Introduction
The normal pancreas is made up of two classes of
cells: endocrine (hormone-secreting) and exocrine (digestive
enzyme-producing). Depending on the cell of origin, pancreatic
cancers can be classified as endocrine or exocrine tumors. Roughly
90% of all pancreatic cancers are pancreatic ductal
adenocarcinomas, an exocrine pancreatic tumor that resembles the
cells lining the pancreatic duct (1). Pancreatic cancer is a highly
aggressive malignancy with a notoriously dismal prognosis.
Pancreatic cancer is the 12th most common cancer. Despite not being
one of the most prevalent cancers, it is by far one of the
deadliest, with a 5-year survival of less than 7% (1,2). It
ranks fourth in terms of cancer mortality and accounts for less
than 7% of all cancer-related death (1,2). A
major contributor to this poor clinical outcome is pancreatic
cancer's prominent chemoresistance (3).
The flavonoid p-hydroxycinnamic acid (HCA),
which is an intermediate-metabolic substance in plants and fruits,
is synthesized from tyrosine (4,5). HCA
has been found to exhibit anabolic effects on bone metabolism in
vitro and preventive effects on bone loss in osteoporosis model
animals including postmenopause and type 1 diabetes in vivo
(6–8). Among the botanical factor cinnamic
acid-related compounds (cinnamic acid, HCA, ferulic acid, caffeic
acid and 3,4-dimethoxycin-namic acid), HCA has been shown to
possess a specific anabolic effect on bone metabolism in
vitro (6). HCA has been shown
to possess suppressive effects on osteoclastogenesis by
antagonizing the receptor activator of nuclear factor-κB ligand
(RANKL)-induced NF-κB activation and potent stimulatory effects on
osteoblastogenesis and mineralization through inhibition of tumor
necrosis factor (TNF)-α-enhanced NF-κB signaling in vitro
(9–11). HCA was also found to suppress
adipogenesis in bone marrow cells by inhibiting MEK/extracellular
signal-regulated kinase (ERK) signaling in vitro (12). Thus, HCA has been demonstrated to
exhibit suppressive effects on multi-signaling pathways in various
types of cells to restore metabolic disorder.
It is well-known that many signaling pathways are
attenuated in cancer cells. The effects of HCA on cancer cells have
not been investigated. We hypothesized that HCA may exhibit
anticancer effects on human pancreatic cancer MIA PaCa-2 cells
in vitro, which possess resistance to drugs and radiation in
pancreatic cancer therapy. This study was undertaken to determine
whether HCA possesses suppressive effects on pancreatic cancer
cells in vitro. HCA was found to suppress the proliferation
and to stimulate apoptotic cell death of human pancreatic cancer
MIA PaCa-2 and Pt45P1 cells in vitro. In this study,
botanical factor HCA was demonstrated to possess anticancer cell
effects.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM) with 4.5
g/l glucose, L-glutamine and sodium pyruvate and antibiotics
(penicillin and streptomycin) were purchased from Corning Cellgro
(Mediatech, Inc., Manassas, VA, USA). α-minimum essential medium
(α-MEM) was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Fetal bovine serum (FBS) was from HyClone Corp. (Logan, UT, USA).
HCA (100% pure) was obtained from Wako Pure Chemical Co., Ltd.
(Osaka, Japan). TNF-α was from R&D Systems (Minneapolis, MN,
USA). PD98059, staurosporine, Bay K 8644, wortmannin or
5,6-dichloro-1-β-D-ribofuranosylbenzimidazole (DRB), sodium
butyrate, roscovitine, sulforaphane, caspase-3 inhibitor and all
other reagents were purchased from Sigma-Aldrich unless otherwise
specified. Gemcitabine was obtained from Hospira, Inc. (Lake
Forest, IL, USA). Gemcitabine and caspase-3 inhibitor were diluted
in phosphate-buffered saline (PBS) and other reagents were
dissolved in 100% ethanol for use in the experiments.
Pancreatic cancer cells
We used human pancreatic cancer MIA Paca-2, Pt45P1
(highly expressing tissue factors; high TF) and Pt45P1 (highly
expressing alternative spliced variant TF; asTF) cells (13,14).
These human pancreatic cancer cell lines were obtained from the
American Type Culture Collection (ATCC; Rockville, MD, USA). MIA
PaCa-2 cells are commonly used as a model for human pancreatic
cancer cells. This cancer cell line possesses resistance for the
therapy with drugs and radiation. This cell line was a useful tool
to determine whether or not HCA exhibits anticancer effects.
Therefore, we used the cell line. Moreover, we used Pt45P1 cells
that highly express tissue factors.
Cell proliferation
Pancreatic cancer cells (1×105/ml per
well) were cultured in a 24-well plate with DMEM containing 10% FBS
and 1% P/S in the absence or presence of HCA (10, 100, 250, 500 or
1,000 nM) for 1, 3, 7 or 14 days in a water-saturated atmosphere
containing 5% CO2 and 95% air at 37°C (15,16).
In separate experiments, pancreatic cancer cells
(1×105/ml per well) were cultured in DMEM containing 10%
FBS and 1% P/S in the presence of sodium butyrate (10 and 100
µM), roscovitine (10 and 100 nM), sulforaphane (1 and 10
nM), TNF-α (1 ng/ml), Bay K 8644 (1 µM), PD98059 (1
µM), staurosporin (0.1 µM), wortmannin (1 µM),
DRB (1 µM), or gemcitabine (100 nM) for 3–7 days. After
culture, the cells were detached from each culture dish and
counted.
Cell death
Pancreatic cancer cells (1×105/ml per
well) were cultured using a 24-well plate in DMEM containing 10%
FBS and 1% P/S in the absence of HCA for 7 days. When reaching
confluency, the cells were additionally cultured in the presence of
HCA (10, 100, 250, 500 or 1,000 nM) with or without gemcitabine
(100 nM) or caspase-3 inhibitor (5 µM) for 3 or 7 days
(17). After culture, the cells
were detached from each culture dish.
Cell counting
After trypsinization of each culture dish using 0.2%
trpysin plus 0.02% EDTA in Ca2+/Mg2+-free PBS
for 2 min at 37°C, the detached cells from the dish were collected
after centrifugation (15–18). The cells were resuspended in PBS
solution and stained with eosin. Cell numbers were counted under a
microscope using a hemocytometer plate. For each dish, we took the
average of two countings. Cell number was expressed as the number
per well of the plate.
Statistical analysis
Statistical significance was determined using
GraphPad InStat version 3 for Windows XP (GraphPad Software Inc.,
La Jolla, CA, USA). Multiple comparisons were performed by one-way
analysis of variance (ANOVA) with Tukey-Kramer multiple comparisons
post test for parametric data as indicated. P<0.05 was
considered to indicate a statistically significant difference.
Results
HCA suppresses the proliferation of
pancreatic cancer cells
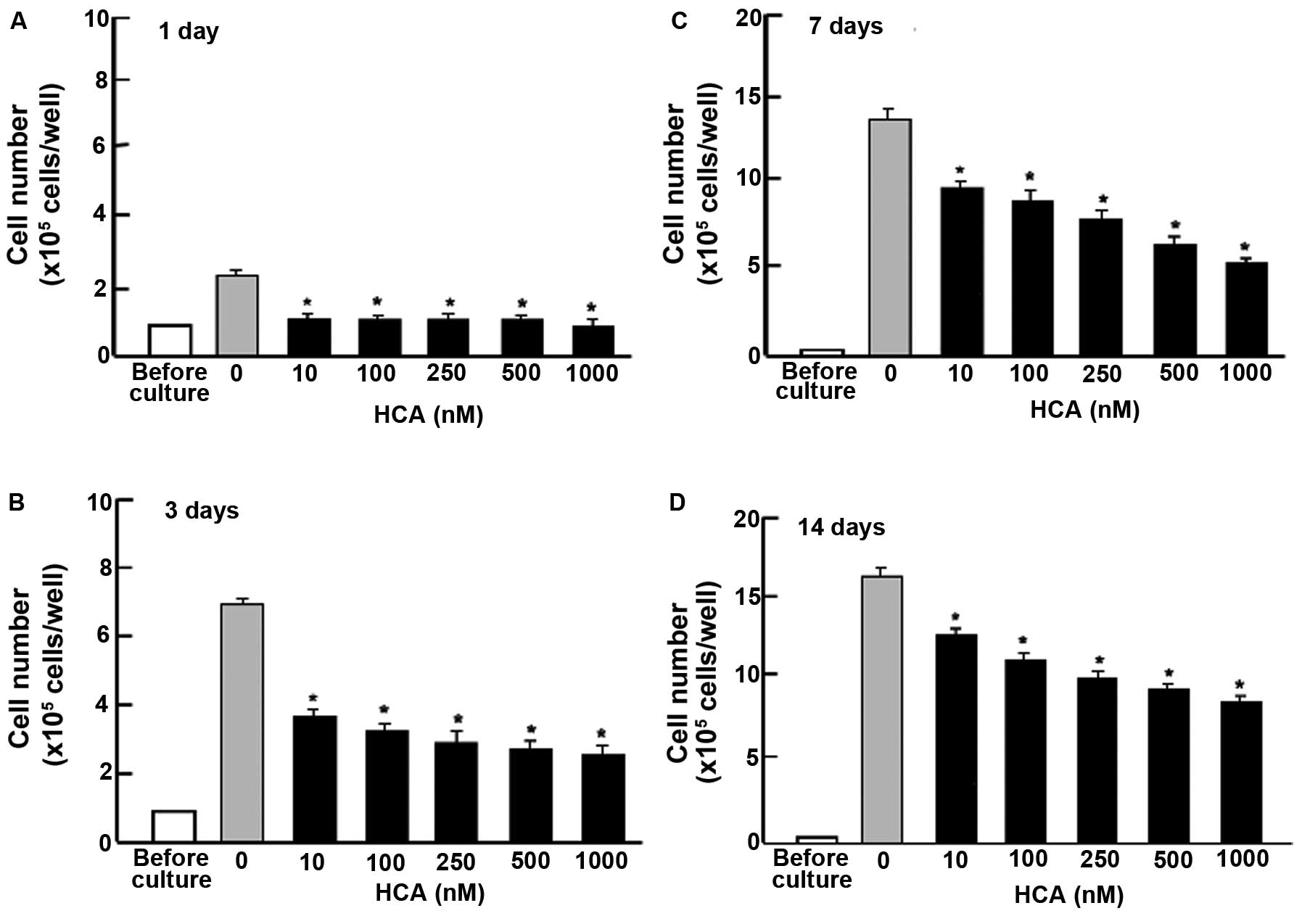
To determine whether HCA possesses suppressive
effects on the proliferation of cloned human pancreatic cancer MIA
PaCa-2 cells in vitro, the cells were cultured in the
presence of HCA (10-1,000 nM) for 1, 3, 7 and 14 days (Fig. 1). Cell numbers were elevated with
increasing culture time. Addition of HCA suppressed the increase in
cell number. Thus, HCA was found to exhibit suppressive effects on
the proliferation of MIA PaCa-2 cells. Moreover, we determined
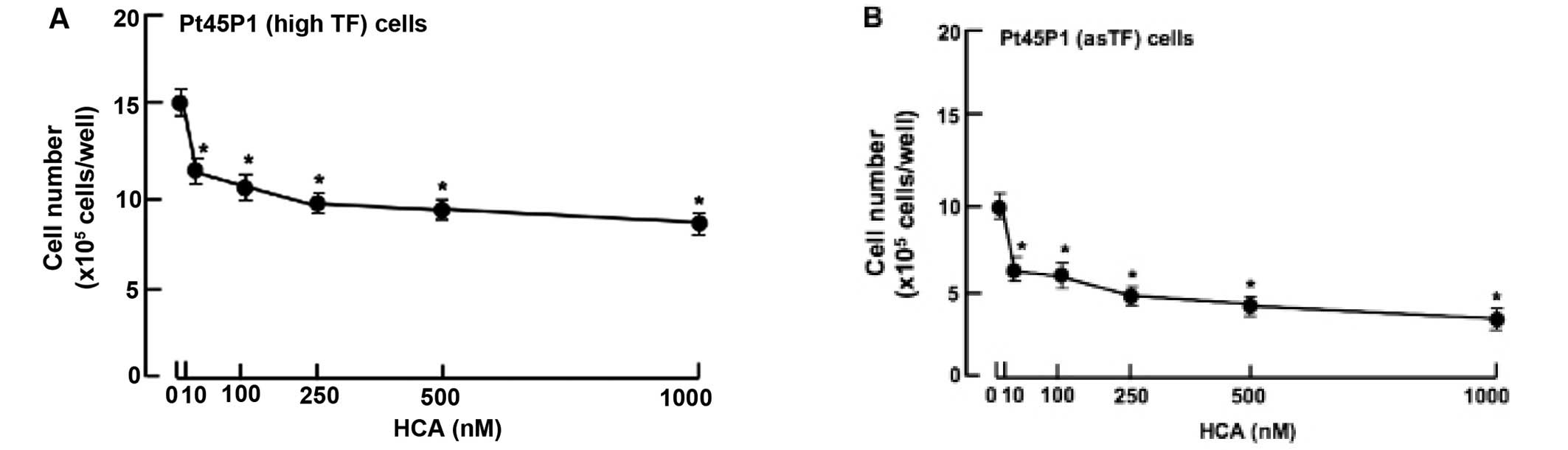
whether HCA possesses anticancer effects on the proliferation in
other human pancreatic cancer cells. We used Pt45P1 (highly
expressing tissue factors; high TF) and Pt45P1 cells (highly
expressing alternative spliced variant tissue factor; asTF). The
suppressive effects of HCA on the proliferation were also noted in
the Pt45P1 (Fig. 2A) and Pt45P1
cells (Fig. 2B) in vitro,
when cells were culture for 7 days in the presence of HCA (10–1,000
nM). Thus, HCA was found to possess anticancer effects in various
types of human pancreatic cancer cells in vitro. This was a
novel finding.
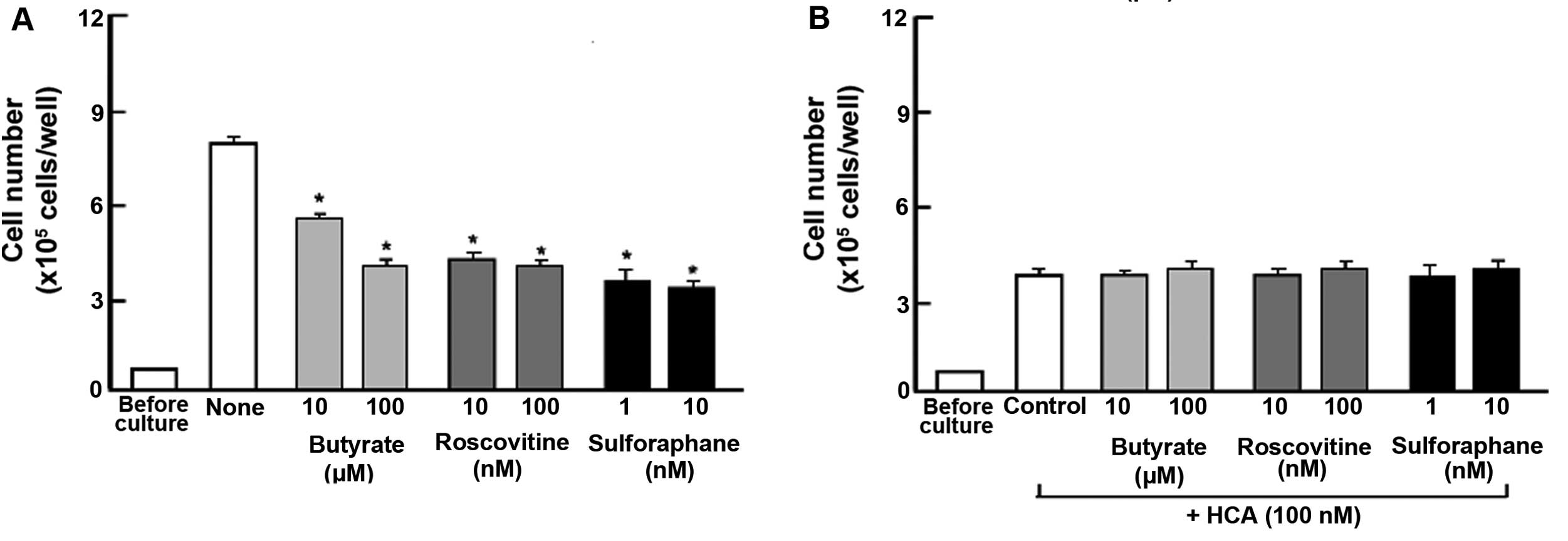
The suppressive effects of HCA on the proliferation
of MIA PaCa-2 cells were determined in the presence of various
inhibitors that induce cell cycle arrest in vitro (Fig. 3). The cells were cultured for 3 days
in the absence (Fig. 3A) or
presence (Fig. 3B) of HCA (100 nM)
with or without butyrate (10 and 100 µM), roscovitine (10
and 100 nM) or sulforaphane (1 and 10 nM) (16,19,20).
Proliferation of the MIA PaCa-2 cells was suppressed in the
presence of these inhibitors (Fig.
3A). The suppressive effects of these inhibitors on cell
proliferation were not observed in the presence of HCA (100 nM)
(Fig. 3B).
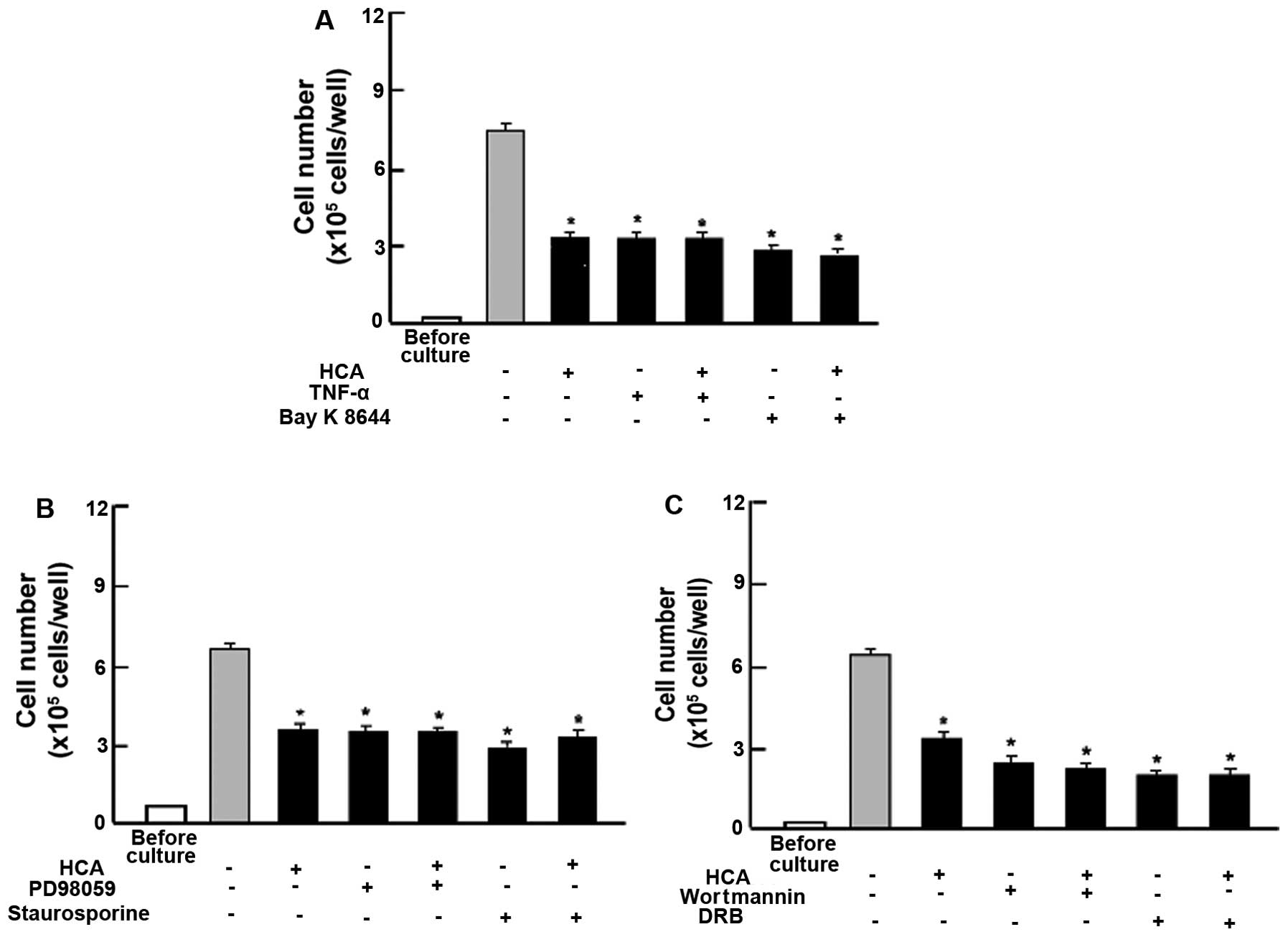
To determine the involved mechanism by which HCA
possesses suppressive effects on cell proliferation, we ascertained
whether HCA regulates intracellular signaling pathways using
various inhibitors for cell signaling. The suppressive effects of
HCA on the proliferation of MIA PaCa-2 cells were not altered in
the presence of TNF-α, an enhancer of NF-κB signaling (21), or Bay K 8644, an agonist of
Ca2+ entry in cells (22) (Fig.
4A). The suppressive effects of HCA (100 nM) on the
proliferation of MIA PaCa-2 cells were not modulated in the
presence of PD98059 (1 µM), an extracellular
signal-regulated kinase (ERK) inhibitor (23) or staurosporin (0.1 µM), an
inhibitor of protein kinase C (24)
(Fig. 4B). In addition, the
suppressive effects of regucalcin on cell proliferation were not
potentiated in the presence of wortmannin (1 µM), an
inhibitor of phosphatidylinositol 3-kinase (PI3K) (25) or DRB (1 µM), an inhibitor of
transcriptional activity with RNA polymerase II inhibition
(26) (Fig. 4C). Thus, HCA was suggested to
inhibit various processes involved in intracellular signaling that
are related to cell proliferation.
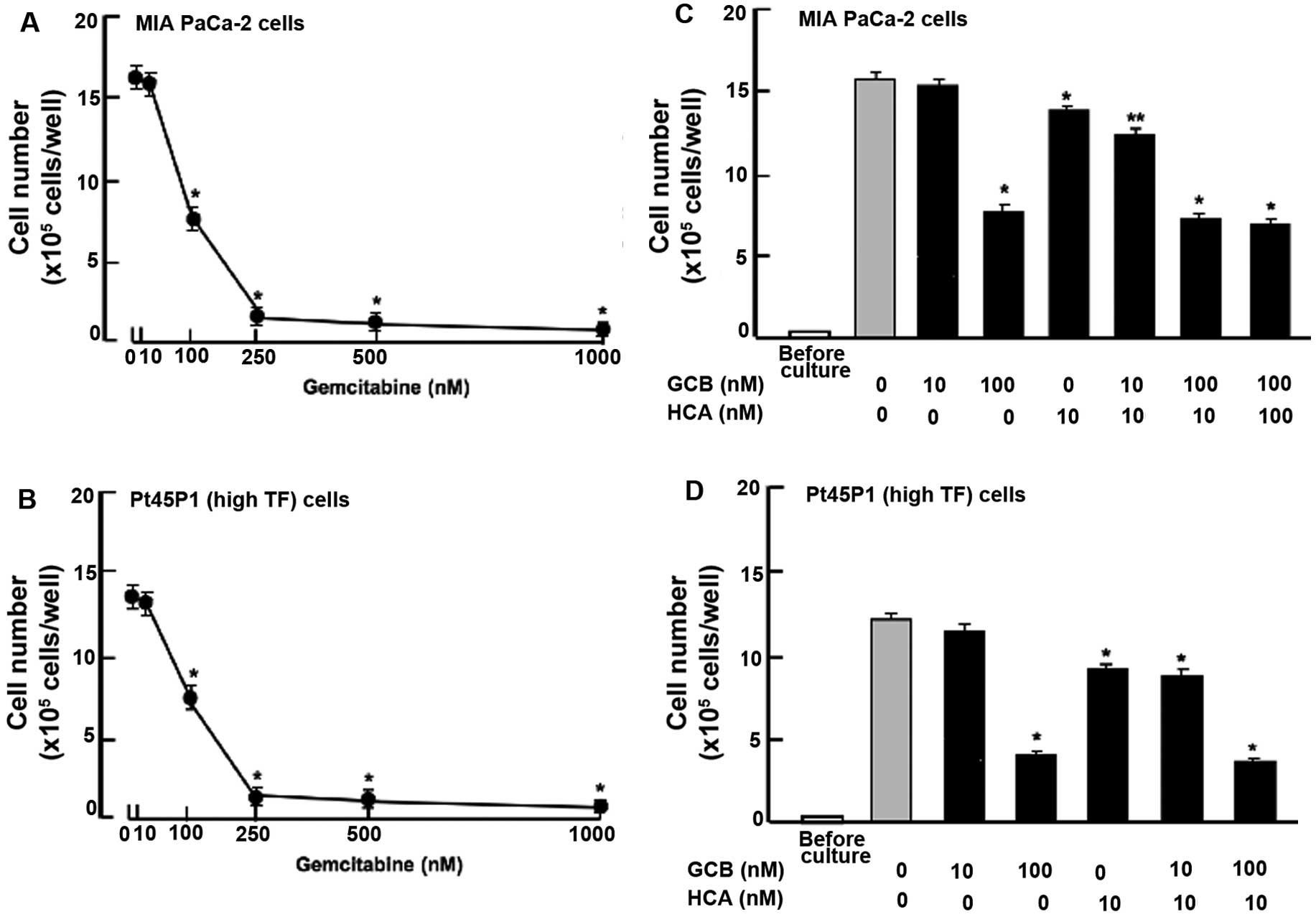
Moreover, we compared the suppressive effects of HCA
on human pancreatic cancer cells using gemcitabine, a potent
antitumor agent that induces nuclear DNA damage (27). Culture with gemcitabine (50-1,000
nM) for 7 days (Fig. 5A) or 14 days
(Fig. 5B) suppressed the
proliferation of the MIA PaCa-2 (Fig.
5A) and Pt45P1 (high TF) (Fig.
5B) cells. Notably, the suppressive effects of HCA (10 nM) on
the proliferation of MIA PaCa-2 cells were significantly
potentiated in the presence of gemcitabine (10 nM) with a
concentration that did not possess suppressive effects on cell
proliferation (Fig. 5C). Such
effects were not observed in the case of Pt45P1 (high TF) cells
(Fig. 5D).
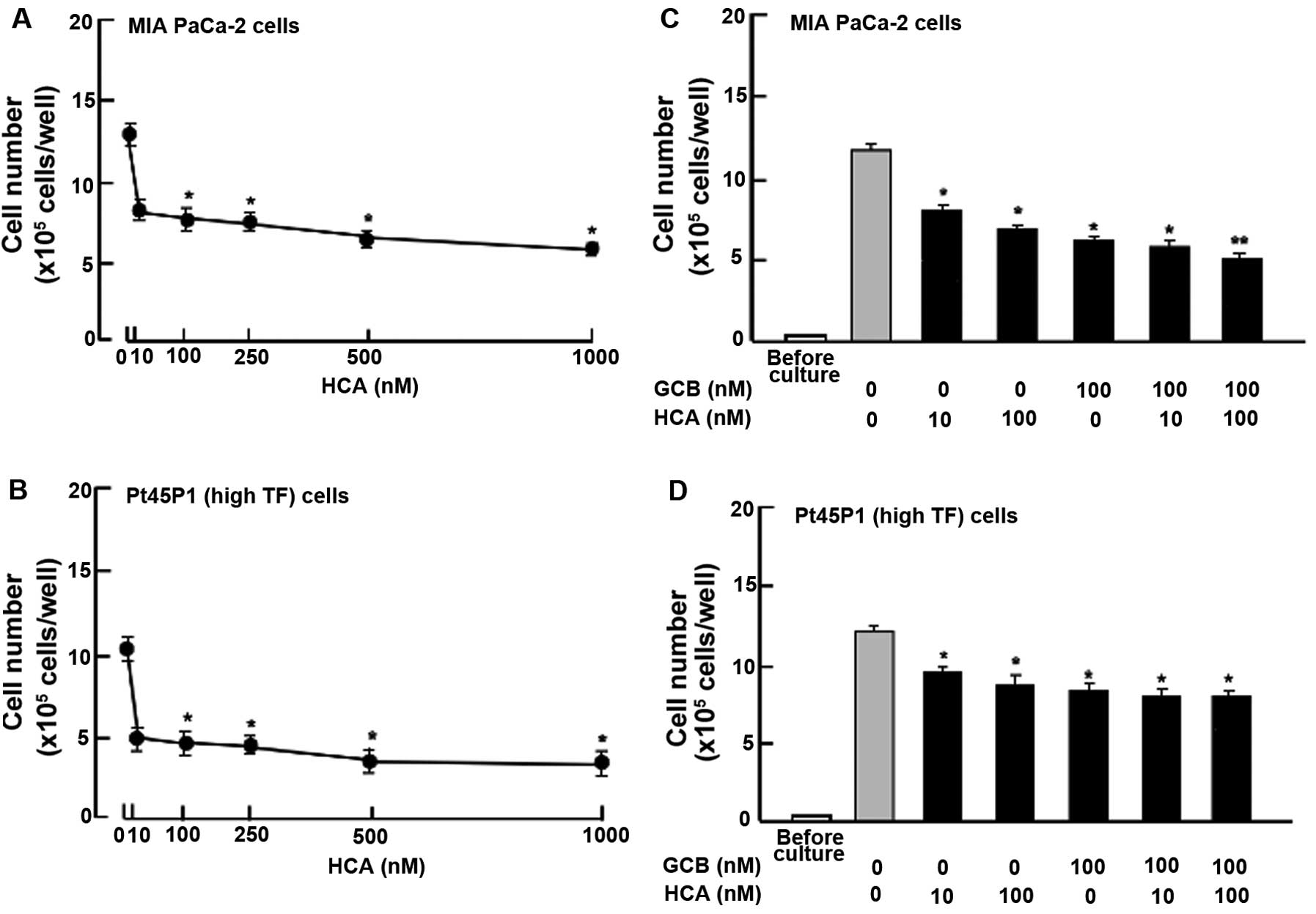
HCA stimulates cell death
To determine the effects of HCA on cell death in
human pancreatic cancer MIA PaCa-2 cells, the cells were cultured
for 7 days. After reaching confluency, the cells were cultured for
an additional 3 days in the presence of HCA (10-1,000 nM) (Fig. 6). Cell number was decreased after
culture with HCA (10-1,000 nM) (Fig.
6A). Similar effects of HCA (10-1,000 nM) on decreasing the
cell number were also noted in human pancreatic cancer Pt45P1
(highly expressing TF) cells (Fig.
6B). The effects of HCA (10 or 100 nM) in decreasing the
numbers of MIA PaCa-2 cells (Fig.
6C) and Pt45P1 (highly expressed TF) cells (Fig. 6D) were not potentiated in the
presence of gemcitabine (100 nM).
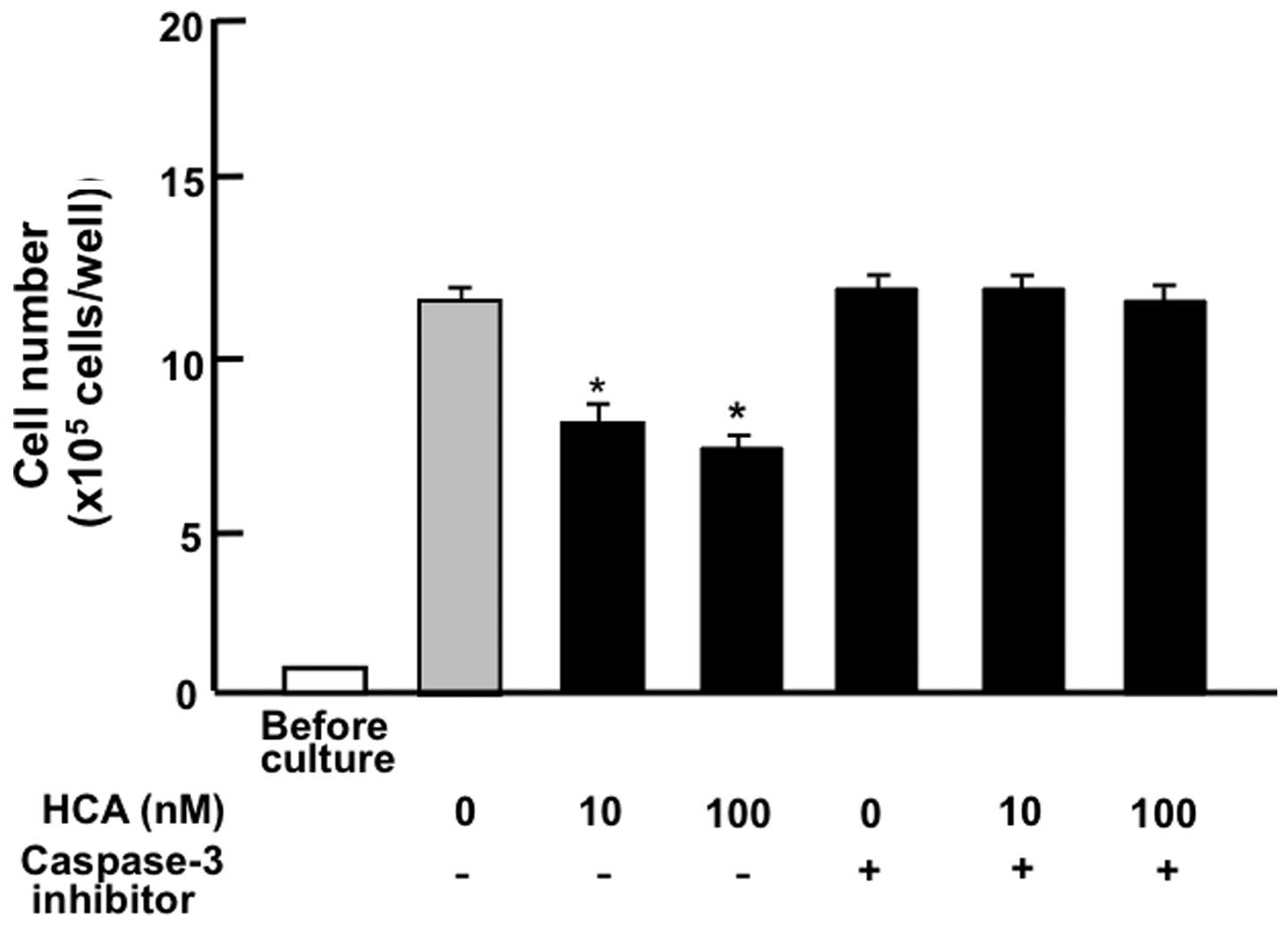
MIA PaCa-2 cells were cultured for 7 days. After
reaching confluency, the cells were cultured for an additional 2
days in the presence of HCA (10 or 100 nM) with or without a
caspase-3 inhibitor (5 µM). The suppressive effects of HCA
on cell death were prevented in the presence of the caspase-3
inhibitor (Fig. 7). Thus, HCA was
suggested to stimulate cell death related to an increase in
caspase-3 activity.
Discussion
The flavonoid HCA, which exhibits an anabolic effect
on osteoporotic bone loss (6–8), has
been demonstrated to stimulate osteoblastic mineralization and to
suppress osteoclastogenesis and adipogenesis in mouse bone marrow
culture in vitro (9–12). In these processes, HCA has been
shown to suppress various signaling pathways including NF-κB and
MAPK/ERK that play a pivotal role in cell regulation of various
types of cells. These signaling systems are known to be disturbed
in cancer cells. We hypothesized that HCA may exhibit anticancer
cell effects by restoring attenuated cell signaling in cancer
cells.
HCA was demonstrated to suppress the proliferation
and stimulate cell death in human pancreatic cancer MIA PaCa-2 and
Pt45P1 (highly expressing TF) cells in vitro, supporting the
view that HCA has an anticancer cell effect on various types of
human pancreatic cancer cells. The suppressive effects of HCA on
the proliferation of MIA PaCa-2 cells were not potentiated in the
presence of butyrate, roscovitine or sulforaphane that induce cell
cycle arrest. Roscovitine is a potent and selective inhibitor of
the cyclin-dependent kinase cdc2, cdk2m and cdk5 (19). Sulforaphane induces G2/M phase cell
cycle arrest by stimulation of p21 and inhibition of cdc2 kinase
(20). Butyrate induces inhibition
of G1 progression by inhibiting Akt (16). HCA may induce G1 and G2/M phase cell
cycle arrest by inhibiting the kinase activities of cdc2 and Akt in
MIA PaCa-2 cells.
Moreover, the suppressive effects of HCA on MIA
PaCa-2 cells were not modulated in the presence of various
inhibitors that regulate intracellular signaling pathways in
vitro. The suppressive effects of HCA on the proliferation of
MIA PaCa-2 cells were not modulated in the presence of TNF-α, an
enhancer of NF-κB signaling (21),
Bay K 8644, an agonist of Ca2+ entry in cells (22), PD98059, an inhibitor of
ERK/mitogen-activated protein (MAP) kinase signaling pathway
(23), staurosporin, an inhibitor
of calcium-dependent protein kinase C signaling pathway (24) and wortmannin, an inhibitor of
PI3/Akt signaling pathway (25).
These findings suggest that HCA exerts suppressive effects mediated
by the inhibition of various signaling pathways related to NF-κB,
ERK, protein kinase C, calcium signaling, or PI3K in MIA PaCa-2
cells. Moreover, the suppressive effects of HCA on cell
proliferation were not enhanced in the presence of DRB, an
inhibitor of transcriptional activity with RNA polymerase II
inhibition (26). HCA may suppress
transcriptional activity in the nucleus of MIA PaCa-2 cells. Thus,
HCA may possess suppressive effects on the proliferation by
inhibiting various signaling processes in human pancreatic cancer
cells. HCA may be a multi-inhibitor in the proliferation of
pancreatic cancer cells. Further research is warranted to elucidate
the molecular mechanisms.
Gemcitabine is used clinically in the therapy of
pancreatic cancer (27).
Gemcitabine is a potent antitumor agent that induces nuclear DNA
damage and apoptotic cell death in cancer cells (27). This agent suppresses cell
proliferation and stimulates apoptotic cell death in cancer cells
of various types (27). The
stimulatory effects of HCA on cell death in the MIA PaCa-2 cells
were significantly enhanced in the presence of gemcitabine. HCA may
possess a different mode of action when compared to that of
gemcitabine. HCA was also found to stimulate cell death in human
pancreatic cancer Pt45P1 (highly expressing TF) cells in
vitro. HCA at a comparative lower concentration (10 nM) was
found to exhibit suppressive effects on the proliferation and
stimulatory effects on apoptotic cell death in the MIA PaCa-2 and
Pt45P1 cells. Such effects were not observed at the same
concentrations of gemcitabine. Notably, the stimulatory effects of
HCA on cell death in the MIA PaCa-2 cells were not noted in the
presence of a caspase-3 inhibitor. HCA may stimulate cell death
through a mechanism by which caspase-3 activity is increased. It is
possible that HCA directly activates this enzyme in the nucleus.
HCA, which is a botanical factor, may have lower toxicity as
compared with that of gemcitabine. HCA may be a useful tool in the
prevention and therapy in human pancreatic cancer.
In conclusion, this study demonstrates that the
flavonoid HCA suppresses the proliferation and stimulates the cell
death of human pancreatic cancer MIA PaCa-2 and Pt45P1 (highly
expressing TF and alternative spliced variant TF) cells in
vitro. Thus, HCA was found to have an anticancer effect in
various types of human pancreatic cancer cells in vitro.
This was a novel finding. HCA may be a useful tool in the
prevention and therapy of human pancreatic cancers in vivo.
Further research is needed to confirm the suppressive effects of
HCA on carcinogenesis in vivo.
References
|
1
|
Sousa CM and Kimmelman AC: The complex
landscape of pancreatic cancer metabolism. Carcinogenesis.
35:1441–1450. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McCarroll JA, Naim S, Sharbeen G, Russia
N, Lee J, Kavallaris M, Goldstein D and Phillips PA: Role of
pancreatic stellate cells in chemoresistance in pancreatic cancer.
Front Physiol. 5:1412014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Suzuki T, Nakayama H and Yamaguchi M:
Effect of wasabi leafstalk (Wasabia japonica Matsum) extract on
bone metabolism in mouse calvaria tissue culture. Food Sci Technol
Int Tokyo. 3:366–369. 1997. View Article : Google Scholar
|
|
5
|
Suzuki T and Yamaguchi M: Purification of
active component in wasabi leafstalk (Wasabia japonica Matsum)
extract in stimulating bone calcification in vitro. J Health Sci.
50:483–490. 2004. View Article : Google Scholar
|
|
6
|
Lai YL and Yamaguchi M: Phytocomponent
p-hydroxycinnamic acid stimulates bone formation and inhibits bone
resorption in rat femoral tissues in vitro. Mol Cell Biochem.
292:45–52. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamaguchi M, Lai YL, Uchiyama S and
Nakagawa T: Oral administration of phytocomponent p-hydroxycinnamic
acid prevents bone loss in ovariectomized rats. Mol Cell Biochem.
311:31–36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamaguchi M, Uchiyama S and Lai YL: Oral
administration of phytocomponent p-hydroxycinnamic acid has a
preventive effect on bone loss in streptozotocin-induced diabetic
rats. Int J Mol Med. 19:803–807. 2007.PubMed/NCBI
|
|
9
|
Lai YL and Yamaguchi M: Phytocomponent
p-hydroxycinnamic acid inhibits osteoclast-like cell formation in
mouse bone marrow cultures. Int J Mol Med. 19:123–128. 2007.
|
|
10
|
Yamaguchi M, Lai YL, Uchiyama S and
Nakagawa T: Phytocomponent p-hydroxycinnamic acid stimulates
mineralization in osteoblastic MC3T3-E1 cells. Int J Mol Med.
22:287–291. 2008.PubMed/NCBI
|
|
11
|
Yamaguchi M and Weitzmann MN: The bone
anabolic carotenoid p-hydroxycinnamic acid promotes osteoblast
mineralization and suppresses osteoclast differentiation by
antagonizing NF-κB activation. Int J Mol Med. 30:708–712.
2012.PubMed/NCBI
|
|
12
|
Yamaguchi M, Baile CA, Zhu S and Shoji M:
Bioactive flavonoid p-hydroxycinnamic acid stimulates
osteoblastogenesis and suppresses adipogenesis in bone marrow
culture. Cell Tissue Res. 354:743–750. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ninomiya I, Yamazaki K, Oyama K, Hayashi
H, Tajima H, Kitagawa H, Fushida S, Fujimura T and Ohta T:
Pioglitazone inhibits the proliferation and metastasis of human
pancreatic cancer cells. Oncol Lett. 8:2709–2714. 2014.PubMed/NCBI
|
|
14
|
Sipos B, Möser S, Kalthoff H, Török V,
Löhr M and Klöppel G: A comprehensive characterization of
pancreatic ductal carcinoma cell lines: Towards the establishment
of an in vitro reseach platform. Virchows Arch. 442:444–452.
2003.PubMed/NCBI
|
|
15
|
Misawa H, Inagaki S and Yamaguchi M:
Suppression of cell proliferation and deoxyribonucleic acid
synthesis in the cloned rat hepatoma H4-II-E cells overexpressing
regucalcin. J Cell Biochem. 84:143–149. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamaguchi M and Daimon Y: Overexpression
of regucalcin suppresses cell proliferation in cloned rat hepatoma
H4-II-E cells: Involvement of intracellular signaling factors and
cell cycle-related genes. J Cell Biochem. 95:1169–1177. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamaguchi M: The anti-apoptotic effect of
regucalcin is mediated through multisignaling pathways. Apoptosis.
18:1145–1153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamaguchi M, Zhu S, Weitzmann MN, Snyder
JP and Shoji M: Curcumin analog UBS109 prevents bone marrow
osteoblastogenesis and osteoclastogenesis disordered by coculture
with breast cancer MDA-MB-231 bone metastatic cells in vitro. Mol
Cell Biochem. 401:1–10. 2015. View Article : Google Scholar
|
|
19
|
Meijer L, Borgne A, Mulner O, Chong JP,
Blow JJ, Inagaki N, Inagaki M, Delcros JG and Moulinoux JP:
Biochemical and cellular effects of roscovitine, a potent and
selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and
cdk5. Eur J Biochem. 243:527–536. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singh SV, Herman-Antosiewicz A, Singh AV,
Lew KL, Srivastava SK, Kamath R, Brown KD, Zhang L and Baskaran R:
Sulforaphane-induced G2/M phase cell cycle arrest involves
checkpoint kinase 2-mediated phosphorylation of cell division cycle
25C. J Biol Chem. 279:25813–25822. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Y, Li A, Strait K, Zhang H, Nanes MS
and Weitzmann MN: Endogenous TNFalpha lowers maximum peak bone mass
and inhibits osteoblastic Smad activation through NF-kappaB. J Bone
Miner Res. 22:646–655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cano-Abad MF, Villarroya M, García AG,
Gabilan NH and López MG: Calcium entry through L-type calcium
channels causes mitochondrial disruption and chromaffin cell death.
J Biol Chem. 276:39695–39704. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen S, Wang Y, Ruan W, Wang X and Pan C:
Reversing multidrug resistance in hepatocellular carcinoma cells by
inhibiting extracellular signal-regulated kinase/mitogen-activated
protein kinase signaling pathway activity. Oncol Lett. 8:2333–2339.
2014.PubMed/NCBI
|
|
24
|
Chen QW, Edvinsson L and Xu CB: Role of
ERK/MAPK in endothelin receptor signaling in human aortic smooth
muscle cells. BMC Cell Biol. 10:522009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Serrano-Nascimento C, da Silva Teixeira S,
Nicola JP, Nachbar RT, Masini-Repiso AM and Nunes MT: The acute
inhibitory effect of iodide excess on sodium/iodide symporter
expression and activity involves the PI3K/Akt signaling pathway.
Endocrinology. 155:1145–1156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Palangat M, Grass JA, Langelier MF,
Coulombe B and Landick R: The RPB2 flap loop of human RNA
polymerase II is dispensable for transcription initiation and
elongation. Mol Cell Biol. 31:3312–3325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang SC and Chen YC: Novel therapeutic
targets for pancreatic cancer. World J Gastroenterol.
20:10825–10844. 2014. View Article : Google Scholar : PubMed/NCBI
|