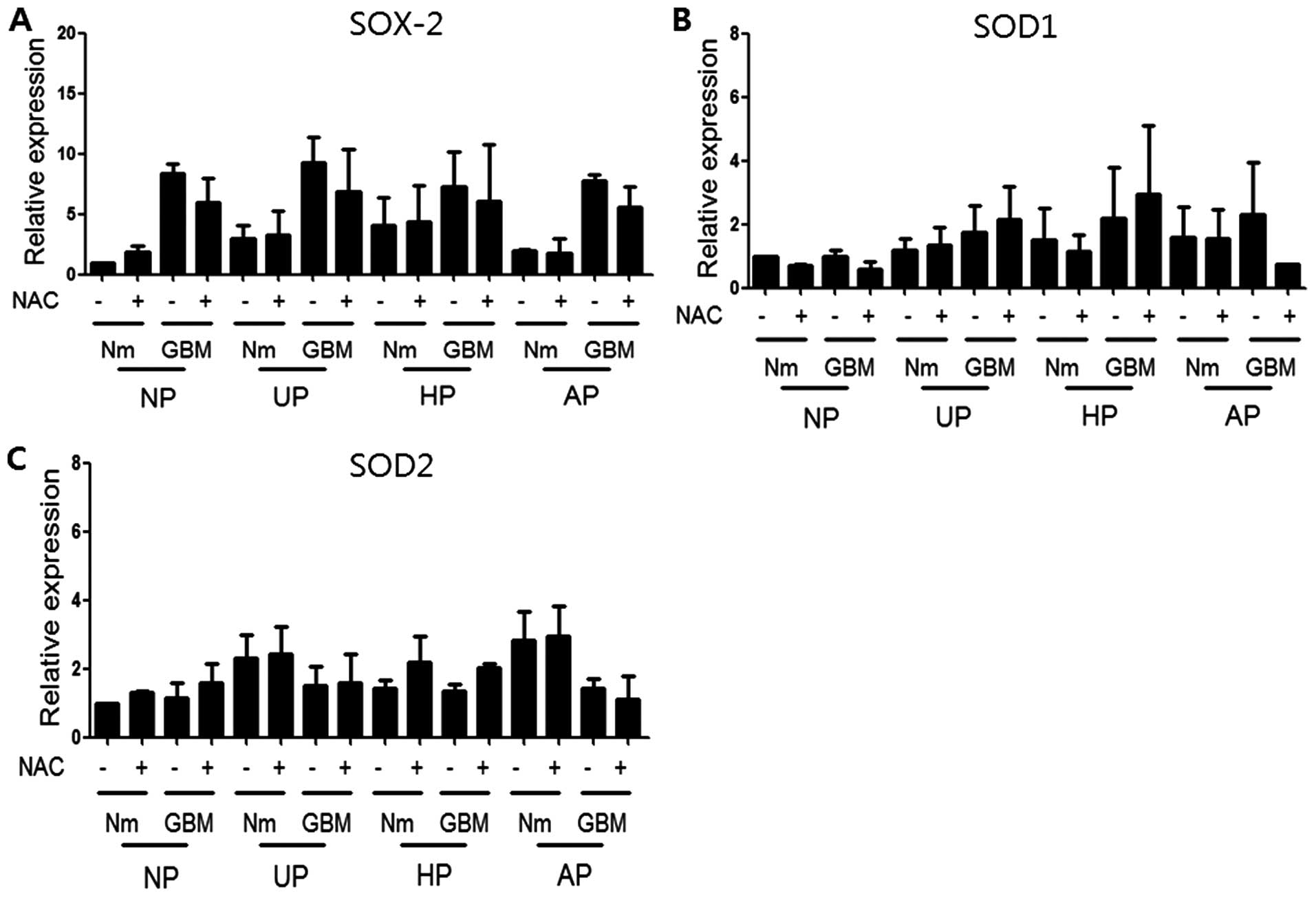
conditions. Finally, cells were treated with NAC, a general ROS
scavenger, to determine whether NAC could reverse the SOX-2
induction observed under our culture conditions. Western blotting
was performed using antibodies to SOX-2, SOD1, and SOD2 after
treatment with 10 mM NAC under the same conditions for 72 h.
Fig. 6 shows that NAC treatment did
not block the induction of SOX-2 or SOD2, suggesting that it was
independent of ROS. Data from qRT-PCR analyses also revealed no
significant recovery of SOX-2 with NAC treatment (data not
shown).
Discussion
The isolation and enrichment of CSCs in vitro
are important for targeting strategies and drug screening for
anticancer therapy. It would be beneficial to be able to
characterize cancer cells under different culture conditions in
vitro to better understand the features of CSCs. This study
examined the combined effects of serum deprivation and culture
plate surface type on the morphology, viability, and markers for
stemness and EMT in A172 glioblastoma cells. These cells were
previously shown to have aggressive migration ability and ROS
accumulation in response to appropriate stimuli (36,37).
In the presence of serum, A172 cells grew as a
monolayer while those on UP, HP and AP did not attach to the bottom
and formed colonies, which led to decreased viability and increased
apoptosis (Figs. 1A and 2A and B). However, most of the cells from
each plate regained the adhesion ability and apparent viability
when allowed to re-adhere to NP after 72 h of culture on each
plate. These observations indicated that the interaction with the
extracellular matrix (ECM) is an important prerequisite for the
survival of cancer cells (Fig.
1A–C). The results also demonstrated that incubation of cells
on various plates in serum-free GBM did not further affect the
viability, except on NP, but delayed the re-adhesion on NP with an
appearance of more elongated phenotypes after 72 h of culture. This
delay is attributed to vitronectin and fibronectin in serum
(38). It is plausible that the
serum-free condition is less critical to the maintenance of
viability but is more effective than the non-adherent condition for
enrichment of cancer stem cell-like cells.
Our results based on the mitochondrial reductase
activity and subG1 fractions were inconsistent for the evaluation
of cell viability. This indicated that the reduced viability
estimated by mitochondrial reductase activity did not necessarily
represent apoptotic cells but rather the metabolically inactive
status, which could be stimulated by environmental changes. These
results also reinforce the heterogeneous and dynamic nature of
cancer cells, which can show different responses under these harsh
conditions. It is significant that cell incubation on AP exhibited
the lowest activity in mitochondrial reductase and highest
proportions of subG1 and highest ROS accumulation among the culture
plate conditions, and that serum deprivation did not have
considerable effects. Therefore, culture on AP rather than HP in
the presence of serum seemed be the most efficient way to induce
anoikis, which is an important defense mechanism for preventing the
anchorage-independent survival of tumor cells (39).
SOX-2 was significantly increased by the GBM
condition, and was especially prominent on UP and HP at both mRNA
and protein levels (Figs. 3B and
5). These results support the
importance of serum-free conditions on enrichment of CSCs. Our
findings were consistent with the previous report that serum
deprivation-induced bone marrow stem cells show upregulated
expression of stemness-related protein, such as SOX-2 (40).
SOX-2 has been suggested to be a marker of stem
cells (7) and CSCs (41,42).
Thus, culture of cancer cells under conditions of serum deprivation
on UP or HP could be useful for the isolation and enrichment of
SOX-2-expressing CSCs. The expression of some EMT-related genes,
such as ZEB2, TWIST and VIMENTIN, was induced by non-adherent
conditions rather than by serum deprivation. However, these genes
were expressed with different statistical significances, which
suggested that the acquisition of both stemness and EMT properties
could be provided to cancer cells only by very subtle and complex
changes in the environment.
Fig. 4 shows that
significantly higher ROS levels were observed in GBM on NP as well
as on non-adherent plates (UP, HP and AP). The higher levels seemed
to be more dependent on serum deprivation than on the presence of a
non-adherent surface. ROS is a critical factor for maintaining
stemness by the expression of SOX-2 (43), and SOD2 promotes migration and
invasion and protects cells from ROS-mediated cell death (44–46).
Therefore, we hypothesized that a ROS scavenger would block SOX-2
induction under our cell culture conditions. NAC treatment,
however, did not prevent SOX-2 levels at the mRNA (data not shown)
or protein level (Fig. 6A). These
results indicated that induction of SOX-2 was not dependent on ROS
under our conditions. However, the induction profiles of SOX-2 with
SOD2 under different culture conditions suggested some influence of
SOD2 on ROS and SOX-2 induction. This indication merits further
investigation.
Although Sauerzweig et al (40) suggested that serum deprivation is an
enrichment process for CSCs, growth factors are also required to
maintain the self-renewal of brain tumor stem cells (23). Therefore, we could not exclude the
effects of the growth factors EGF and bFGF, which were included in
GBM in the present study, on the expression of SOX-2 as well as on
ROS accumulation. Another limitation of our study was that the
viability and expression of markers for CSC and EMT were examined
after only 72 h of incubation; this is probably insufficient time
to fully select the CSCs. Therefore, the effects of serum
deprivation only and long-term culture on different plate
conditions should also be examined to determine the most efficient
culture conditions for CSCs.
Acknowledgments
This research was supported by the Basic Science
Research Program through the National Research Foundation of korea
(NRF) funded by the Ministry of Science, ICT and Future Planning
(2014R1A1A1006961 and 2012R1A5A2047939).
References
|
1
|
Jordan CT, Guzman ML and Noble M: Cancer
stem cells. N Engl J Med. 355:1253–1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kapoor A and Kumar S: Cancer stem cell: A
rogue responsible for tumor development and metastasis. Indian J
Cancer. 51:282–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chinn SB, Darr OA, Peters RD and Prince
ME: The role of head and neck squamous cell carcinoma cancer stem
cells in tumori-genesis, metastasis, and treatment failure. Front
Endocrinol (Lausanne). 3:902012.
|
|
4
|
Sahlberg SH, Spiegelberg D, Glimelius B,
Stenerlöw B and Nestor M: Evaluation of cancer stem cell markers
CD133, CD44, CD24: Association with AkT isoforms and radiation
resistance in colon cancer cells. PLoS One. 9:e946212014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He J, Shan Z, Li L, Liu F, Liu Z, Song M
and Zhu H: Expression of glioma stem cell marker CD133 and
O6-methylguanine-DNA methyltransferase is associated
with resistance to radiotherapy in gliomas. Oncol Rep.
26:1305–1313. 2011.PubMed/NCBI
|
|
6
|
Tam WL and Ng HH: Sox2: Masterminding the
root of cancer. Cancer Cell. 26:3–5. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen S, Choo AB, Nai-Dy W, Heng-Phon T and
Oh SK: Knockdown of Oct-4 or Sox-2 attenuates neurogenesis of mouse
embryonic stem cells. Stem Cells Dev. 16:413–420. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chiou SH, Yu CC, Huang CY, Lin SC, Liu CJ,
Tsai TH, Chou SH, Chien CS, Ku HH and Lo JF: Positive correlations
of Oct-4 and Nanog in oral cancer stem-like cells and high-grade
oral squamous cell carcinoma. Clin Cancer Res. 14:4085–4095. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ishiwata T, Teduka K, Yamamoto T, Kawahara
K, Matsuda Y and Naito Z: Neuroepithelial stem cell marker nestin
regulates the migration, invasion and growth of human gliomas.
Oncol Rep. 26:91–99. 2011.PubMed/NCBI
|
|
10
|
Jung MJ, Rho JK, Kim YM, Jung JE, Jin YB,
Ko YG, Lee JS, Lee SJ, Lee JC and Park MJ: Upregulation of CXCR4 is
functionally crucial for maintenance of stemness in drug-resistant
non-small cell lung cancer cells. Oncogene. 32:209–221. 2013.
View Article : Google Scholar
|
|
11
|
Smit MA, Geiger TR, Song JY, Gitelman I
and Peeper DS: A Twist-Snail axis critical for TrkB-induced
epithelial-mesen-chymal transition-like transformation, anoikis
resistance, and metastasis. Mol Cell Biol. 29:3722–3737. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng H and Kang Y: Multilayer control of
the EMT master regulators. Oncogene. 33:1755–1763. 2014. View Article : Google Scholar
|
|
13
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
14
|
Clément V, Dutoit V, Marino D, Dietrich PY
and Radovanovic I: Limits of CD133 as a marker of glioma
self-renewing cells. Int J Cancer. 125:244–248. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Davies MA, Lu Y, Sano T, Fang X, Tang P,
LaPushin R, Koul D, Bookstein R, Stokoe D, Yung WK, et al:
Adenoviral transgene expression of MMAC/PTEN in human glioma cells
inhibits Akt activation and induces anoikis. Cancer Res.
58:5285–5290. 1998.PubMed/NCBI
|
|
16
|
Minett TW, Tighe BJ, Lydon MJ and Rees DA:
Requirements for cell spreading on polyHEMA coated culture
substrates. Cell Biol Int Rep. 8:151–159. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fiucci G, Ravid D, Reich R and Liscovitch
M: Caveolin-1 inhibits anchorage-independent growth, anoikis and
invasiveness in MCF-7 human breast cancer cells. Oncogene.
21:2365–2375. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie TX, Zhou G, Zhao M, Sano D, Jasser SA,
Brennan RG and Myers JN: Serine substitution of proline at codon
151 of TP53 confers gain of function activity leading to anoikis
resistance and tumor progression of head and neck cancer cells.
Laryngoscope. 123:1416–1423. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gomez-Casal R, Bhattacharya C, Ganesh N,
Bailey L, Basse P, Gibson M, Epperly M and Levina V: Non-small cell
lung cancer cells survived ionizing radiation treatment display
cancer stem cell and epithelial-mesenchymal transition phenotypes.
Mol Cancer. 12:942013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Krishnamurthy S, Dong Z, Vodopyanov D,
Imai A, Helman JI, Prince ME, Wicha MS and Nör JE: Endothelial
cell-initiated signaling promotes the survival and self-renewal of
cancer stem cells. Cancer Res. 70:9969–9978. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kesanakurti D, Chetty C, Rajasekhar
Maddirela D, Gujrati M and Rao JS: Functional cooperativity by
direct interaction between PAk4 and MMP-2 in the regulation of
anoikis resistance, migration and invasion in glioma. Cell Death
Dis. 3:e4452012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo D, Xu BL, Zhang XH and Dong MM: Cancer
stem-like side population cells in the human nasopharyngeal
carcinoma cell line CNE-2 possess epithelial mesenchymal transition
properties in association with metastasis. Oncol Rep. 28:241–247.
2012.PubMed/NCBI
|
|
23
|
Pestereva E, Kanakasabai S and Bright JJ:
PPARγ agonists regulate the expression of stemness and
differentiation genes in brain tumour stem cells. Br J Cancer.
106:1702–1712. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Frisch SM and Screaton RA: Anoikis
mechanisms. Curr Opin Cell Biol. 13:555–562. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grossmann J: Molecular mechanisms of
‘detachment-induced apoptosis – Anoikis’. Apoptosis. 7:247–260.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhong X and Rescorla FJ: Cell surface
adhesion molecules and adhesion-initiated signaling: understanding
of anoikis resistance mechanisms and therapeutic opportunities.
Cell Signal. 24:393–401. 2012. View Article : Google Scholar
|
|
27
|
Zhan M, Zhao H and Han ZC: Signalling
mechanisms of anoikis. Histol Histopathol. 19:973–983.
2004.PubMed/NCBI
|
|
28
|
Shen M and Horbett TA: The effects of
surface chemistry and adsorbed proteins on monocyte/macrophage
adhesion to chemically modified polystyrene surfaces. J Biomed
Mater Res. 57:336–345. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hardee ME, Marciscano AE, Medina-Ramirez
CM, Zagzag D, Narayana A, Lonning SM and Barcellos-Hoff MH:
Resistance of glioblastoma-initiating cells to radiation mediated
by the tumor microenvironment can be abolished by inhibiting
transforming growth factor-β. Cancer Res. 72:4119–4129. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Silginer M, Weller M, Ziegler U and Roth
P: Integrin inhibition promotes atypical anoikis in glioma cells.
Cell Death Dis. 5:e10122014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hong X, Chedid K and Kalkanis SN:
Glioblastoma cell line-derived spheres in serum-containing medium
versus serum-free medium: A comparison of cancer stem cell
properties. Int J Oncol. 41:1693–1700. 2012.PubMed/NCBI
|
|
32
|
Bigarella CL, Liang R and Ghaffari S: Stem
cells and the impact of ROS signaling. Development. 141:4206–4218.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Suzuki S, Okada M, Shibuya K, Seino M,
Sato A, Takeda H, Seino S, Yoshioka T and Kitanaka C: JNk
suppression of chemotherapeutic agents-induced ROS confers
chemoresistance on pancreatic cancer stem cells. Oncotarget.
6:458–470. 2014.PubMed/NCBI
|
|
34
|
Ali Azouaou S, Emhemmed F, Idris-khodja N,
Lobstein A, Schini-kerth V, Muller CD and Fuhrmann G: Selective
ROS-dependent p53-associated anticancer effects of the hypoxoside
derivative rooperol on human teratocarcinomal cancer stem-like
cells. Invest New Drugs. 33:64–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Myhre O, Andersen JM, Aarnes H and Fonnum
F: Evaluation of the probes 2′,7′-dichlorofluorescin diacetate,
luminol, and lucigenin as indicators of reactive species formation.
Biochem Pharmacol. 65:1575–1582. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee YD, Cui MN, Yoon HH, Kim HY, Oh IH and
Lee JH: Down-modulation of Bis reduces the invasive ability of
glioma cells induced by TPA, through NF-κB mediated activation of
MMP-9. BMB Rep. 47:262–267. 2014. View Article : Google Scholar :
|
|
37
|
Yoo HJ, Im CN, Youn DY, Yun HH and Lee JH:
Bis is induced by oxidative stress via activation of HSF1. korean J
Physiol Pharmacol. 18:403–409. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hayman EG, Pierschbacher MD, Suzuki S and
Ruoslahti E: Vitronectin - a major cell attachment-promoting
protein in fetal bovine serum. Exp Cell Res. 160:245–258. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Paoli P, Giannoni E and Chiarugi P:
Anoikis molecular pathways and its role in cancer progression.
Biochim Biophys Acta. 1833:3481–3498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sauerzweig S, Munsch T, Lessmann V,
Reymann KG and Braun H: A population of serum deprivation-induced
bone marrow stem cells (SD-BMSC) expresses marker typical for
embryonic and neural stem cells. Exp Cell Res. 315:50–66. 2009.
View Article : Google Scholar
|
|
41
|
Ling GQ, Chen DB, Wang BQ and Zhang LS:
Expression of the pluripotency markers Oct3/4, Nanog and Sox2 in
human breast cancer cell lines. Oncol Lett. 4:1264–1268.
2012.PubMed/NCBI
|
|
42
|
Favaro R, Appolloni I, Pellegatta S, Sanga
AB, Pagella P, Gambini E, Pisati F, Ottolenghi S, Foti M,
Finocchiaro G, et al: Sox2 is required to maintain cancer stem
cells in a mouse model of high-grade oligodendroglioma. Cancer Res.
74:1833–1844. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim MC, Cui FJ and Kim Y: Hydrogen
peroxide promotes epithelial to mesenchymal transition and stemness
in human malignant mesothelioma cells. Asian Pac J Cancer Prev.
14:3625–3630. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu Z, He Q, Ding X, Zhao T, Zhao L and
Wang A: SOD2 is a C-myc target gene that promotes the migration and
invasion of tongue squamous cell carcinoma involving cancer
stem-like cells. Int J Biochem Cell Biol. 60:139–146. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Im CN, Lee JS, Zheng Y and Seo JS: Iron
chelation study in a normal human hepatocyte cell line suggests
that tumor necrosis factor receptor-associated protein 1 (TRAP1)
regulates production of reactive oxygen species. J Cell Biochem.
100:474–486. 2007. View Article : Google Scholar
|
|
46
|
Ruggeri P, Farina AR, Di Ianni N,
Cappabianca L, Ragone M, Ianni G, Gulino A and Mackay AR: The
TrkAIII oncoprotein inhibits mitochondrial free radical ROS-induced
death of SH-SY5Y neuroblastoma cells by augmenting SOD2 expression
and activity at the mitochondria, within the context of a tumour
stem cell-like phenotype. PLoS One. 9:e945682014. View Article : Google Scholar : PubMed/NCBI
|