Introduction
Due to its high metastasis and invasion,
glioblastoma is the most common malignant tumor of the central
nervous system (CNS) in adults with poor prognoses (1), and a median survival of ~10–14 months
after the initial diagnosis (2).
Accordingly, the recurrence of gliomas remains unavoidable.
Currently, early diagnosis and a multimodal approach are the basic
requirements for glioma treatments, which include surgical
resection, chemotherapeutics and radiotherapy and have an extremely
poor prognosis. The major challenges for glioma treatment include
many barriers, such as the extent of surgical resection and
resistance to radiotherapy and chemotherapy (3). Thus, it is important to explore more
efficacious measures against glioma to address the aforementioned
issues, and there is an urgent need to develop new compounds
against glioma.
Epidermal growth factor receptor (EGFR) is a member
of the HER family, which also includes HER2, HER3 and HER4, and
EGFR has been identified as an oncogene in many malignant tumors
(4,5). EGFR and its downstream signaling
pathways are pivotal regulators of many cellular processes,
including cell differentiation, metabolism, proliferation and
survival, in a large variety of tumors (6–8). EGFR
is also overexpressed in most gliomas (9). Therefore, therapies that target EGFR
and its downstream signaling pathways may be potential treatments
for glioblastoma.
Oxymatrine (OM), a natural quinolizidine alkaloid
that is the principal component of the traditional Chinese herb
Sophora flavescens, has been reported to produce a great
diversity of pharmacological effects, such as anti-inflammatory,
antiviral, immunoregulatory and anti-apoptosis effects. Originally,
OM was used for the treatment of viral hepatitis (10–14).
More recent in vitro studies have revealed the significant
antitumor activity of OM in the cells of many human cancers,
including hepatic (15), pancreatic
(16,17), gastric (18) and colorectal carcinoma (19), as well as mastocarcinoma (20). However, the antitumor activity of OM
in glioma cells has been rarely studied and the mechanism by which
OM exerts its antitumor effect against glioma has not been
reported.
In the present study, we studied the effect of OM on
glioblastoma cells. We found that OM markedly suppressed the
proliferation and invasion, as well as promoted the apoptosis of
glioma cells. Further studies revealed that OM arrested the cell
cycle at the G0/G1 phase and decreased the protein expression
levels of cyclin D1 and CDK4/6. In addition, our findings are the
first to demonstrate that the EGFR/PI3K/Akt/mTOR signaling pathway
and STAT3 are involved in the OM-induced inhibition in glioma
cells. In summary, these results indicated that OM may be a
promising antineoplastic agent against gliomas.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM) and fetal
bovine serum (FBS) were purchased from Corning Cellgro (Mediatech,
Inc., Manassas, VA, USA), and antibiotics (streptomycin and
penicillin) were purchased from Corning Incorporated (Corning, NY,
USA). OM was purchased from Shanghai Aladdin Bio-Chem Technology
Co., Ltd. (Shanghai, China), and the purity (over 98%) was
confirmed by high-performance liquid chromatography (HPLC). OM was
dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis,
MO, USA) at a concentration of 10 mg/ml, stored at −20°C, and then
freshly diluted with culture medium for each experiment. An Annexin
V-FITC Apoptosis Detection kit and a Cycletest Plus DNA Reagent kit
were purchased from BD Biosciences (Franklin Lakes, NJ, USA).
Cell culture
The U251MG human malignant glioma cell line was
obtained from the Institute of Brain Science of Harbin Medical
University (Harbin, China). The cells were cultured in DMEM
supplemented with 10% FBS and 1% streptomycin/penicillin at 37°C in
an incubator with a humidified atmosphere of 5% CO2 and
95% air.
Cell viability assay
A Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) assay was performed to explore
the cytotoxic effects of OM and/or erlotinib on human glioblastoma
cells. U251MG cells were seeded in a 96-well plate at a density of
1,000 cells/well and then treated with OM at various concentrations
(0, 0.25, 0.5, 1, 2 and 4 mg/ml) for 24, 48 and 72 h, or treated
with 2 µM erlotinib in the absence or presence of 1 mg/ml OM for 48
h. Following the treatment with OM and/or erlotinib, 10 µl of CCK-8
was added to each well, and the cells were incubated at 37°C for 1
h. A microplate reader (Tecan Group Ltd., Männedorf, Switzerland)
was used to measure the absorbance of each well at 450 nm to assess
the proliferation.
Detection of cell apoptosis by flow
cytometry
An Annexin V-fluorescein isothiocyanate (FITC) assay
was employed to quantify apoptotic cells by flow cytometry. U251MG
cells were seeded into 6-well plates at a density of
2×105 cells/well, cultured overnight, and then cultured
with OM at a final concentration of 0.5, 1, or 2 mg/ml or with DMSO
for 48 h. Then, the cells were harvested by trypsin, washed with
ice-cold PBS twice, collected, fixed and stained using an Annexin
V-FITC/PI double fluorescence apoptosis detection kit according to
the manufacturer's instructions. The samples were then analyzed by
flow cytometry (BD FACSCalibur System; BD Biosciences).
TUNEL assay
DNA fragmentation was performed using a One-Step
TUNEL kit (Beyotime Institute of Biotechnology, Shanghai, China)
following the manufacturer's recommendations. Briefly, U251MG cells
were exposed to OM (0.5, 1 or 2 mg/ml) for 48 h and then fixed in
4% paraformaldehyde for 10 min at room temperature. Subsequently,
the cells were washed with PBS three times and permeabilized for 2
min on ice and then the cells were resuspended in TUNEL working
solution. Following incubation for 1 h in a humidified atmosphere
at 37°C in the dark, the cells were counterstained with DAPI
staining solution for 5 min at room temperature, and then,
TUNEL-positive cells were visualized under a fluorescence
microscope. DAPI was used for the nuclear staining.
Cell cycle analysis by flow
cytometry
The cell cycle phase distribution was evaluated by
flow cytometric analysis of the DNA content of cells with the BD
Cycletest Plus DNA Reagent kit (BD Biosciences) according to the
manufacturer's instructions. In brief, cells were plated in 6-well
plates and treated with different concentrations of OM (0.5, 1 and
2 mg/ml) for 48 h, harvested, fixed in 70% pre-chilled ethanol
(−20°C) and maintained at 4°C overnight. Then, the cells were
resuspended in propidium iodide (PI) buffer (50 g/ml PI and 100
µg/ml RNase) and incubated for 30 min shielded from light at room
temperature. The cells were then washed twice with PBS. The
percentages of cells in each phase of the cell cycle were
determined by BD FACSCalibur flow cytometer (BD Biosciences,
Fraklin Lakes, NJ, USA) and analyzed by ModFit LT version 4.1
software (Verity Software House, Topsham, ME, USA).
Cell invasion assay
Cell invasion assays were performed using a Corning
Matrigel invasion assay system according to the manufacturer's
instructions. Following treatment with OM at various concentrations
(0.25, 0.5, 1 and 2 mg/ml) for 24 h or 48 h, cells were seeded for
24 h and suspended in serum-free DMEM. Then, the cells were placed
in the upper part of each chamber, whereas the lower chambers
contained FBS (10%). After incubation in a humidified 5%
CO2 atmosphere at 37°C for 24 or 48 h, the cells in the
upper chamber and on the Matrigel were mechanically removed with a
cotton swab, and the cells on the bottom side of the filter were
fixed in 4% paraformaldehyde for 30 min at room temperature.
Subsequently, the cells were washed with PBS three times and
stained with 0.1% crystal violet for 15 min at room temperature.
Then, the cells were washed three times with PBS and counted under
a inverted phase contrast microscope (magnification, ×200).
Western blot analysis
Following treatment with different concentrations of
OM (0.5, 1 and 2 mg/ml), or with 2 µM erlotinib in the absence or
presence of 1 mg/ml OM for 48 h, cells were collected, washed with
ice-cold PBS and then lysed in lysis buffer [5 mmol/l EDTA, 40
mmol/l Tris (pH 8.0), 150 mmol/l NaCl, 1% NP-40, 2 mg/ml leupeptin,
2 mg/ml aprotinin, 5 mg/ml benzamidine and 0.5 mmol/l
phenylmethylsulfonyl fluoride]. The protein concentration was
determined using a BCA protein assay kit (Beyotime Biotechnology,
Shanghai, China). An equal amount of protein (60 µg) from each
group was separated using 10% SDS-polyacrylamide gel
electrophoresis (PAGE), transferred to a PVDF membrane (EMD
Millipore, Billerica, MA, USA), blocked with 5% skim milk in
TBS-Tween 20 (0.05%, v/v) for 1 h, and then incubated with primary
antibodies at 4°C for 16 h. After washing with TBS-T, the membranes
were subsequently incubated with horseradish peroxidase-conjugated
secondary antibodies for 1 h at room temperature. GAPDH or β-actin
was used as protein loading controls. After three washes, the
target proteins were visualized using an enhanced chemiluminescent
(ECL) kit (Thermo Fisher Scientific; Pierce, Rockford, IL, USA).
The band intensities were assessed with the Gel-Pro Analyzer
Software version 4.0 (Media Cybernetics, Rockville, MD, USA).
Primary antibodies against cyclin D1 (1:1,000; cat. no. 2922), CDK4
(1:1,000; cat. no. 12790), CDK6 (1:1,000; cat. no. 13331), p-Akt
(Ser473; 1:500; cat. no. 9271), Akt (1:500; cat. no. 9272), STAT3
(1:1,000; cat. no. 12640), p-STAT3 (Ser727; 1:1,000; cat. no.
9134), caspase-3 (1:1,000; cat. no. 9662), Bax (1:1,000; cat. no.
2774), Bcl-2 (1:1,000; cat. no. 2872), GAPDH (1:1,000; cat. no.
2118) and β-actin (1:1,000; cat. no. 4970) were purchased from Cell
Signaling Technology (Danvers, MA, USA). EGFR (1:1,000; cat. no.
GTX628887), p-EGFR (Tyr1068; 1:1,000; cat. no. GTX132810), mTOR
(1:1,000; cat. no. GTX101557) and p-mTOR (Ser2448; 1:1,000; cat.
no. GTX132803) antibodies were purchased from GeneTex Inc. (Irvine,
CA, USA). Secondary antibodies goat anti-rabbit IgG HRP (1:5,000;
cat. no. ab6721) and goat anti-mouse IgG HRP (1:5,000; cat. no.
ab205719) were purchased from Abcam (Cambridge, UK).
Statistical analysis
All statistical analyses were carried out using the
SPSS 22.0 statistical software package (IBM Corp., Armonk, NY,
USA). Data were expressed as the mean ± standard deviation (SD).
Differences among groups were analyzed by one-way analysis of
variance (ANOVA) followed by Student-Newman-Keuls (SNK) multiple
comparison test. Differences between two groups were assessed using
the Student's t-test. For each measurement, at least three
independent experiments were performed. P<0.05 was considered to
indicate a statistically significant difference.
Results
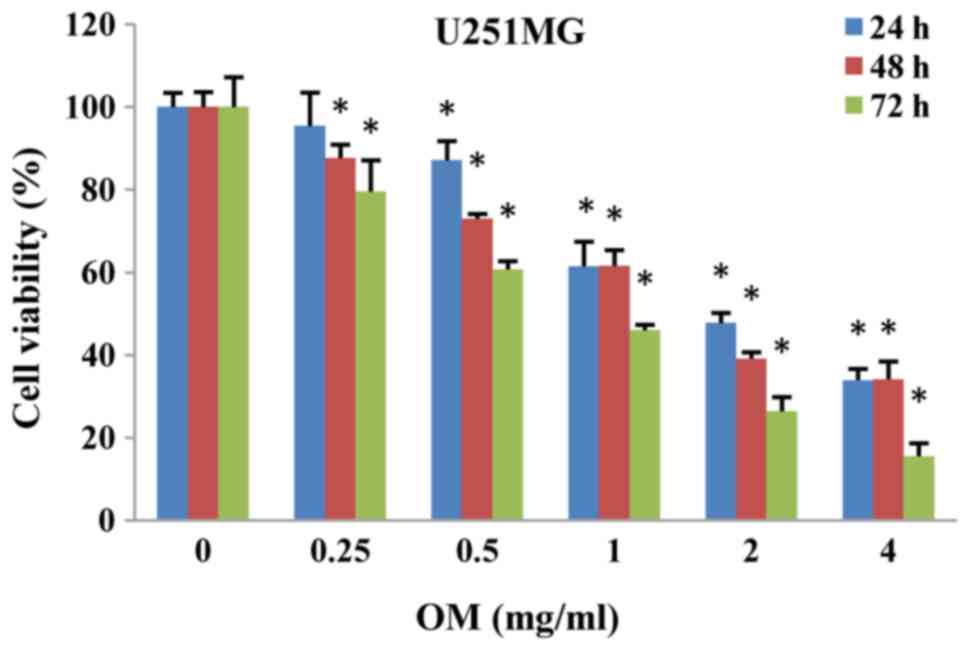
OM potently inhibits the proliferation
of glioblastoma cells
The cytotoxic effects of OM on cells were first
evaluated by the CCK-8 cell viability assay. U251MG cells were
incubated with OM at various concentrations for 24, 48 and 72 h,
and the results revealed that cell viability was reduced in a dose-
and time-dependent manner in U251MG cells (Fig. 1).
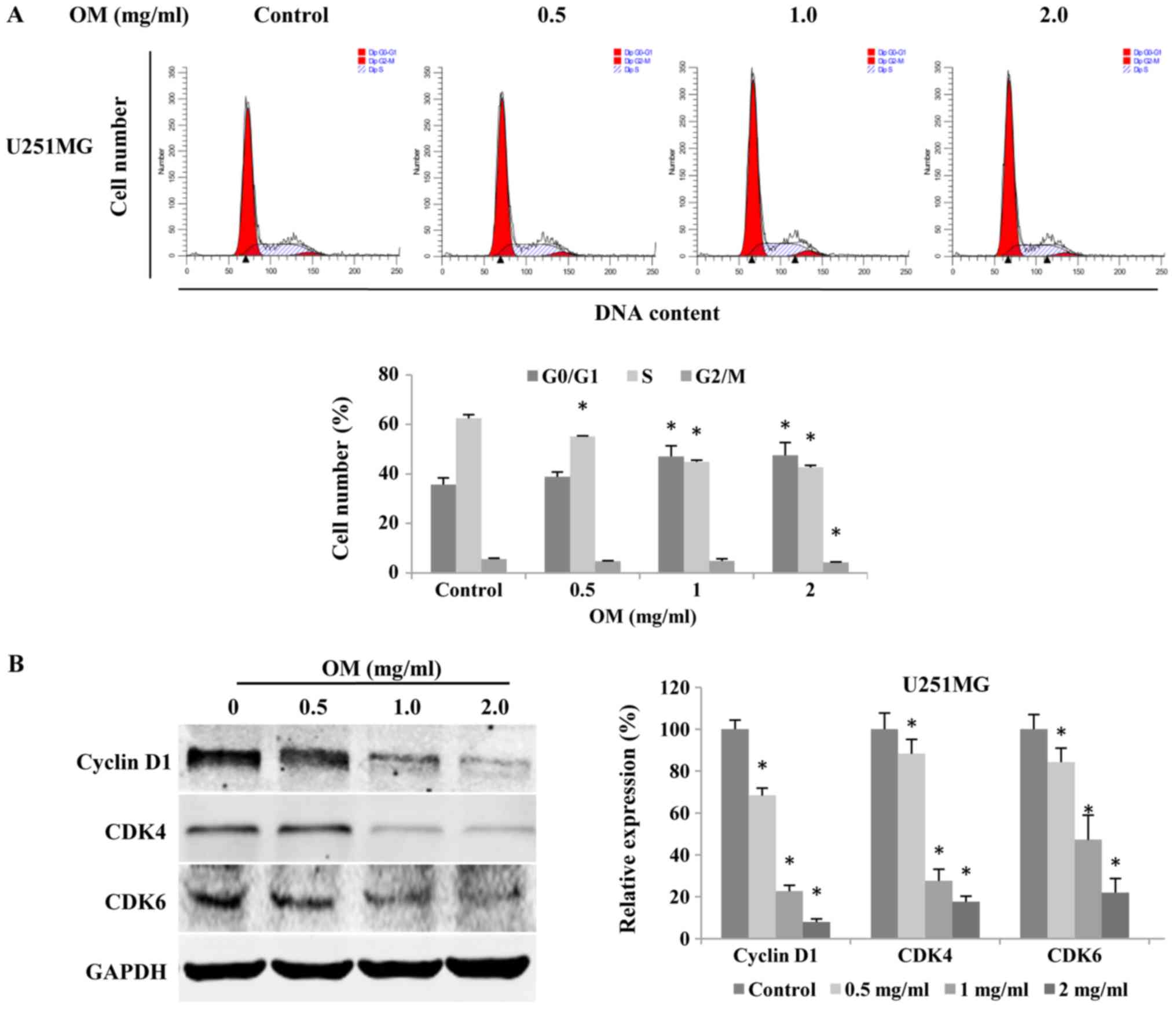
OM induces cell cycle arrest in
glioblastoma cells
To reveal the underlying mechanisms of OM in the
inhibition of the growth of glioblastoma cells, we performed cell
cycle analysis. Cells of the glioblastoma cell line U251MG were
exposed to different concentrations of OM for 48 h, and flow
cytometric analysis was then performed. Our data revealed that OM
led to cell accumulation in the G1 phase with a concomitant
decrease in the number of cells in the S phase in a dose-dependent
manner (Fig. 2A). The results
indicated that the mechanism of the OM-induced suppression of
glioblastoma cell viability involved arresting the cell cycle at
the G0/G1 phase.
Furthermore, since cyclin D1, CDK4 and CDK6 are the
key regulators of the G0/G1 phase of the cell cycle, we performed
western blot analysis to determine the expression level of the
proteins in OM-treated glioblastoma cells. The results revealed
that the protein expression levels of cyclin D1, CDK4 and CDK6 were
significantly reduced by OM (Fig.
2B), indicating that OM arrests glioma cells at the G0/G1 phase
by downregulating CDK4, CDK6 and cyclin D1. Collectively, our data
revealed that OM could potently arrest the proliferation of
glioblastoma cells.
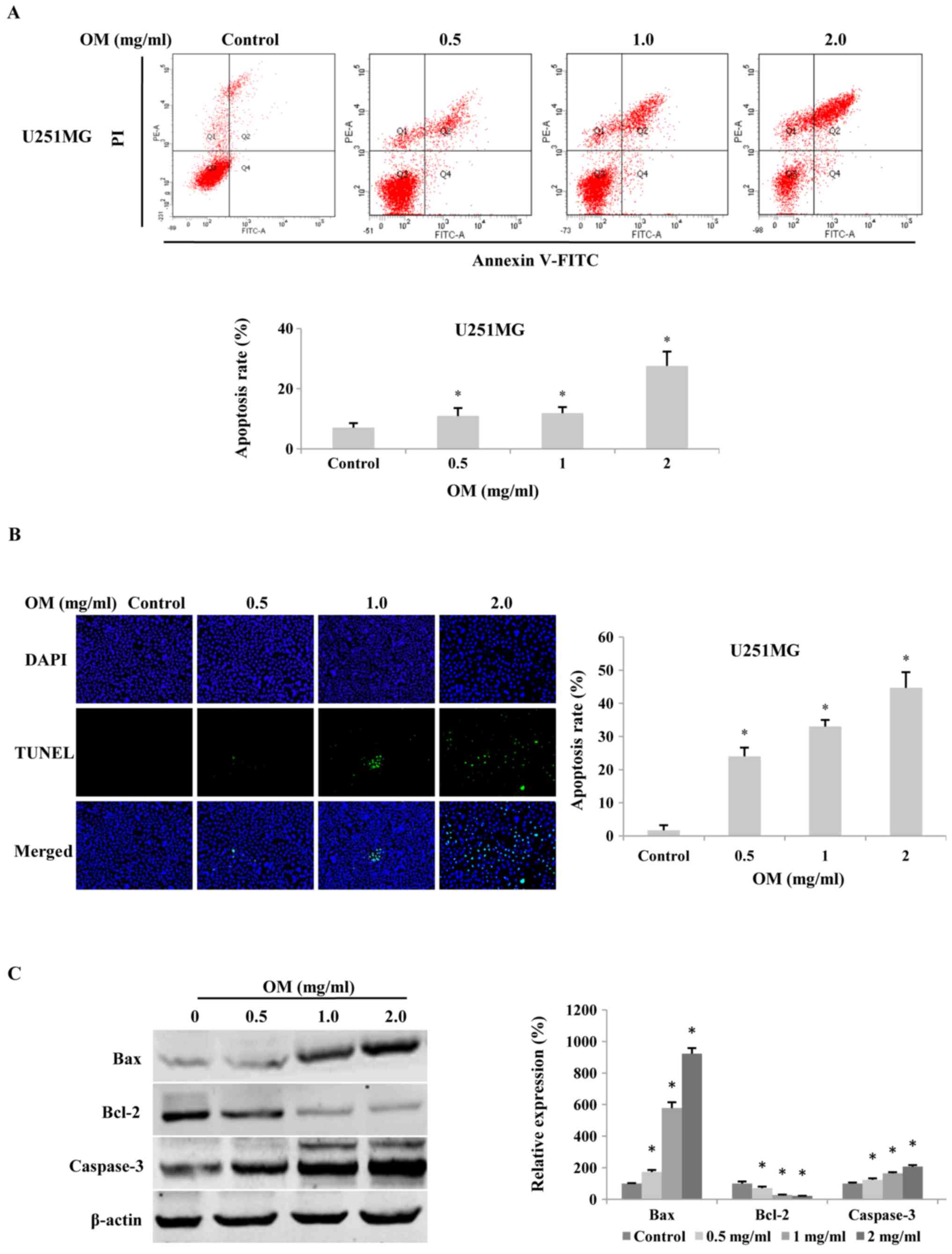
OM induces the apoptosis of
glioblastoma cells
To evaluate whether OM induced the apoptosis of
glioma cells, cells were stained with Annexin V-FITC/PI and then
analyzed by flow cytometry. The results revealed that after
treatment with various concentrations of OM for 48 h, the
percentage of apoptotic cells was significantly increased in a
dose-dependent manner compared to that of the control cells
(Fig. 3A).
Furthermore, as fragmented nuclei are typical
characteristics of apoptosis, we performed DAPI staining and a
TUNEL assay to detect alterations in the nuclei. The results
revealed that after treatment with various concentrations of OM for
48 h, the number of TUNEL-positive cells was significantly higher
in the OM-treated cells (Fig. 3B).
Furthermore, to reveal the potential mechanism of OM-induced
apoptosis in glioma cells, we assessed the protein expression
levels of Bax, Bcl-2, and caspase-3, which are important regulators
of apoptosis, by western blot analysis. The results revealed that
the expression levels of Bax and caspase-3 were significantly
increased, whereas the level of Bcl-2 was decreased after
incubation with OM for 48 h, thus leading to an increased ratio of
Bax to Bcl-2 (Fig. 3C).
Collectively, the results indicated that OM had pro-apoptotic
effects on glioblastoma cells.
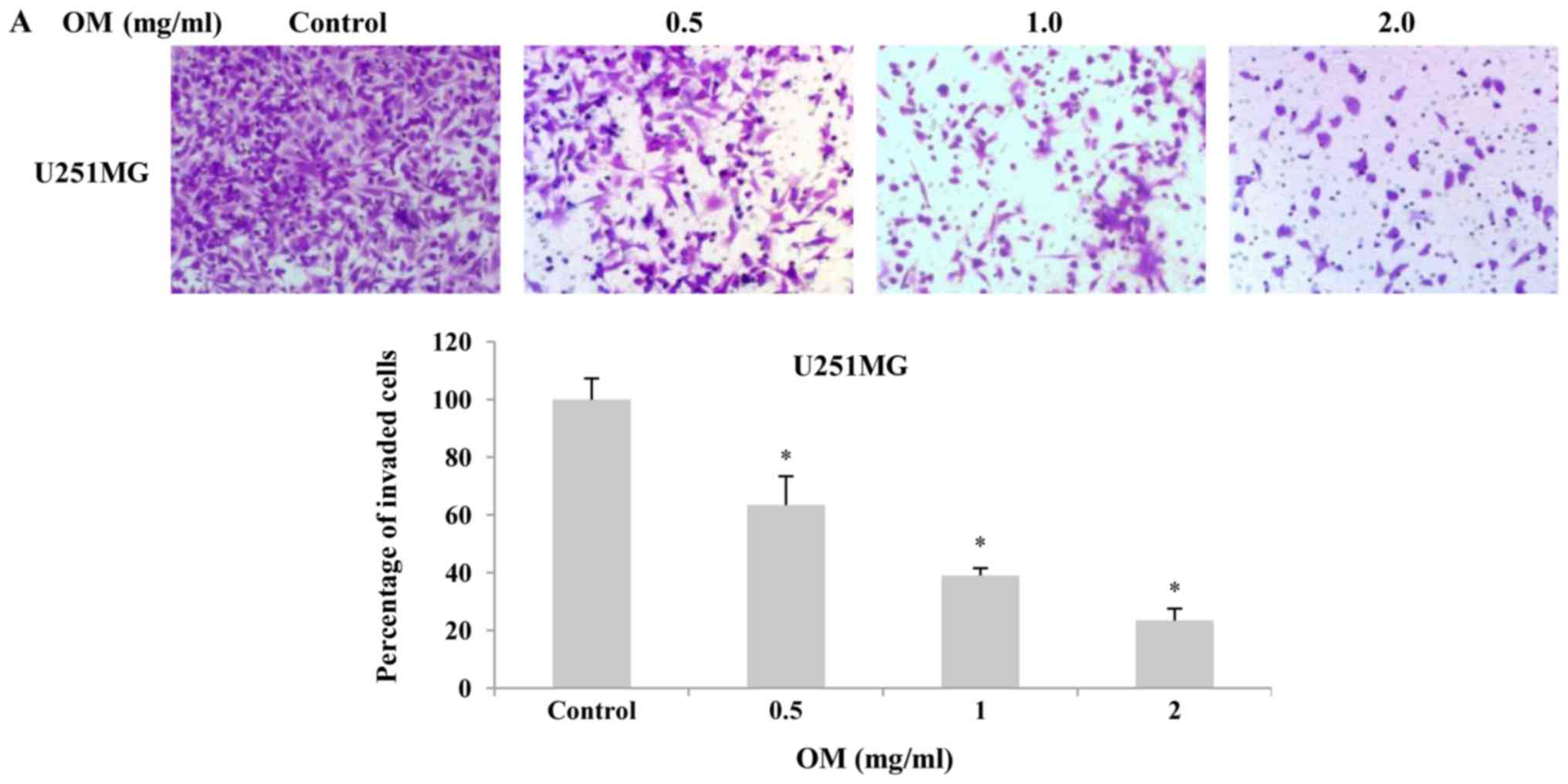
OM suppresses the invasion of
glioblastoma cells
Since invasion ability is one of the
pathophysiological features of malignant gliomas, an assay using
Matrigel-coated Transwell membranes was performed to evaluate the
effects of OM on invasion. As displayed in Fig. 4, the percentage of invading cells on
the lower side of the membrane was decreased in a dose-dependent
manner after treatment with OM for 24 h (Fig. 4B) and 48 h (Fig. 4A) compared with that in the control
group. The results indicated that the invasion of glioblastoma
cells could be significantly suppressed by OM.
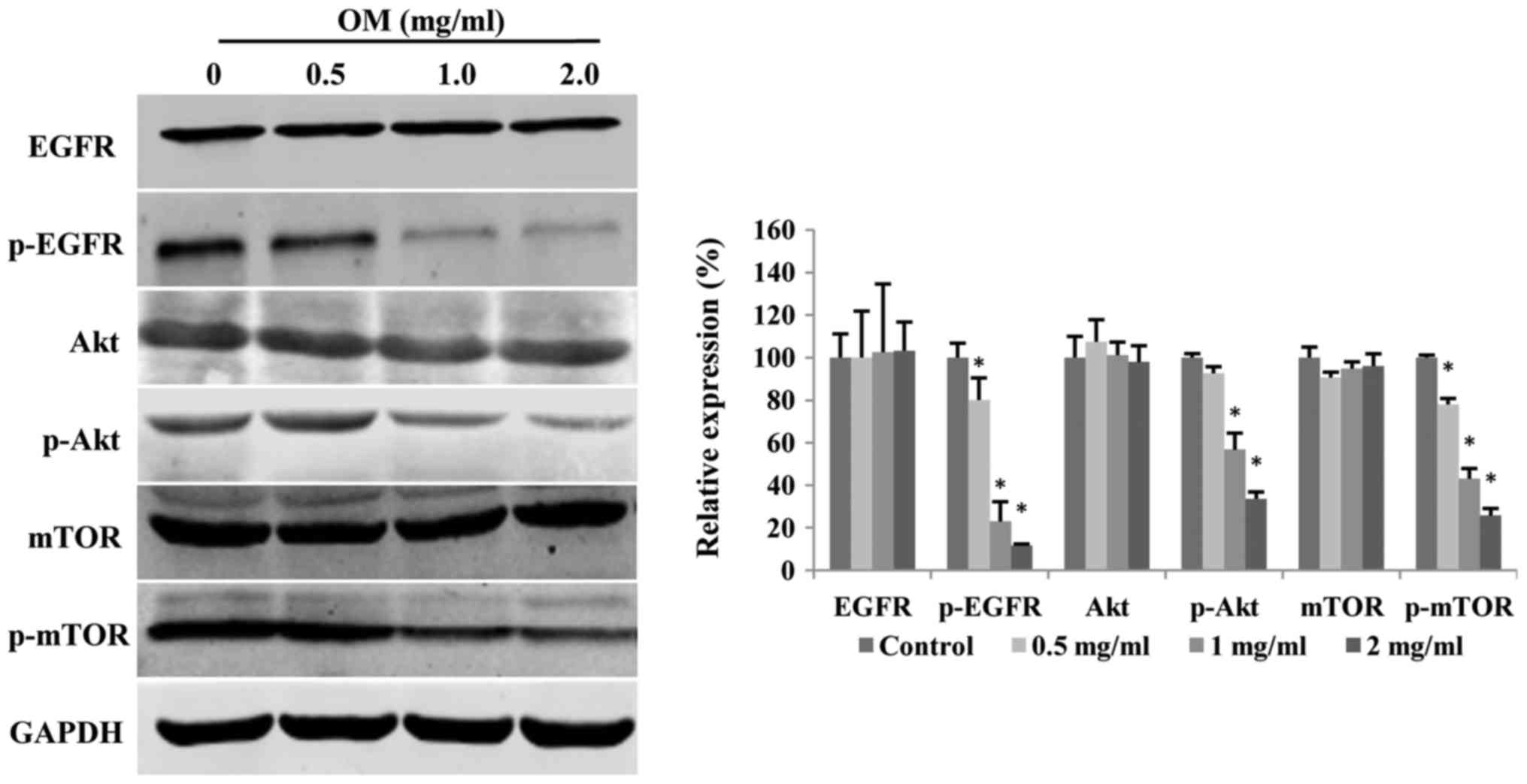
The EGFR/PI3K/Akt/mTOR signaling
pathway is suppressed by OM
EGFR amplification is one of the most common genetic
alterations in glioblastoma. Gain-of-function of EGFR can lead to
upregulation of the PI3K/Akt/mTOR signaling pathway, which is
involved in cell survival and proliferation. Therefore, we assessed
whether the EGFR/PI3K/Akt/mTOR pathway is altered in glioblastoma
cells treated with different concentrations of OM for 48 h. As
displayed in Fig. 5, OM
significantly decreased the expression levels of p-EGFR, p-Akt and
p-mTOR in a dose-dependent manner, without affecting total EGFR,
Akt and mTOR levels.
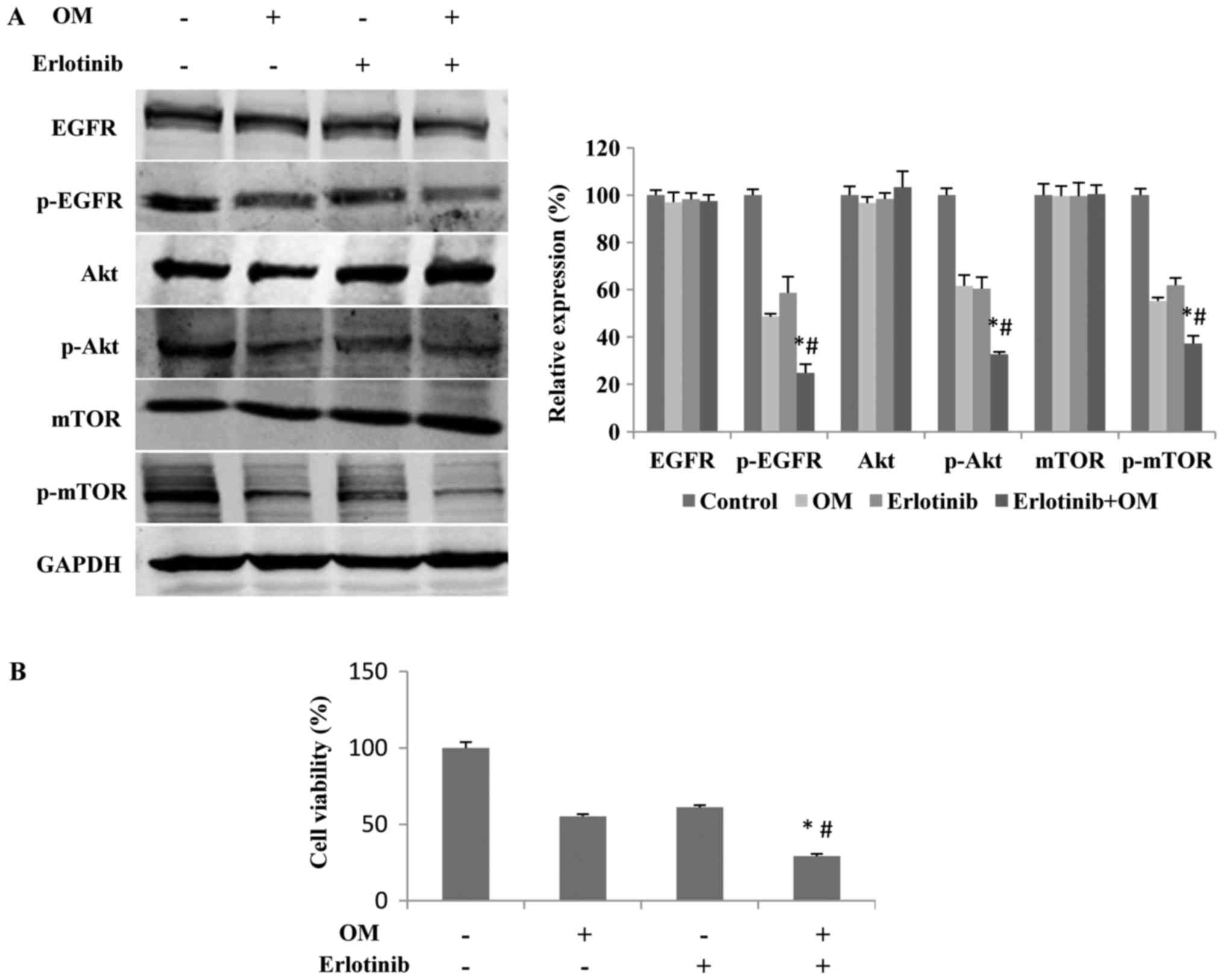
Downregulation of the
EGFR/PI3K/Akt/mTOR signaling pathway mediates the OM-induced
inhibition of proliferation
To further test whether downregulation of the EGFR
pathway is responsible for the OM-induced inhibition of glioma cell
proliferation, we treated the cells with erlotinib, an inhibitor of
EGFR, in the absence or presence of OM for 48 h. The results
indicated that in the presence of both erlotinib and OM, the levels
of p-EGFR, p-Akt and p-mTOR were significantly lower than those in
the presence of erlotinib or OM alone (Fig. 6A). Additionally, compared with the
treatment with either erlotinib or OM alone, the combined treatment
with erlotinib and OM exhibited a stronger effect that inhibited
the proliferation of glioma cells (Fig.
6B).
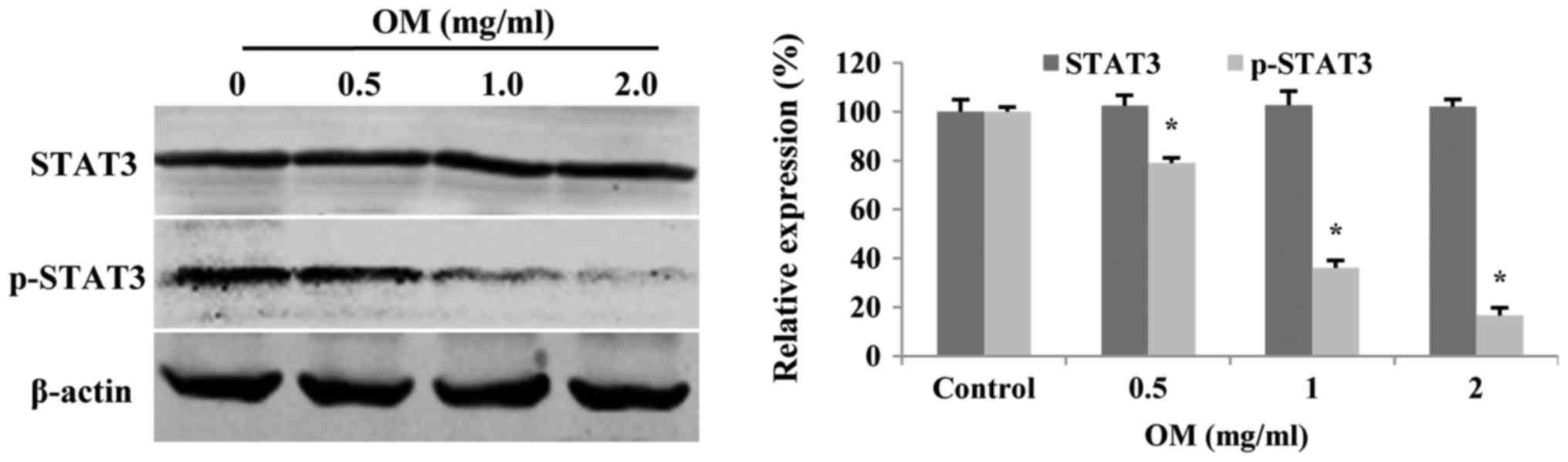
OM modulates STAT3 in glioblastoma
cells
Upregulated STAT3 activity is associated with many
human tumors, and STAT3 suppression can mediate tumor regression
(21). Therefore, we assessed the
expression levels of p-STAT3 and total STAT3 in glioblastoma cells
treated with OM for 48 h. Western blot analysis demonstrated that
OM significantly decreased the expression levels of p-STAT3, but
did not affect the total STAT3 level (Fig. 7).
Discussion
Glioblastomas are characterized by aggressive
proliferation and high invasion, which play an important role in
preventing complete tumor resection, thus leading to tumor
recurrence and poor prognosis (22,23).
In the present study, our results revealed that OM exerted a potent
antitumor effect against U251MG glioblastoma cells. OM effectively
inhibited proliferation, suppressed invasion and induced apoptosis
in glioblastoma cells, suggesting that OM may be a potential agent
for use in clinics to treat glioma in the future.
The essential attribute of tumorigenesis is
uncontrolled and unlimited proliferation, and inhibiting the
proliferation of tumor cells can lead to growth arrest. In the
present study, we assessed cell viability of glioblastoma cells
after incubation with OM. The results revealed that the
proliferation of U251MG cells was inhibited in a dose-dependent and
time-dependent manner.
EGFR is overexpressed in most human glioblastomas,
and it plays a critical role in tumor formation (24). As a result, treatments that
particularly aim at EGFR and its downstream pathways in
glioblastoma cells may have a potential therapeutic effect. It is
well known that the PI3K/Akt/mTOR signaling pathway is regulated by
EGFR and is intimately associated with cell growth, autophagy and
proliferation. In the present study, key proteins of the
EGFR/PI3K/Akt/mTOR signaling pathway, such as p-EGFR, p-Akt and
p-mTOR, were inhibited by OM in a dose-dependent manner (Fig. 5). In addition, OM had a similar
effect as erlotinib, an EGFR inhibitor, on the EGFR pathway. In
combination with erlotinib, OM had a stronger effect that inhibited
the proliferation of glioma cells (Fig.
6). These results indicated that OM is a potential
antiproliferative agent against glioblastoma, and the potential
mechanism of this antiproliferative effect is interfering with the
EGFR/PI3K/Akt/mTOR pathway. In addition, it has been documented
that the expression of STAT3 is regulated by mTOR signaling
(25,26). In the present study, we determined
that the expression level of p-STAT3 was decreased following
treatment with OM (Fig. 7),
indicating that the effect of OM on glioma cells may be mediated by
the EGFR/Akt/mTOR/STAT3 pathway. In addition, dual inhibition of
the EGFR/PI3K/Akt/mTOR pathway by erlotinib with OM would also be a
reasonable treatment for glioblastoma.
As is well known, dysregulation of cell cycle
progression is a basic reason for the abnormal proliferation of
tumor cells (27). For cell cycle
progression, cell cycle-regulating proteins, such as CDK4, CDK6 and
cyclin D1, and their inhibitors play an important role in the G1 to
S phase transition (28),
especially cyclin D1, which regulates this transition. The present
study revealed that OM leads to G0/G1 cell-cycle arrest, which is
accompanied by a decrease in the proportion of U251MG cells in the
S phase (Fig. 2A). We also observed
that OM decreased the expression level of cyclin D1 and CDK4/6
(Fig. 2B), suggesting that cell
cycle arrest may be one of the underlying mechanisms by which OM
exerts its antiproliferative effect on glioblastoma cells as a
result of the downregulation of positive cell cycle regulatory
proteins.
In addition, cyclin D1 is a downstream protein of
Akt, which plays an important role in the cell cycle through the G1
phase. The dephosphorylation of EGFR and Akt results in the
downregulation of cyclin D1, consequently arresting the cell cycle
at the G1 phase (29–32). Consistently, in the present study,
we found that OM decreased the expression levels of p-EGFR, p-Akt
and cyclin D1 (Figs. 5 and 2B). Similarly, previous studies revealed
that OM could induce G1 phase cell-cycle arrest by targeting EGFR
in gastric cancer cells (33).
Therefore, we hypothesized that the effect of OM on cell cycle
arrest in glioblastoma cells may be activated by suppression of the
EGFR/Akt/cyclin D1 signaling pathway.
Apoptosis is a type of programmed cell death
controlled by genes to maintain the internal environmental
stability, and inducing apoptosis should be extremely beneficial
for antitumor therapy. Previous studies demonstrated that many
antitumor drugs exert therapeutic effects by inducing apoptosis in
tumor cells. In the present study, flow cytometry and a TUNEL assay
revealed that apoptosis was induced in glioma cells following
treatment with OM (Fig. 3A and B),
suggesting that OM inhibits the proliferation of glioblastoma cells
by inducing apoptosis.
To investigate potential mechanisms for OM-induced
apoptosis, we evaluated the regulators of apoptosis by western blot
analysis. As we know, the Bcl-2 family members play a key role in
apoptosis such as Bax and Bcl-2, and Bax can promote cell death,
while Bcl-2 can inhibit it. Therefore, the Bax/Bcl-2 ratio
determines the susceptibility of tumor cells to drug-mediated
apoptosis (34). Furthermore, the
caspase family that includes at least 11 members to date, also
plays an important role in apoptosis implementing, and caspase-3
due to its characteristic of leading to final disruption of the
target cell, is recognized as the key member (35,36).
Notably, our study revealed that the Bax/Bcl-2 ratio and caspase-3
increased in glioblastoma cells after treatment with OM (Fig. 3C), which indicate that regulation of
the Bcl-2 family protein expression and activation of caspase-3 may
lead to OM-induced apoptosis in glioma cells. In addition, the
PI3K/Akt/Bcl-2 pathway which can be initiated from EGFR is involved
in various cellular processes including cell apoptosis and the
activation of PI3K/Akt/Bcl-2 leads to cell survival (32,37,38).
In the present study, we found that OM markedly suppressed the
expression levels of p-EGFR, p-Akt and Bcl-2 (Figs. 5 and 3C). It is reasonable to conclude that OM
induced the apoptosis of glioblastoma cells probably by
inactivating the EGFR/PI3K/Akt/Bcl-2 pathway.
Invasion, as one of the main malignant phenotypes of
glioblastoma, is a formidable barrier to the treatment of
glioblastoma, leading to a poor prognosis. Although distant
metastasis of glioma is rare, the pathophysiological features and
the ability to invade adjacent areas makes complete surgical
resection impossible and increases the risk of tumor recurrence
(39–41). Therefore, disrupting the invasion
process could be an effective way to treat glioma. Previous studies
revealed that OM interrupted tumor invasion in human gastric
cancer, colorectal carcinoma and glioma cells (33,42,43).
Consistently, the Transwell results of the present study
demonstrated that OM significantly inhibited the invasion of
glioblastoma cells in a dose-dependent manner (Fig. 4). However, the exact mechanism by
which OM inhibits invasion warrants further exploration.
In conclusion, OM effectively inhibited the
proliferation and invasion of malignant glioma cells and promoted
their apoptosis by suppressing the expression of STAT3 and altering
the expression of the cell cycle and apoptosis regulators.
Furthermore, the EGFR/PI3K/Akt/mTOR pathway is involved in the
OM-mediated antitumor effect in glioblastoma cells, and OM combined
with erlotinib (a chemotherapeutic agent for tumors) had a more
powerful effect that decreased the survival of glioma cells. These
findings indicated that the EGFR/PI3K/Akt/mTOR signaling pathway
and STAT3 suppression may be a potential mechanism by which OM
exerted its antitumor effect against glioma and that EGFR may be
the target of OM. Although these results need to be confirmed in
vivo, OM, a traditional Chinese herbal product, may be a
promising drug and offer a novel therapeutic strategy for malignant
gliomas.
Acknowledgements
The authors thank Dr Zengxiang Dong for providing
technical assistance with FACS. We would also like to thank Dr
Xiaolei Zhang for helpful discussion. We thank American Journal
Experts (AJE) for English language editing.
Funding
The present study was supported by the Special Fund
for Translational Research of Sino-Russia Medical Research Center
in Harbin Medical University (nos. CR201410 and CR201512 to
S.Z.).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
ZD and SZ designed the research; ZD, LW, XW, BZ, WZ,
SSB, JY, ZY and JZ performed the experiments; LW, XW, BZ and WZ
provided reagents and technical support; ZD, XW and SZ analyzed the
data; ZD and SZ wrote and revised the manuscript. SZ supervised the
work. All authors have read and approved the final manuscript. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gunther W, Pawlak E, Damasceno R, Arnold H
and Terzis AJ: Temozolomide induces apoptosis and senescence in
glioma cells cultured as multicellular spheroids. Br J Cancer.
88:463–469. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saito N, Fu J, Zheng S, Yao J, Wang S, Liu
DD, Yuan Y, Sulman EP, Lang FF, Colman H, et al: A high Notch
pathway activation predicts response to γ secretase inhibitors in
proneural subtype of glioma tumor-initiating cells. Stem Cells.
32:301–312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hynes NE and Lane HA: ERBB receptors and
cancer: The complexity of targeted inhibitors. Nat Rev Cancer.
5:341–354. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lieto E, Ferraraccio F, Orditura M,
Castellano P, Mura AL, Pinto M, Zamboli A, De Vita F and Galizia G:
Expression of vascular endothelial growth factor (VEGF) and
epidermal growth factor receptor (EGFR) is an independent
prognostic indicator of worse outcome in gastric cancer patients.
Ann Surg Oncol. 15:69–79. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gusterson BA: Identification and
interpretation of epidermal growth factor and c-erbB-2
overexpression. Eur J Cancer. 28:263–267. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiao LJ, Lin P, Lin F, Liu X, Qin W, Zou
HF, Guo L, Liu W, Wang SJ and Yu XG: ADAM17 targets MMP-2 and MMP-9
via EGFR-MEK-ERK pathway activation to promote prostate cancer cell
invasion. Int J Oncol. 40:1714–1724. 2012.PubMed/NCBI
|
|
8
|
Taylor TE, Furnari FB and Cavenee WK:
Targeting EGFR for treatment of glioblastoma: Molecular basis to
overcome resistance. Curr Cancer Drug Targets. 12:197–209. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Libermann TA, Nusbaum HR, Razon N, Kris R,
Lax I, Soreq H, Whittle N, Waterfield MD, Ullrich A and
Schlessinger J: Amplification, enhanced expression and possible
rearrangement of EGF receptor gene in primary human brain tumours
of glial origin. Nature. 313:144–147. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang B, Wang G and Xu J: Inhibitory effect
of oxymatrine on vascular endothelial cell proliferation induced by
tumor. J Pract Oncol. 15:297–300. 2000.
|
|
11
|
Cao YG, Jing S, Li L, Gao JQ, Shen ZY, Liu
Y, Xing Y, Wu ML, Wang Y, Xu CQ, et al: Antiarrhythmic effects and
ionic mechanisms of oxymatrine from sophora flavescens. Phytother
Res. 24:1844–1849. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cui X, Wang Y, Kokudo N, Fang D and Tang
W: Traditional Chinese medicine and related active compounds
against hepatitis B virus infection. Biosci Trends. 4:39–47.
2010.PubMed/NCBI
|
|
13
|
Deng ZY, Li J, Jin Y, Chen XL and Lu XW:
Effect of oxymatrine on the p38 mitogen-activated protein kinases
signalling pathway in rats with CCl4 induced hepatic fibrosis. Chin
Med J (Engl). 122:1449–1454. 2009.PubMed/NCBI
|
|
14
|
Fan H, Li L, Zhang X, Liu Y, Yang C, Yang
Y and Yin J: Oxymatrine downregulates TLR4, TLR2, MyD88, and
NF-kappaB and protects rat brains against focal ischemia. Mediators
Inflamm. 2009:7047062009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ho JW, Ngan Hon PL and Chim WO: Effects of
oxymatrine from Ku Shen on cancer cells. Anticancer Agents Med
Chem. 9:823–826. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen H, Zhang J, Luo J, Lai F, Wang Z,
Tong H, Lu D, Bu H, Zhang R and Lin S: Antiangiogenic effects of
oxymatrine on pancreatic cancer by inhibition of the NF-κB-mediated
VEGF signaling pathway. Oncol Rep. 30:589–595. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ling Q, Xu X, Wei X, Wang W, Zhou B, Wang
B and Zheng S: Oxymatrine induces human pancreatic cancer PANC-1
cells apoptosis via regulating expression of Bcl-2 and IAP
families, and releasing of cytochrome c. J Exp Clin Cancer Res.
30:662011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song MQ, Zhu JS, Chen JL, Wang L, Da W,
Zhu L and Zhang WP: Synergistic effect of oxymatrine and
angiogenesis inhibitor NM-3 on modulating apoptosis in human
gastric cancer cells. World J Gastroenterol. 13:1788–1793. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zou J, Ran ZH, Xu Q and Xiao SD:
Experimental study of the killing effects of oxymatrine on human
colon cancer cell line SW1116. Chin J Dig Dis. 6:15–20. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y, Piao B, Zhang Y, Hua B, Hou W, Xu
W, Qi X, Zhu X, Pei Y and Lin H: Oxymatrine diminishes the side
population and inhibits the expression of β-catenin in MCF-7 breast
cancer cells. Med Oncol. 28 (Suppl 1):S99–S107. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Inghirami G, Chiarle R, Simmons WJ, Piva
R, Schlessinger K and Levy DE: New and old functions of STAT3: A
pivotal target for individualized treatment of cancer. Cell cycle.
4:1131–1133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ulrich TA, de Juan Pardo EM and Kumar S:
The mechanical rigidity of the extracellular matrix regulates the
structure, motility, and proliferation of glioma cells. Cancer Res.
69:4167–4174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tu Y, Gao X, Li G, Fu H, Cui D, Liu H, Jin
W and Zhang Y: MicroRNA-218 inhibits glioma invasion, migration,
proliferation, and cancer stem-like cell self-renewal by targeting
the polycomb group gene Bmi1. Cancer Res. 73:6046–6055. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schlegel J, Merdes A, Stumm G, Albert FK,
Forsting M, Hynes N and Kiessling M: Amplification of the
epidermal-growth-factor-receptor gene correlates with different
growth behaviour in human glioblastoma. Int J Cancer. 56:72–77.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goncharova EA, Goncharov DA, Damera G,
Tliba O, Amrani Y, Panettieri RA Jr and Krymskaya VP: Signal
transducer and activator of transcription 3 is required for
abnormal proliferation and survival of TSC2-deficient cells:
Relevance to pulmonary lymphangioleiomyomatosis. Mol Pharmacol.
76:766–777. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang
Y, Deng J, Margolick JB, Liotta LA, Petricoin E III and Zhang Y:
Activation of the PTEN/mTOR/STAT3 pathway in breast cancer
stem-like cells is required for viability and maintenance. Proc
Natl Acad Sci USA. 104:16158–16163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Massagué J: G1 cell-cycle control and
cancer. Nature. 432:298–306. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kastan MB and Bartek J: Cell-cycle
checkpoints and cancer. Nature. 432:316–323. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ye X, Guo Y, Zhang Q, Chen W, Hua X, Liu
W, Yang Y and Chen G: βKlotho suppresses tumor growth in
hepatocellular carcinoma by regulating Akt/GSK-3β/cyclin D1
signaling pathway. PLoS One. 8:e556152013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu M, Gong X, Lu Y, Guo J, Wang C and Pan
Y: Molecular cloning and functional characterization of a
cell-permeable superoxide dismutase targeted to lung adenocarcinoma
cells. Inhibition cell proliferation through the Akt/p27kip1
pathway. J Biol Chem. 281:13620–13627. 2006.
|
|
31
|
Zhao W, Zhou SF, Zhang ZP, Xu GP, Li XB
and Yan JL: Gambogic acid inhibits the growth of osteosarcoma cells
in vitro by inducing apoptosis and cell cycle arrest. Oncol Rep.
25:1289–1295. 2011.PubMed/NCBI
|
|
32
|
Pal HC, Sharma S, Strickland LR, Agarwal
J, Athar M, Elmets CA and Afaq F: Delphinidin reduces cell
proliferation and induces apoptosis of non-small-cell lung cancer
cells by targeting EGFR/VEGFR2 signaling pathways. PLoS One.
8:e772702013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo B, Zhang T, Su J, Wang K and Li X:
Oxymatrine targets EGFRp-Tyr845 and inhibits
EGFR-related signaling pathways to suppress the proliferation and
invasion of gastric cancer cells. Cancer Chemother Pharmacol.
75:353–363. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Burlacu A: Regulation of apoptosis by
Bcl-2 family proteins. J Cell Mol Med. 7:249–257. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stennicke HR and Salvesen GS: Properties
of the caspases. Biochim Biophys Acta. 1387:17–31. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Budihardjo I, Oliver H, Lutter M, Luo X
and Wang X: Biochemical pathways of caspase activation during
apoptosis. Annu Rev Cell Dev Biol. 15:269–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Morozevich GE, Kozlova NI, Ushakova NA,
Preobrazhenskaya ME and Berman AE: Integrin α5β1 simultaneously
controls EGFR-dependent proliferation and Akt-dependent
pro-survival signaling in epidermoid carcinoma cells. Aging.
4:368–374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang C, Li C, Chen S, Li Z, Jia X, Wang
K, Bao J, Liang Y, Wang X, Chen M, et al: Berberine protects
against 6-OHDA-induced neurotoxicity in PC12 cells and zebrafish
through hormetic mechanisms involving PI3K/AKT/Bcl-2 and Nrf2/HO-1
pathways. Redox Biol. 11:1–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Giese A, Bjerkvig R, Berens ME and
Westphal M: Cost of migration: Invasion of malignant gliomas and
implications for treatment. J Clin Oncol. 21:1624–1636. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Louis DN, Pomeroy SL and Cairncross JG:
Focus on central nervous system neoplasia. Cancer Cell. 1:125–128.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Paw I, Carpenter RC, Watabe K, Debinski W
and Lo HW: Mechanisms regulating glioma invasion. Cancer Lett.
362:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang X, Liu C, Wang J, Fan Y, Wang Z and
Wang Y: Oxymatrine inhibits the migration of human colorectal
carcinoma RKO cells via inhibition of PAI-1 and the TGF-β1/Smad
signaling pathway. Oncol Rep. 37:747–753. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu F, Wang B, Wang J, Ling X, Li Q, Meng
W and Ma J: Oxymatrine inhibits proliferation and migration while
inducing apoptosis in human glioblastoma cells. Biomed Res Int.
2016:17841612016. View Article : Google Scholar : PubMed/NCBI
|