Introduction
The aim of the present study was to identify an
effective chemotherapy regimen that suppresses the local recurrence
and metastasis of head and neck squamous cell carcinoma (HNSCC).
The main HNSCC treatments include surgery, radiotherapy and
chemotherapy; however, many refractory tumors recur and metastasize
despite treatment. Moreover, the head and neck contain many organs
that play important roles in daily functions such as chewing,
swallowing, vocalization and respiration. Damage to these organs
from side-effects of surgery, radiotherapy or chemotherapy
seriously reduces the quality of life of these HNSCC patients
(1,2). The first-line chemotherapy for HNSCC
is the platinum-containing drug cisplatin, and in cases of
recurrence or metastasis, combination chemotherapy consisting of
cisplatin and fluorouracil (5-FU) or cetuximab, which is an
epidermal growth factor receptor (EGFR) inhibitor, is used
(3,4).
In recent years, it has been appreciated that there
is a hierarchy to the cells that constitute cancer tissues; most
cancer cells are thought to arise from a small number of cancer
stem cells, which are located at the top of this hierarchy
(5). Studies have demonstrated that
cancer stem cells possess properties similar to the
self-replication phenotypes of conventional stem cells. Cancer stem
cells also have a high differentiation capacity and are resistant
to standard anticancer treatments, such as radiotherapy and
chemotherapy (6). Therefore, even
though it may appear that all cancer cells have been eradicated by
what appears to be curative treatment, in most cases, a small
number of cancer stem cells remain. When these cancer stem cells
regenerate to reform the primary tumor, it is classified as
recurrence, while metastasis is defined as the migration of cancer
stem cells to other organs, producing a tumor at these sites
(5,7). Currently, there are no treatments to
control HNSCC recurrence and metastasis.
Biomarkers of HNSCC cancer stem cells include CD44,
aldehyde dehydrogenase (ALDH), CD133, c-Met (a tyrosine kinase
receptor for hepatocyte growth factor), and drug efflux
transporters such as MDR1 and ABCG2 (8). CD44 is a large cell surface
glycoprotein associated with tumor growth and migration (9) and the most well-known cancer stem
marker in HNSCC. CD44 binds growth factors, such as EGFR, and
metalloproteinases, such as MMP9, resulting in reduced apoptosis,
and the induction of angiogenesis and cancer cell invasion
(8,10–12).
Our previous study revealed that CD44 inhibits apoptosis by
modulating DNA damage responses during the G2/M phase of the cell
cycle (13). Clinically, it has
been found that CD44-positive cells are associated with poor
prognosis, more aggressive tumors and higher recurrence rates after
radiation therapy (14). Other
studies have reported that the microRNA miR-34a is a novel
therapeutic target that negatively regulates CD44-positive cells
(15).
The underlying question for the present study is
whether there is a more effective combination therapy for HNSCC
among clinically approved chemotherapeutic agents. While many
researchers have reported that cancer stem cells influence
recurrence, metastasis and prognosis, it is unclear how existing
chemotherapies influence cancer stem cells. Therefore, in the
present study, we searched for effective therapies for HNSCC among
conventional chemotherapeutic agents and clarified the effects of
these drugs on cancer stem cells.
Materials and methods
Reagents and cell culture
The HNSCC cell lines HSC-2, HSC-3, SAS and Ca9-22
were obtained from the Riken Cell Bank (Ibaraki, Japan). The human
immortalized non-tumorigenic keratinocyte cell line, HaCaT was
purchased from DKFZ (Heidelberg, Germany). Cells were cultured in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (FBS) (both from Life Technologies Japan Ltd.,
Yokohama, Japan), 100 U/ml penicillin and 100 µg/ml streptomycin at
37°C in a humidified atmosphere containing 5%.
Evaluation of cell growth and
determination of drug synergy
Following in vitro drug treatment, the number
of viable cells was measured using
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay and the Cell Proliferation Kit I (Roche Diagnostics,
Mannheim, Germany) according to the manufacturer's instructions.
The number of viable cells was assessed by measuring the absorbance
of formazan crystals at 595 nm with TECAN SpectraFluor Plus XFluor4
software (Tecan Japan Co., Ltd., Kawasaki, Japan). Synergetic drug
effects were assessed with the CompuSyn™ program (Biosoft,
Ferguson, MO, USA) using a combination index (CI) that is based
upon the Chou and Talalay median-effect principle (16). The CompuSyn™ software was used to
calculate the dose-effect relationship and quantitate synergism and
antagonism in drug combinations from IC50,
ED50 or LD50 data based on Chou's
median-effect equation (17). CI
was used to identify synergistic, additive and antagonistic drug
interaction; CI <1 was considered a synergistic effect.
Mice and in vivo tumor growth
analysis
Male BALB/c nude mice, 6- to 8-weeks old, were
obtained from Sankyo Labo Service Corporation, Inc. (Tokyo, Japan).
The present study was approved by the Animal Ethics Committee of
Meikai University (no. C1201). HSC-2 cells (3×106) were
resuspended in 0.1 ml phosphate-buffered saline (PBS) and
subcutaneously injected into the lateroabdominal region of male
BALB/c nude mice. Tumor volume was calculated using the formula a ×
b2/2 where ‘a’ is the length and ‘b’ is the width of the
tumor diameter. Drug administration was initiated when tumor
volumes reached ~400–500 mm3. SN-38 (Tokyo Chemical
Industry Co., Ltd., Tokyo, Japan) and gefitinib (LC Laboratories,
Woburn, MA, USA) were dissolved in 0.5% carboxymethylcellulose
sodium. SN-38 (10 mg/kg) was administered by intraperitoneal
injection, and gefitinib (100 mg/kg) was administered by
subcutaneous injection. The SN-38 concentration was based on the
study by Guo et al (18),
and that of gefitinib was based on the study by Stewart et
al (19). When used as
combination therapy, SN-38 (10 mg/kg) and gefitinib (100 mg/kg)
were administered by independent injections to the same mouse. For
vehicle controls, 0.5% carboxymethylcellulose sodium was
administered in equal volumes via the respective routes. Each group
contained 5 mice that received drug or vehicle injections 5
days/week for 3 weeks. Tumor size was measured every 3 days and
this observation continued for 60 days. There were no significant
differences in the body weights of the mice. At the end of this
experiment, mice were given 5% isoflurane (Wako Pure Chemical
Industries, Ltd., Osaka, Japan) inhalation anesthesia and
euthanized by pentobarbital Na (Nakalai Tesuque Co., Kyoto, Japan)
overdose (~100 mg/kg) via intraperitoneal administration.
Immunohistochemistry
Mice were sacrificed 21 days after finishing
treatment (42 days from the initiation of drug administration), and
tumor tissues from the gefitinib-only and combination treatment
groups were fixed in 10% formalin buffer, embedded in paraffin, and
5-µm sections were placed on positively charged glass slides. EGFR
immunohistochemical staining was performed using the EGFR pharmDX
kit (Dako, Glostrup, Denmark) according to the manufacturer's
protocol. The primary antibody contained in this kit is ready to
use, and the response time was 30 min. CD44 was stained separately
using a mouse monoclonal anti-human CD44 phagocytic glycolin-1
antibody (DF1485; Dako) at 1:50 concentration for 30 min, which was
applied to tissue sections followed by secondary biotinylated
antibody and streptavidin-HRP conjugate complex contained in the
kit. After washing with buffer, diaminobenzidine was applied
followed by a counterstain with Mayer's hematoxylin.
Isolation of CD44-positive cells
CD44-positive cells were isolated from the HSC-2
cell line using magnetic anti-CD44 immunobeads (MACS; Miltenyi
Biotec, Berdish-Gladbach, Germany) according to the manufacturer's
instructions. Cells that passed through the MACS magnetic bead
system without attaching to the magnetic anti-CD44 immunobeads were
designated as CD44-negative cells.
Migration assay
Cell migration analysis was performed using BD
Falcon cell culture inserts (Becton-Dickinson and Company, Franklin
Lakes, NJ, USA), which allows cells to migrate through 8-µm pores
in polyethylene terephthalate membranes. Briefly, 1×105
cells were resuspended in serum-free medium and added to the upper
Transwell chamber into a 12-well plate. The lower chamber was
filled with serum-free DMEM or DMEM containing 10% FBS. After 24 h,
cells remaining on the upper surface of the membrane were removed
with a cell scraper. Cells beneath the membrane were fixed with
cold 6.0% (v/v) glutaraldehyde for 30 min and stained with 0.5%
(w/v) crystal violet. Cells were counted in 10 high-power
microscope fields.
CD44 knockdown by siRNA
transfection
Small interfering RNA (siRNA) specifically targeting
human CD44 [HCAM siRNA (h): sc-29342] was obtained from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). HSC-2 cells
(1×105/well) were plated into 6-well plates and
transfected with CD44 siRNA for 24 h in antibiotic-free media using
siRNA Lipofectamine RNAiMAX and Opti-MEM I reduced serum medium
(Invitrogen, Carlsbad, CA, USA) following the manufacturer's
protocol. After a 24-h transfection, the cells were used for
experimentation.
Immunoblot analysis
Whole-cell extracts were obtained in lysis buffer
(10X cell lysis buffer; Cell Signaling Technology, Danvers, MA,
USA) supplemented with 1 mM phenylmethylsulfonyl fluoride (PMSF)
plus one protease inhibitor cocktail tablet (Complete EDTA-free;
Roche Diagnostics). Protein concentration in the lysates was
assayed, and equal amounts of protein for each sample were
subjected to SDS-polyacrylamide gel electrophoresis, followed by
immunoblotting with the following primary antibodies: mouse
monoclonal anti-CD44, rabbit monoclonal anti-cleaved PARP, rabbit
polyclonal anti-EGFR, rabbit monoclonal anti-phospho-EGFR
(Tyr1068), rabbit monoclonal anti-HER2/ErbB2 (all from Cell
Signaling Technology and used at a concentration of 1:1,000),
rabbit polyclonal anti-phospho-HER2 (Tyr1248) (1:50,000; Merck
KGaA, Darmstadt, Germany) and anti-β-actin (1:10,000;
Sigma-Aldrich, St. Louis, MO, USA). Signals were detected using the
corresponding peroxidase-conjugated secondary antibodies
(anti-rabbit IgG or anti-mouse IgG; Cell Signaling Technology), and
immunoreactive bands were visualized by chemiluminescence (Clarity™
Western ECL substrate; Bio-Rad, Hercules, CA, USA). Membranes and
images were developed with a ChemoDoc™ Imaging System (Bio-Rad) or
ImageQuant™ LAS500 Imaging System (GE Healthcare Bio-Sciences AB,
Uppsala, Sweden).
Flow cytometry
HSC-2 cells were harvested by trypsinization,
centrifuged into cell pellets and resuspended in FACS buffer (PBS
containing 0.5% bovine serum albumin). The cells were stained for
30 min at 4°C with FITC-conjugated anti-human CD44 antibody at a
concentration of 1:50 or an isotype-matched FITC-conjugated IgG
control antibody (both from BD Biosciences, San Jose, CA, USA).
Flow cytometry was performed using an EPICS Altra flow cytometer,
and data were analyzed using Expo-3 v1.2B software (Beckman
Coulter, Brea, CA, USA).
Cell cycle analysis
HSC-2 cells were treated with SN-38, gefitinib or
the combination for 24 h at 37°C. Nuclei were labeled with
propidium iodide (BD Biosciences), and the DNA contents of
propidium iodide-labeled nuclei were measured via flow cytometry
according to the manufacturer's instructions. Data acquisition and
analysis were performed using an EC800 flow cytometer with EC800
analysis software (Sony Biotechnology, Tokyo, Japan).
Proteasome and lysosome
inhibition
The proteasome inhibitor MG132 was purchased from
Selleckchem (Houston, TX, USA). HSC-2 cells were pretreated with
the inhibitors for 30 min before stimulation with chemotherapeutic
agents. The lysosome inhibitor chloroquine was purchased from Tokyo
Chemical Industry Co., Ltd. HSC-2 cells were pretreated for 1 h
before stimulation with chemotherapeutic agents.
Statistical analysis
All quantitative data are presented as the mean ±
standard deviation. Data were evaluated using one-way analysis of
variance followed by the Turkey-Kramer post-test. P-values <0.05
were accepted as statistically significant.
Results
Combined SN-38 and gefitinib treatment
inhibits HNSCC cell growth in a synergistic manner
First, we screened combinations of existing
chemotherapeutic agents to find those that synergistically
inhibited HNSCC cell growth. Among them, we focused on SN-38, which
is the active metabolite produced from irinotecan hydrochloride - a
type I DNA topoisomerase inhibitor -after it is metabolized by
carboxylesterase in the liver (20)
and gefitinib, which is a specific EGFR tyrosine kinase inhibitor
(21). The half-maximal inhibitory
concentration (IC50) of SN-38 and gefitinib in the HNSCC
cell line HSC-2 were 6.2±0.9 nM and 38.5±18.8 µM, respectively
(Table I). We next investigated the
cell growth inhibition achieved by combinations at different
concentrations: one quarter, one half, IC50, and 2- and
4-fold of the IC50. Combinatorial treatment at each
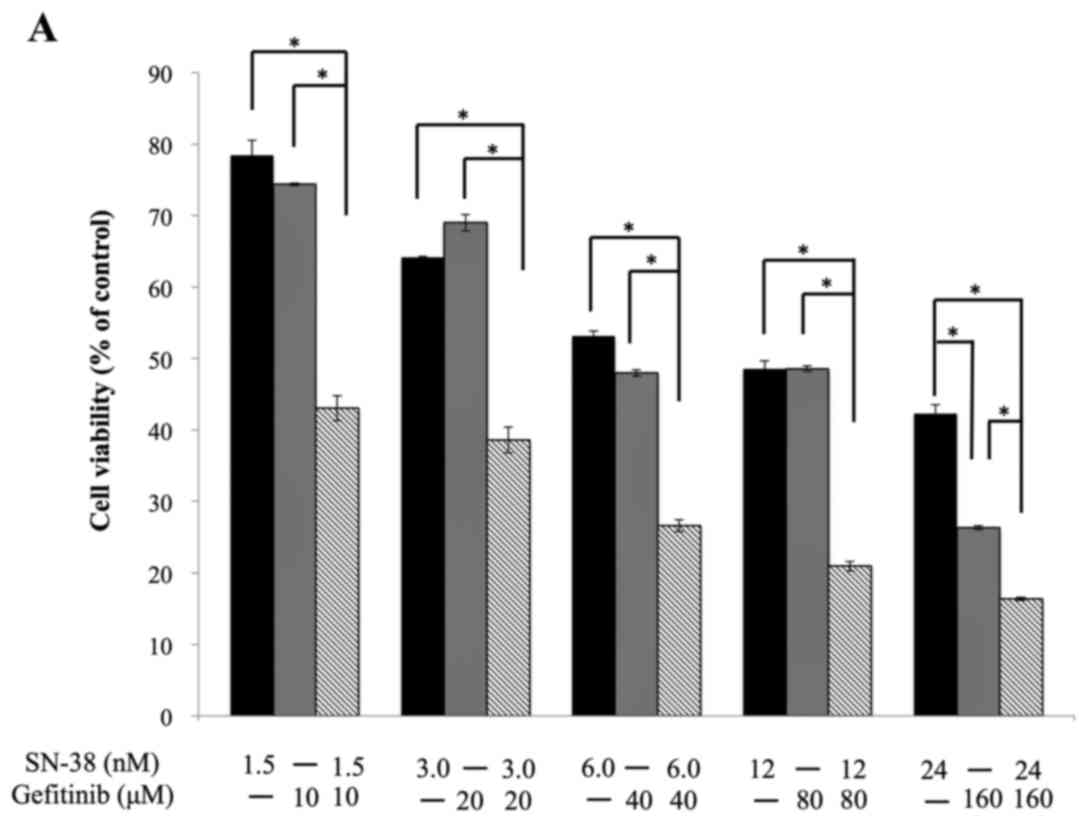
concentration significantly inhibited cell proliferation (Fig. 1A). Furthermore, we investigated
whether the combined treatment showed additive or synergistic
effects by determining the CI. A CI value <1 indicates a
synergistic effect, and CI values of 1 indicate an additive effect.
All combined SN-38 and gefitinib treatments (different doses)
showed CI values <1, revealing that the inhibitory effects on
HNSCC cell proliferation were synergistic for this combination
(Fig. 1B and C, and Table II). We also analyzed cleaved PARP
levels, a marker of apoptosis, and the data suggested that the
combination treatment enhanced HNSCC cell apoptosis (Fig. 1D). These data suggested that the
combined SN-38 and gefitinib treatment synergistically inhibited
HNSCC cell growth and induced apoptosis.
 | Table I.The half-maximal inhibitory
concentration (IC50) of SN-38 and gefitinib for HSC-2
and HaCaT cells. |
Table I.
The half-maximal inhibitory
concentration (IC50) of SN-38 and gefitinib for HSC-2
and HaCaT cells.
| The half-maximal
inhibitory concentration (IC50) | HSC-2 (head and
neck squamous cell carcinoma cell line) | HaCaT (human skin
keratinocyte cell line) |
|---|
| SN-38 (nM) | 6.2±0.9 | 32.5±8.6 |
| Gefitinib (µM) | 38.5±18.8 | 0.63±0.01 |
 | Table II.The CI index of SN-38 and gefitinib
for HSC-2 cells using the Chou and Talalay method. CI values for
actual experimental points. |
Table II.
The CI index of SN-38 and gefitinib
for HSC-2 cells using the Chou and Talalay method. CI values for
actual experimental points.
| Total dose
(µM) | Fa | CI value |
|---|
| 10.0149 | 0.506 | 0.35601 |
| 20.0298 | 0.463 | 0.53007 |
| 40.0596 | 0.358 | 0.52431 |
| 80.1192 | 0.264 | 0.54891 |
| 160.238 | 0.195 | 0.65120 |
CD44 is expressed in regrowth tumor
tissue
Our in vitro data suggested that the combined
SN-38 and gefitinib treatment could be effective against HNSCC.
Therefore, we attempted to verify these findings in vivo
using a xenograft mouse model. Contrary to our expectation, there
was no significant difference between gefitinib single treatment
and combined gefitinib and SN-38 treatment in this model (Fig. 2A). However, tumors in 3 of 5 mice
from the gefitinib single administration group showed renewed
growth 6 weeks (42 days) after terminating treatment (Fig. 2A). In contrast, post-treatment tumor
growth was not observed by the end of observation in the combined
treatment group. We next investigated EGFR and CD44 expression in
tumors that were removed 42 days after treatment termination by
immunohistochemistry. These results demonstrated that EGFR
(Fig. 2B) and CD44 (Fig. 2C) were more highly expressed in the
tumor tissues from the gefitinib-only treated mice compared with
the combined treatment mice. In the tumor tissues from the
gefitinib-only treated mice, both EGFR and CD44 were clearly
expressed along the cell membrane (Fig.
2B and C).
Combined SN-38 and gefitinib treatment
inhibits CD44 expression in HNSCC cells
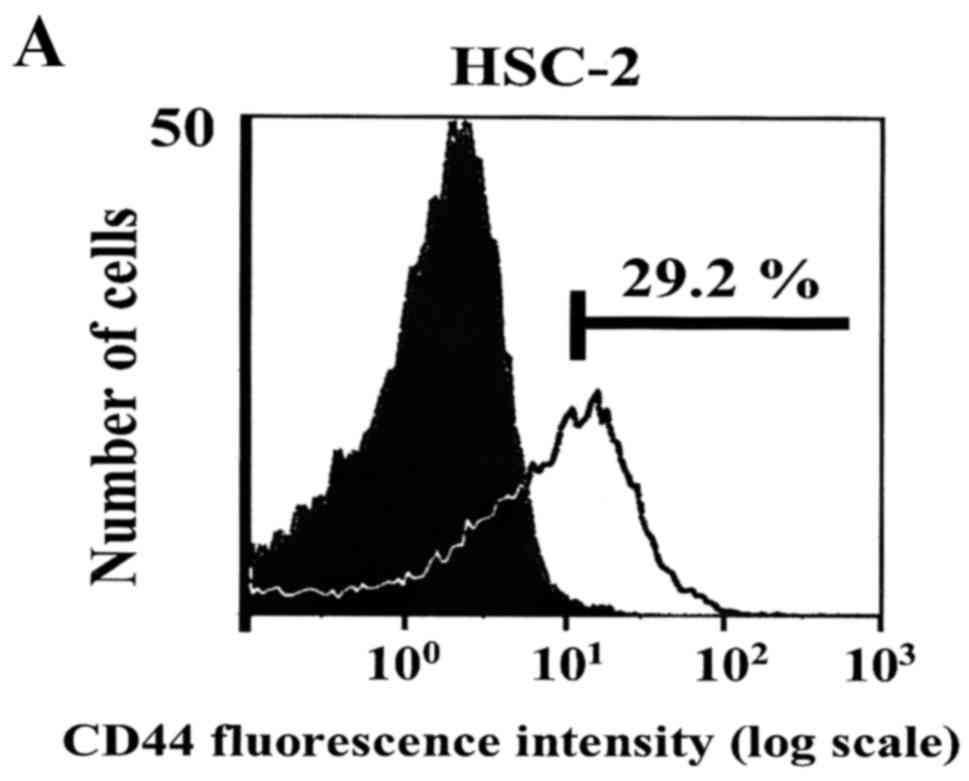
Next, we examined the proportion of CD44-positive
cells in different HNSCC cell lines. These data showed that ~30% of
HSC-2 cells were CD44-positive (Fig.
3A). We also found that CD44 expression was higher in HSC-3
cells than in HSC-2 cells, while the SAS and Ca9-22 cell lines had
less CD44 expression than HSC-2. In summary, CD44 expression was
confirmed in all 4 HNSCC cell lines (Fig. 3B).
We then examined the molecular mechanism by which
the combined treatment inhibited HNSCC cell proliferation. In
accordance with previously published results, cell cycle analysis
revealed that SN-38 decreased the G0/G1 phase from 66 to 52%, while
the S phase population increased from 16 to 21% (Table III). Following gefitinib
treatment, the percentage of G0/G1 phase HSC-2 cells was
significantly increased to 81%, and the S phase population was
significantly decreased to 4% (Table
III). The combination treatment group showed the same tendency
as gefitinib, and there were no significant differences between the
two (Table III). EGFR and HER2
phosphorylation were inhibited by the gefitinib single treatment,
and there was no significant difference between the gefitinib
single treatment and the combined treatment (Fig. 3C). However, CD44 expression was
inhibited in the combined gefitinib and SN-38 group (Fig. 3C, lane 4). Furthermore, when CD44
expression was inhibited by siRNA transfection (Fig. 3D compare lanes 1 and 3), EGFR
expression was also inhibited (Fig.
3D, lane 3). In the context of CD44 silencing, we were able to
further reduce expression of the remaining protein by SN-38 or
gefitinib treatment alone (Fig. 3D,
compare lanes 4 and 5 with lane 3). Notably, when the combined
treatment was applied to CD44-knockdown cells, CD44 and EGFR were
more strongly inhibited, and apoptosis was also induced to a
greater extent (Fig. 3D, lane 6).
These data revealed that combined SN-38 and gefitinib treatment
inhibited CD44 expression in vitro.
 | Table III.Cell cycle distributions of HSC-2
cells following SN-38, gefitinib or combined treatment. Cell cycle
distribution: average (%) ± SD. |
Table III.
Cell cycle distributions of HSC-2
cells following SN-38, gefitinib or combined treatment. Cell cycle
distribution: average (%) ± SD.
| Cell cycle
phase | Control | SN-38 (6 nM) | Gefitinib (40
µM) | Combination |
|---|
| G0+G1 | 66.5±7.2 | 51.8±2.4 | 80.7±2.0 | 76.0±2.0 |
| S | 15.8±2.3 | 21.4±2.6 | 4.2±0.3 | 7.6±2.3 |
| G2+M | 16.9±8.1 | 26.7±4.5 | 14.8±2.0 | 15.1±4.1 |
CD44-positive HNSCC cells have
characteristics of cancer stem-like cells
It has been reported that CD44 expression in HNSCC
cells is a marker of cancer stem cells that has a significant
relationship with prognosis (14,22).
Our data showed that CD44 expression was markedly high in the
growing tumor tissues from xenograft models post-treatment
(Fig. 2C). Therefore, we next
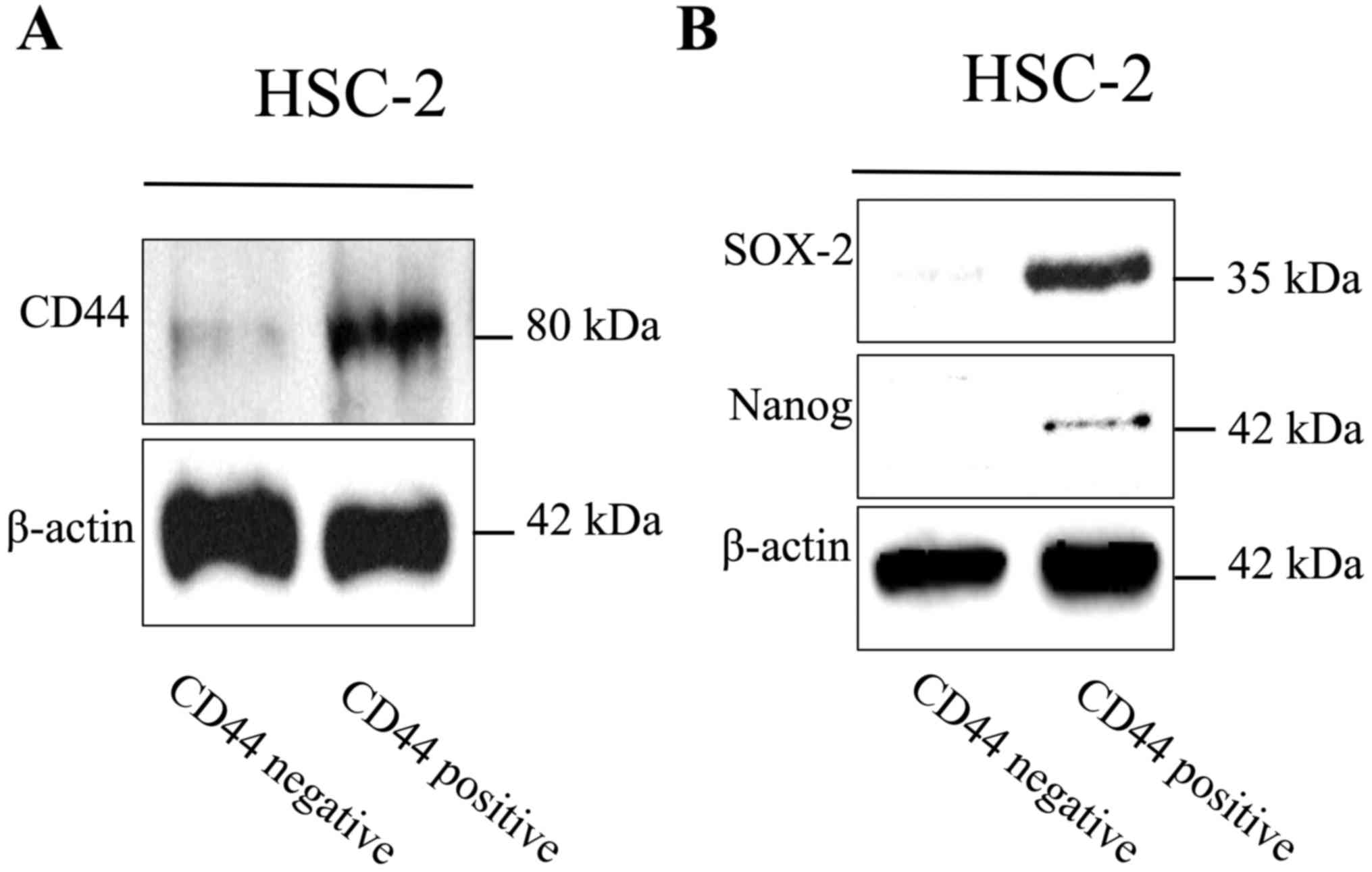
examined whether CD44-positive HNSCC cells showed cancer stem cell
characteristics. CD44-positive cells that were isolated by the MACS
magnetic bead system strongly expressed SOX-2 and Nanog, which are
known transcription factors expressed in stem cells (23,24)
(Fig. 4A and B). Moreover,
CD44-positive cells showed significantly increased migratory
ability (Fig. 4C). These data
revealed that CD44-positive HNSCC cells have characteristics of
cancer stem-like cells.
Lysosome inhibition enhances CD44
expression in HNSCC cells
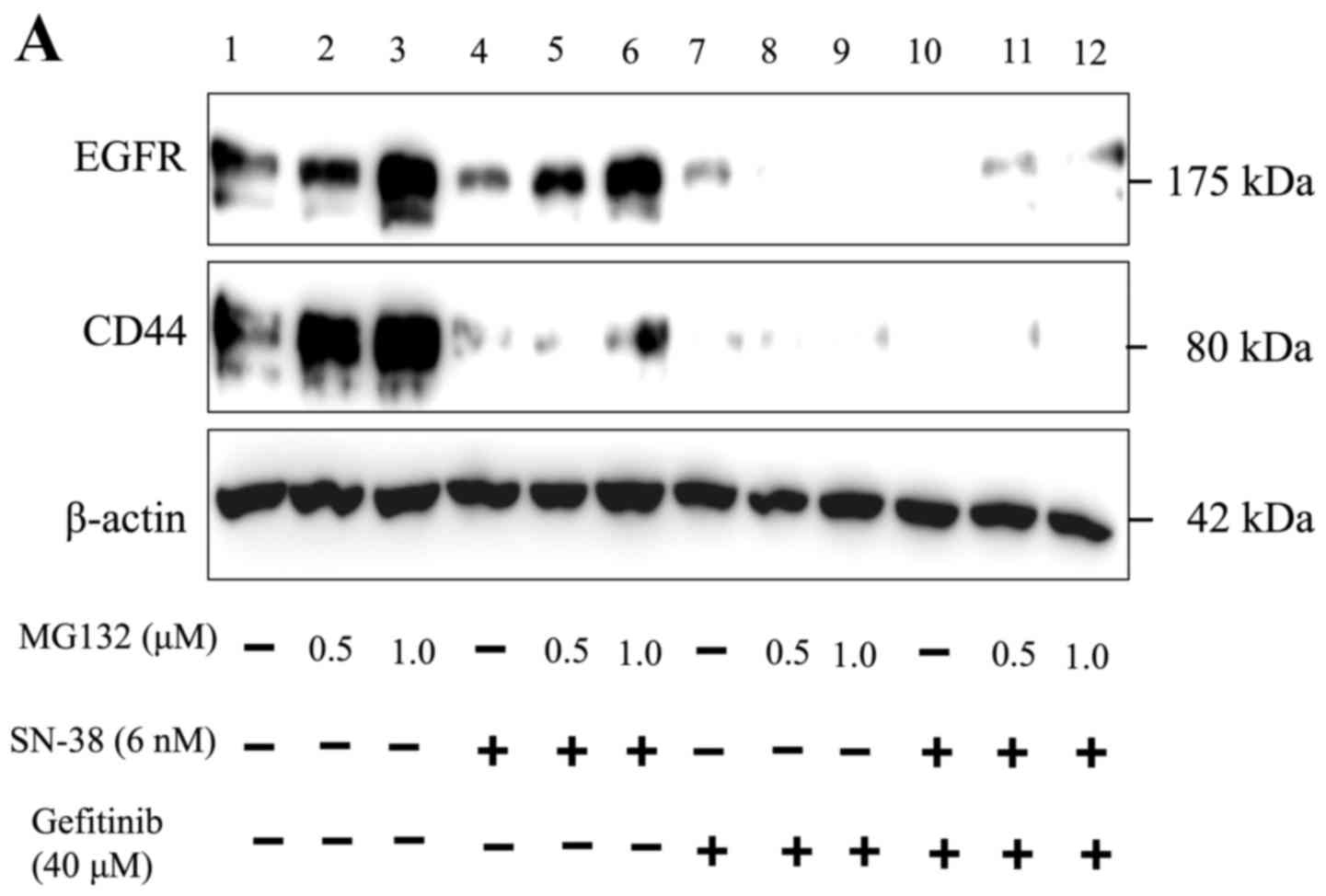
Next, we examined how the combination treatment
affects CD44 expression, testing the hypothesis that it may promote
CD44 degradation. Therefore, we tested whether the reduction in
CD44 and EGFR expression could be recovered by inhibiting the
proteasome. These results showed that the reduced EGFR expression
following treatment was recovered, and the reduction of CD44 by
SN-38 single treatment was partially recovered when combined with
MG132 (Fig. 5A, compare lanes 6 and
4). However, the reduction of CD44 expression by gefitinib single
treatment (Fig. 5A, compare lanes 9
and 7) and the combination treatment were not recovered by MG132
pretreatment (Fig. 5A, compare
lanes 12 and 10). Next, we investigated whether the reduction of
CD44 and EGFR expression could be recovered when the lysosome was
inhibited. There was no effect on EGFR expression when lysosomes
were inhibited by chloroquine in the absence of the chemotherapy
drugs (Fig. 5B). In contrast, the
reduction of CD44 expression by gefitinib treatment alone and
gefitinib plus SN-38 treatment was partially recovered by
chloroquine (Fig. 5B, compare lanes
12 and 10).
Discussion
Initially, we used existing chemotherapeutic agents
to identify an effective combination that inhibited HNSCC cell
proliferation and induced apoptosis. Among those tested, SN-38, an
active metabolite of irinotecan hydrochloride, and gefitinib, an
EGFR tyrosine kinase inhibitor, were found to inhibit cell
proliferation and induce apoptosis in HNSCC cells in a synergistic
manner (Fig. 1). We then tested the
effectiveness of this combination therapy on an in vivo
xenograft mouse model. Contrary to our expectations, there was no
significant difference in tumor growth inhibition between gefitinib
treatment and combined gefitinib and SN-38 administration (Fig. 2A). However, by continuing tumor
measurements after terminating chemotherapy, we found that tumors
from the gefitinib-only treatment group resumed growth. Conversely,
tumors from the combined treatment group did not show growth after
treatment had stopped. Investigating tumor tissues from the
gefitinib-only group that had begun to progress revealed that
expression of the major cancer stem cell marker CD44 was increased
in these tissues compared with tumor tissues from the combined
treatment group (Fig. 2C).
Therefore, we next focused on elucidating how combined SN-38 and
gefitinib treatment affected CD44 expression in HNSCC cells. Our
data showed that CD44 degradation following combined SN-38 and
gefitinib treatment was partially blocked by pretreatment with the
lysosome inhibitor chloroquine (Fig.
5B, compare lanes 12 and 10), while CD44 degradation was not
blocked by pretreatment with the proteasome inhibitor MG132
(Fig. 5A, compare lanes 12 and 10).
These studies clearly demonstrated that SN-38 treatment partially
promoted the proteasomal degradation of CD44, while gefitinib and
the combined treatment promoted lysosomal CD44 degradation
(Fig. 5).
Many researchers have reported that CD44 is an
important cancer stem cell marker in HNSCC and that CD44 expression
is closely related to prognosis (22,25,26).
It has also been shown that cancer stem cells have an increased
capacity for self-renewal, drug resistance, metastasis and tumor
recurrence (27). In the present
study, we demonstrated that CD44-positive HNSCC cells have cancer
stem-like characteristics (Fig. 4).
We also showed that combined SN-38 and gefitinib treatment reduced
CD44 expression in HNSCC cells. Furthermore, our data suggested
that reduced CD44 expression was attributable to lysosomal CD44
degradation (Fig. 5).
However, when considering whether combined
irinotecan hydrochloride (CPT-11), which becomes SN-38 when
metabolized, and gefitinib treatment may be effective for
clinically staged HNSCC, it should be noted that gefitinib has no
significant clinical effect on HNSCC (28). In our data, the IC50 of
HaCaT cells, which are normal human skin keratinocytes, was 0.63
µM, which is ~60 times more sensitive than HSC-2 cells, whose
IC50 was 38.5 µM (Table
I). These data indicate that the proliferation of normal
squamous cells could be inhibited before HNSCC cells are inhibited.
Therefore, it is expected that the combined treatment of irinotecan
hydrochloride and gefitinib may not be realistic for HNSCC in the
clinic.
Nevertheless, other studies have reported that
lysosomal CD44 degradation in ovarian cancer cells inhibits
metastasis (29). Therefore,
inhibition of CD44 expression by enhancing lysosomal CD44
degradation may also inhibit HNSCC tumor metastasis. Additionally,
CD44 was highly expressed in tumors that showed renewed growth
after gefitinib treatment (Fig. 2),
while the combination therapy, in which CD44 expression was
inhibited, did not show tumor regrowth after finishing chemotherapy
(Fig. 2). Therefore, the reduced
CD44 expression due to enhanced lysosomal degradation may inhibit
tumor regrowth after therapy.
Studies have shown that there are several HNSCC
cancer stem cell markers in addition to CD44, including ALDH
(30) and CD133 (31); double-positive cells (CD44 plus ALDH
or CD133) have also been shown to be more tumorigenic and prone to
metastasis compared with CD44 single-positive cells. However, we
did not investigate these double-positive cells in the present
study. Thus, in future studies we may consider these other cancer
stem cell markers. Additionally, we showed that inhibition of CD44
expression may inhibit tumor regrowth after treatment, but it may
be necessary to verify this finding in greater detail in the
future.
In conclusion, we demonstrated that combined SN-38
and gefitinib treatment enhanced the lysosomal degradation of CD44,
which is a known marker of cancer stem cells. This combination
chemotherapy was also shown to inhibit tumor regrowth after
treatment. Together, these data indicate the potential for
chemotherapies that enhance the lysosomal degradation of CD44 to
inhibit tumor metastasis and recurrence.
Acknowledgements
The present study was supported in part by JSPS
KAKENHI (grant nos. 26462854, 26861748 and 17K11691). We wish to
thank Kentaro Kikuchi, PhD and Kaoru Kusama, PhD from the Division
of Oral Pathology, Meikai University for their technical advice
regarding immunohistochemistry procedures. We also thank James P.
Mahaffey, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this
manuscript.
References
|
1
|
Sanderson RJ and Ironside JA: Squamous
cell carcinomas of the head and neck. BMJ. 325:822–827. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stewart JS, Cohen EE, Licitra L, van
Herpen CM, Khorprasert C, Soulieres D, Vodvarka P, Rischin D, Garin
AM, Hirsch FR, et al: Phase III study of gefitinib compared with
intravenous methotrexate for recurrent squamous cell carcinoma of
the head and neck [corrected]. J Clin Oncol. 27:1864–1871. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vermorken JB, Remenar E, van Herpen C,
Gorlia T, Mesia R, Degardin M, Stewart JS, Jelic S, Betka J, Preiss
JH, et al EORTC 24971/TAX 323 Study Group, : Cisplatin,
fluorouracil, and docetaxel in unresectable head and neck cancer. N
Engl J Med. 357:1695–1704. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vermorken JB, Mesia R, Rivera F, Remenar
E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol
D, et al: Platinum-based chemotherapy plus cetuximab in head and
neck cancer. N Engl J Med. 359:1116–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: Accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zöller M: CD44: Can a cancer-initiating
cell profit from an abundantly expressed molecule? Nat Rev Cancer.
11:254–267. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gupta PB, Onder TT, Jiang G, Tao K,
Kuperwasser C, Weinberg RA and Lander ES: Identification of
selective inhibitors of cancer stem cells by high-throughput
screening. Cell. 138:645–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Major AG, Pitty LP and Farah CS: Cancer
stem cell markers in head and neck squamous cell carcinoma. Stem
Cells Int. 2013:3194892013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Orian-Rousseau V: CD44, a therapeutic
target for metastasising tumours. Eur J Cancer. 46:1271–1277. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kajita M, Itoh Y, Chiba T, Mori H, Okada
A, Kinoh H and Seiki M: Membrane-type 1 matrix metalloproteinase
cleaves CD44 and promotes cell migration. J Cell Biol. 153:893–904.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim HR, Wheeler MA, Wilson CM, Iida J, Eng
D, Simpson MA, McCarthy JB and Bullard KM: Hyaluronan facilitates
invasion of colon carcinoma cells in vitro via interaction with
CD44. Cancer Res. 64:4569–4576. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Golshani R, Lopez L, Estrella V, Kramer M,
Iida N and Lokeshwar VB: Hyaluronic acid synthase-1 expression
regulates bladder cancer growth, invasion, and angiogenesis through
CD44. Cancer Res. 68:483–491. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ohkoshi E and Umemura N: Induced
overexpression of CD44 associated with resistance to apoptosis on
DNA damage response in human head and neck squamous cell carcinoma
cells. Int J Oncol. 50:387–395. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Joshua B, Kaplan MJ, Doweck I, Pai R,
Weissman IL, Prince ME and Ailles LE: Frequency of cells expressing
CD44, a head and neck cancer stem cell marker: Correlation with
tumor aggressiveness. Head Neck. 34:42–49. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu C, Kelnar K, Liu B, Chen X,
Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, et
al: The microRNA miR-34a inhibits prostate cancer stem cells and
metastasis by directly repressing CD44. Nat Med. 17:211–215. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chou TC: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chou TC: Theoretical basis, experimental
design, and computerized simulation of synergism and antagonism in
drug combination studies. Pharmacol Rev. 58:621–681. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo B, Cao S, Tóth K, Azrak RG and Rustum
YM: Overexpression of Bax enhances antitumor activity of
chemotherapeutic agents in human head and neck squamous cell
carcinoma. Clin Cancer Res. 6:718–724. 2000.PubMed/NCBI
|
|
19
|
Stewart CF, Leggas M, Schuetz JD, Panetta
JC, Cheshire PJ, Peterson J, Daw N, Jenkins JJ III, Gilbertson R,
Germain GS, et al: Gefitinib enhances the antitumor activity and
oral bioavailability of irinotecan in mice. Cancer Res.
64:7491–7499. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Azrak RG, Cao S, Durrani FA, Toth K,
Bhattacharya A and Rustum YM: Augmented therapeutic efficacy of
irinotecan is associated with enhanced drug accumulation. Cancer
Lett. 311:219–229. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Prahallad A, Sun C, Huang S, Di
Nicolantonio F, Salazar R, Zecchin D, Beijersbergen RL, Bardelli A
and Bernards R: Unresponsiveness of colon cancer to BRAF(V600E)
inhibition through feedback activation of EGFR. Nature.
483:100–103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kokko LL, Hurme S, Maula SM, Alanen K,
Grénman R, Kinnunen I and Ventelä S: Significance of site-specific
prognosis of cancer stem cell marker CD44 in head and neck
squamous-cell carcinoma. Oral Oncol. 47:510–516. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qin D, Gan Y, Shao K, Wang H, Li W, Wang
T, He W, Xu J, Zhang Y, Kou Z, et al: Mouse meningiocytes express
Sox2 and yield high efficiency of chimeras after nuclear
reprogramming with exogenous factors. J Biol Chem. 283:33730–33735.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ang YS, Tsai SY, Lee DF, Monk J, Su J,
Ratnakumar K, Ding J, Ge Y, Darr H, Chang B, et al: Wdr5 mediates
self-renewal and reprogramming via the embryonic stem cell core
transcriptional network. Cell. 145:183–197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Prince ME, Sivanandan R, Kaczorowski A,
Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF and Ailles
LE: Identification of a subpopulation of cells with cancer stem
cell properties in head and neck squamous cell carcinoma. Proc Natl
Acad Sci USA. 104:pp. 973–978. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Faber A, Barth C, Hörmann K, Kassner S,
Schultz JD, Sommer U, Stern-Straeter J, Thorn C and Goessler UR:
CD44 as a stem cell marker in head and neck squamous cell
carcinoma. Oncol Rep. 26:321–326. 2011.PubMed/NCBI
|
|
27
|
Giancotti FG: Mechanisms governing
metastatic dormancy and reactivation. Cell. 155:750–764. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cohen EE, Kane MA, List MA, Brockstein BE,
Mehrotra B, Huo D, Mauer AM, Pierce C, Dekker A and Vokes EE: Phase
II trial of gefitinib 250 mg daily in patients with recurrent
and/or metastatic squamous cell carcinoma of the head and neck.
Clin Cancer Res. 11:8418–8424. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Haakenson JK, Khokhlatchev AV, Choi YJ,
Linton SS, Zhang P, Zaki PM, Fu C, Cooper TK, Manni A, Zhu J, et
al: Lysosomal degradation of CD44 mediates ceramide
nanoliposome-induced anoikis and diminished extravasation in
metastatic carcinoma cells. J Biol Chem. 290:8632–8643. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chinn SB, Darr OA, Owen JH, Bellile E,
McHugh JB, Spector ME, Papagerakis SM, Chepeha DB, Bradford CR,
Carey TE, et al: Cancer stem cells: Mediators of tumorigenesis and
metastasis in head and neck squamous cell carcinoma. Head Neck.
37:317–326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oliveira LR, Castilho-Fernandes A,
Oliveira-Costa JP, Soares FA, Zucoloto S and Ribeiro-Silva A:
CD44+/CD133+ immunophenotype and matrix metalloproteinase-9:
Influence on prognosis in early-stage oral squamous cell carcinoma.
Head Neck. 36:1718–1726. 2014. View Article : Google Scholar : PubMed/NCBI
|