Introduction
Prostate cancer (PCa) growth is primarily driven by
androgens (1). Therefore, androgen
deprivation therapy (ADT), accomplished by surgical or medical
castration, is the first-line therapeutic approach for the
treatment of oligometastatic or high-risk localized PCa. ADT
generally leads to remissions that last 1.5–3 years (2). However, despite the high initial
response rate (3), the cancer
almost invariably relapses, progressing to a hormone refractory
state manifested by increased proliferation and invasion capacity.
Hormone-refractory PCa is unresponsive to further hormonal
manipulation and has a high resistance to cytotoxic drugs (4,5).
Although the molecular basis for such relapse is not
fully defined, several signaling pathways have been identified by
which androgen receptors are activated in the absence of ligands.
Understanding these pathways is highly important for the
development of new strategies for second-line therapies. One known
mechanism responsible for androgen-independent growth involves
alterations of growth factor receptor signaling (6,7). In
particular, the epidermal growth factor receptor (EGFR) is one of
the frequently deregulated gene products, and EGFR overexpression
is associated with advanced stage PCa, progression towards
androgen-independence and a poor clinical outcome (8–10).
EGFR, also known as ErbB1 or HER1, is a member of
the tyrosine kinase EGFR family of receptors that also includes
HER2/c-neu, HER3 and HER4. EGFR is activated by binding of specific
ligands (EGF and TGFα are the preferred ligands), and three
additional ligands that it shares with HER3 (11). Recently, the role of EGFR in cancer
is a subject of extensive investigations. Ligand binding to EGFR
triggers its dimerization or heterodimerization with other members
of the EGFR family and activates the intracellular tyrosine kinase
domain. This further recruits downstream signaling proteins and
triggers several signaling cascades that regulate growth, survival,
proliferation, motility and differentiation (12). The understanding of these mechanisms
has resulted in the development of drugs targeting EGFR and its
signaling.
For patients, a confirmed EGFR overexpression in PCa
could reveal new second line-treatment options involving monoclonal
antibodies (mAb) such as the already established cetuximab
(Erbitux®) (13,14) and small molecule tyrosine kinase
inhibitors (TKIs) such as erlotinib (Tarceva®) (15,16) or
gefitinib (Iressa®) (17,18).
Many other new targeting drugs are also in preclinical and clinical
development, either specifically targeting EGFR, or targeting
several intracellular signaling pathways simultaneously (19). All these approaches are highly
selective and the successful implementation of anti-EGFR therapies
requires an accurate detection of EGFR expression in primary tumors
and metastasis.
Currently, the most common and established method
for the detection of EGFR expression involves analyses of biopsy
material. However, biopsy procedures are invasive, painful, and can
lead to severe complications. Repetitive biopsies and biopsies from
bone metastases are difficult. Additionally, EGFR expression in PCa
is a relatively late event and the target expression heterogeneity
can lead to false-negative results. EGFR expression may also change
during the course of the disease due to the genetic instability of
the cancer or in response to treatment (20). There is a clear unmet clinical need
for non-invasive robust methods to determine EGFR expression
status, both initially and as a consequence of treatment.
Imaging of EGFR aberrant expression in PCa using
radiolabeled EGFR-specific agents is a promising approach that
allows repetitive, non-invasive investigations of receptor status
in multiple lesions simultaneously. Potential radioimaging agents
include the natural ligands of EGFR and the anti-EGFR mAbs. The use
of radiolabeled epidermal growth factor (EGF) is a straightforward
approach, but is limited by its physiological activity that may
cause adverse reactions (nausea, vomiting, diarrhea, hypotension,
fever and chills) (21).
Radiolabeled intact anti-EGFR mAbs have been extensively
investigated (22–28) demonstrating their capacity to
specifically accumulate in tumors proportionally to EGFR
expression. However, the sensitivity of mAb-mediated imaging is
limited by the long blood residence time and slow tumor
penetration, which reduces the imaging contrast, a major limitation
for targets with low expression. A smaller targeting agent without
physiological activity would be desirable in order to obtain a
higher contrast than antibodies can provide.
Affibody molecules are a promising class of imaging
agents that are characterized by small size and high affinity to
the intended target. They are scaffold proteins composed of
anti-parallel three-helix cysteine-free bundles, derived from the
immunoglobulin-binding B domain of staphylococcal protein A.
Randomization of 13 surface-exposed amino acids on helices 1 and 2
are used for generation of large combinatorial libraries from which
high affinity binders can be selected. The small size (7 kDa) of
affibody molecules facilitates rapid extravasation, rapid tumor
penetration and fast clearance of unbound tracer from blood. This
results in high contrast imaging only a few hours after injection,
as seen in both preclinical and clinical studies (29–31).
Nonetheless, imaging of EGFR in PCa is challenging
due to the relatively low expression in PCa (in comparison to other
cancers). The feasibility of affibody-mediated imaging of EGFR
expression was previously demonstrated using
DOTA-ZEGFR:2377. This construct was initially
radiolabeled with 111In, exhibiting favorable
pharmacokinetic properties, with higher tumor-to-organ ratios than
any anti-EGFR monoclonal antibody (32). In a further attempt to improve the
sensitivity of EGFR detection in vivo, the use of
positron-emitting labels was considered. Positron emission
tomography (PET) provides better spatial resolution, higher
sensitivity and more accurate quantification compared to
single-photon emission computed tomography (SPECT). Several imaging
probes based on ZEGFR:2377 for PET imaging of EGFR were
proposed for labeling with 68Ga, 55Co, and
89Zr (33,34). Among them, the radiocobalt labeled
affibody molecule had the most favorable biodistribution when
evaluated on an epidermoid carcinoma model, A-431, with high
EGFR-expression [2×106 receptors/cell (35)]. In this tumor model, radiocobalt
labeled ZEGFR:2377 demonstrated 2-fold higher
tumor-to-blood, tumor-to-bone and tumor-to-muscle ratios compared
to the same construct radiolabeled with 68Ga and
five-fold higher compared to
89Zr-DFO-ZEGFR:2377 at 3 h post injection
(pi) (33,34).
The promising results obtained during the initial
validation of radiocobalt-labeled DOTA-ZEGFR:2377 in
epidermoid carcinoma, provided the incentive for its evaluation in
a more challenging, low receptor-expressing PCa model. In PCa, the
high tumor-to-blood, tumor-to-bone and tumor-to-muscle ratios are
particularly important, since the majority of metastases occur in
the abdominal lymph nodes and bones. Moreover, the significantly
lower liver uptake of 57Co-DOTA-ZEGFR:2377
compared to the 68Ga-labeled analogue could be
particularly relevant in a low EGFR-expressing tumors where protein
dosing is crucial, as more injected compound would be available for
specific binding to EGFR-expressing tumors and tissues.
The aim of the present study was to test the
hypothesis that radiocobalt-labeled ZEGFR:2377 affibody
molecule would enable PET imaging of EGFR receptor expression in
PCa xenografts where EGFR expression is low. A long-lived PET
radionuclide, 55Co has a half-life of 17.5 h, and would
allow imaging the next day after injection when better contrast
could be achieved (32). Cobalt-55
is not commercially available, which is an obstacle for
pre-clinical investigations. However, we have recently demonstrated
that data obtained for a bombesin analogue labeled with cobalt-57
(T½=272 d) were fully translatable to cobalt-55
(36). Therefore, 57Co
was chosen as a surrogate for 55Co in the present
study.
Materials and methods
Labeling of DOTA-ZEGFR:2377
with cobalt-57
The C-terminal cysteine-containing anti-EGFR
affibody molecule Z2377, conjugated with
maleimido-derivative of DOTA, was produced as previously reported
(32). Buffers for labeling were
purified from metal contamination using Chelex 100 resin (Bio-Rad
Laboratories, Inc., Richmond, CA, USA). Labeling with radiocobalt
and quality control methods were performed as previously described
(33). Briefly,
DOTA-ZEGFR:2377 (50 µg) in 40 µl of 0.2 M ammonium
acetate buffer, pH 5.5, was mixed with 57Co stock
solution (20 µl, 20–40 MBq). The reaction mixture was incubated for
30 min at 60°C. After labeling, the conjugate was purified using
disposable NAP-5 size-exclusion columns pre-equilibrated with
phosphate-buffered saline (PBS). The yield and radiochemical purity
of the conjugate were evaluated by instant thin-layer
chromatography (ITLC) using Tec-Control Chromatography 150–771
strips (Biodex Medical Systems, Shirley, NY, USA) eluted with 0.2 M
citric acid, pH 2.0. The distribution of radioactivity along the
ITLC strips was measured on a Cyclone Storage Phosphor System
(PerkinElmer, Inc., Waltham, MA, USA).
Cell culture
Human PCa cell line DU-145 [American Type Tissue
Culture Collection (ATCC) via LGC Promochem, Borås, Sweden] was
used. The EGFR expression in this cell line was ~2×105
receptors/cell (37). Roswell Park
Memorial Institute medium (RPMI-1640) supplemented with 10% fetal
calf serum (FCS), 2 mM L-glutamine and PEST (penicillin 100 IU/ml,
streptomycin 100 µg/ml) (all from Biochrom AG, Berlin, Germany) was
used for cell culturing. This medium was referred to as complete
medium in the text. The cells were detached using a trypsin-EDTA
solution (0.05% trypsin, 0.02% EDTA; Biochrom AG) and were counted
using an electronic cell counter (Beckman Coulter, Inc., Brea, CA,
USA).
In vitro cell studies
The in vitro binding specificity assay was
designed to determine whether the binding of
57Co-DOTA-ZEGFR:2377 to EGFR-expressing
DU-145 cells was receptor-mediated. In this experiment, the cells
were incubated with 1 nM 57Co-DOTA-ZEGFR:2377
for 2 h at room temperature. One set of dishes was pre-incubated
with i) 0.5 µM unlabeled affibody molecule, ii) one with 0.5 µM of
cetuximab, and iii) one with 0.5 µM bevacizumab for 10 min at room
temperature. After incubation with
57Co-DOTA-ZEGFR:2377, the cells were washed
with serum-free media and detached using 0.5 ml trypsin-EDTA
solution. The cell-associated radioactivity was assessed using a
γ-counter (2480 WIZARD2; PerkinElmer, Inc.) and
presented as a percentage from added radioactivity.
Cellular processing was evaluated using DU-145
cells. The cells were incubated at 37°C with 1.5 nM of
57Co-DOTA-ZEGFR:2377. At predetermined
time-points (1, 2, 4, 8 and 24 h after the start of incubation) the
membrane-bound and internalized radioactivity fractions were
collected and calculated as previously described (37).
To assess the cellular retention of radioactivity
after interrupted incubation with radiocobalt-labeled affibody
molecules, cultured DU-145 cells were incubated for 1 h at 4°C with
1.5 nM 57Co-DOTA-ZEGFR:2377. The cell dishes
were subsequently washed with serum-free media, fresh complete
media was added, and the dishes were incubated at 37°C. At
predetermined time-points, membrane-bound and internalized
radioactivity fractions were collected and measured using the
γ-counter.
The dissociation constant (KD) of
57Co-labeled DOTA- ZEGFR:2377 binding to
living DU-145 cells was measured using LigandTracer Yellow
instruments (Ridgeview Instruments AB, Uppsala, Sweden) as
previously described (38).
Briefly, DU-145 cells were seeded in one area of the Petri dish
(Nunclon™; 100-mm diameter; Thermo Fisher Scientific, Hvidovre,
Denmark). After the addition of 3 ml of complete media, the dish
was placed on the rotating table of the instrument. After a 15-min
baseline run, 57Co-labeled DOTA-ZEGFR:2377
was added to the media to obtain a ligand concentration of 1 and
then 5 nM. For each concentration, the uptake curves were measured
for 120 min. Subsequently, the 57Co-labeled
DOTA-ZEGFR:2377-containing medium was removed, fresh
media (3 ml) was added to the dish, and the dissociation curve was
recorded for 11 h. Two runs were performed at room temperature. The
calculation of equilibrium dissociation constant was performed
using TracerDrawer software (Ridgeview Instruments AB, Vänge,
Sweden).
In vivo studies
All animal experiments were planned and performed in
accordance with the Swedish national legislation on the protection
of laboratory animals and the study plans were approved by the
Ethics Committee for Animal Research in Uppsala. Euthanasia was
performed by intraperitoneal injection of a ketamine-xylazine
(Ketalar-Rompun) solution (200 mg/kg ketamine/Ketalar and 20 mg/kg
xylazine/Rompun), and all efforts were made to minimize suffering.
Female outbred BALB/c nu/nu mice (6 weeks of age) were purchased
from Taconic M&B (Ry, Denmark). The animals (n=25) were housed
at 23°C, 45% humidity, 12-h light/dark cycle, food and water ad
libitum. Prior to implantation the mice were quarantined for 1
week. At the time of experiment the average animal weight was 19±1
g. EGFR-expressing xenografts were established by subcutaneous
injection of ~1×107 DU-145 cells/mouse. The tumors were
grown for 8 days before the experiment (in Matrigel, BD
Biosciences) and the average tumor weight was 0.13±0.08 g. Before
the experiments the animals were randomized into groups of
four.
The mice were intravenously injected with 30 kBq of
radiolabeled conjugate per mouse in 100 µl PBS with 35 µg/mouse
(one group) or 10 µg/mouse (three groups). The protein dose was
adjusted by the addition of non-labeled DOTA-ZEGFR:2377.
The animals were sacrificed at 3 h pi (10- and 35-µg groups) and 7
and 24 h pi (10-µg groups). Blood, tumors and organ samples were
collected and weighed, and their radioactivity was measured. Tissue
uptake was calculated as the percent of injected dose per gram (%
ID/g).
To verify specificity of in vivo targeting, a
group of six mice was pre-injected with an excess amount of
anti-EGFR monoclonal antibody cetuximab (5 mg/ml; cat. no. 64293;
Merk KGaA, Darmstadt, Germany) 24 h prior to the injection of 10 µg
(30 kBq) of 57Co-DOTA-ZEGFR:2377. The animals
were sacrificed at 3 h pi. Cetuximab does not cross-react with
murine EGFR, therefore only blood and tumors were collected for the
blocking group.
Whole body scans of the subjects were performed
using the Triumph™ Trimodality system (Gamma Medica, Inc., Salem,
NH, USA), an integrated microSPECT/PET/CT platform. The mice
bearing DU-145 ×enografts were euthanized by CO2
asphyxiation at 3, 7, and 24 h pi of 10 µg (4 MBq)
57Co-DOTA-ZEGFR:2377. The computed tomography
(CT) acquisition was carried out at the following parameters: Field
of view (FOV), 80 mm; magnification, 1.48; one projection and 512
frames for 2.13 min. SPECT acquisition was performed at the
following parameters: FOV, 80 mm; 75A10 collimators (5 pinhole); 64
projections, 2 h scan time. CT raw files were reconstructed by
Filter Back Projection (FBP). SPECT raw data was reconstructed by
the FLEX™ SPECT software, which uses an ordered Subset Expectation
Maximization (OSEM) iterative reconstruction algorithm. SPECT and
CT data were fused and analyzed using PMOD v3.508 (PMOD
Technologies Ltd., Zurich, Switzerland).
Statistical analysis
Data were assessed either by one-way ANOVA with
Dunnett or with Bonferroni correction for multiple comparisons
using GraphPad Prism 7.03 (GraphPad Software, Inc., La Jolla, CA,
USA) in order to determine significant differences (P<0.05).
Results
Labeling with 57Co under the selected
conditions provided an average yield of 95±5%. After purification
using disposable NAP-5 size-exclusion columns, the radiochemical
purity was 100%.
In vitro cell studies
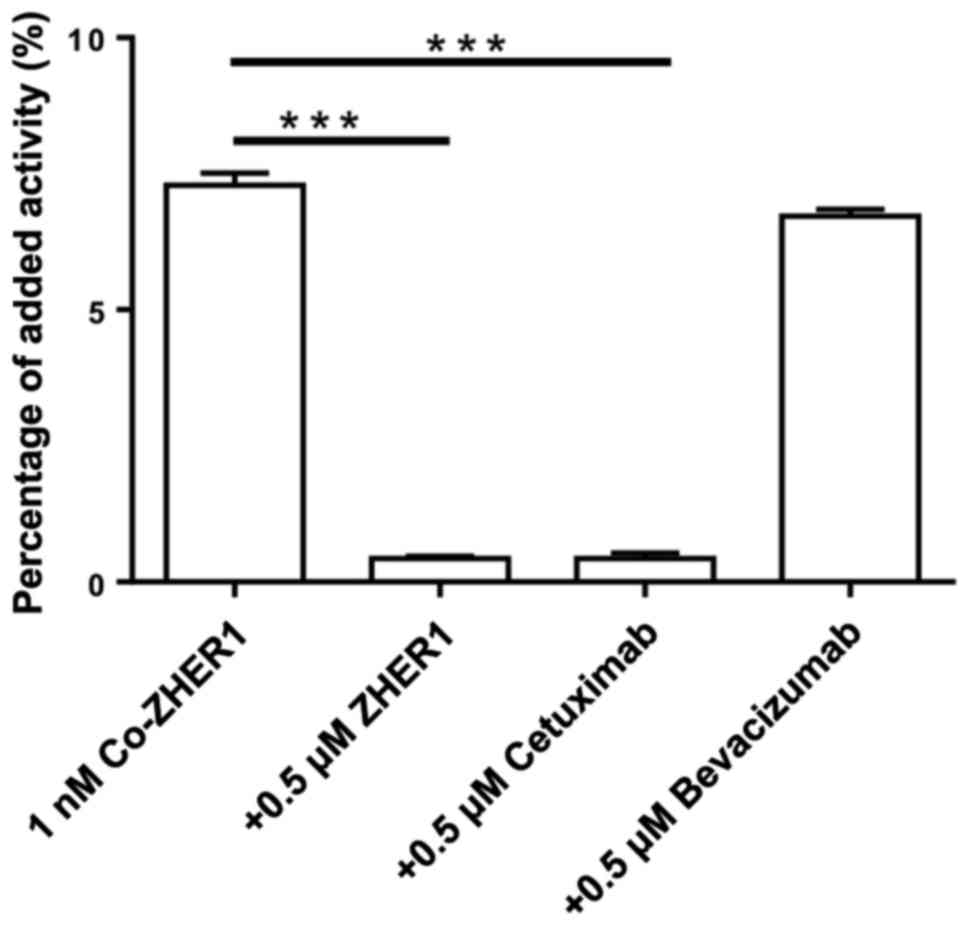
The results of binding specificity tests are
presented in Fig. 1. Pre-saturation
of EGFR with a large molar excess of non-labeled affibody molecules
or EGFR-targeting mAb cetuximab resulted in a significantly reduced
cell-associated radioactivity after 2 h incubation (n=3,
P<0.05). On the contrary, the non-relevant mAb bevacizumab
(anti-VEGF) did not influence the binding of the radioconjugate to
cells. This finding indicated EGFR-mediated binding of
57Co-DOTA-ZEGFR:2377 to DU-145 cells.
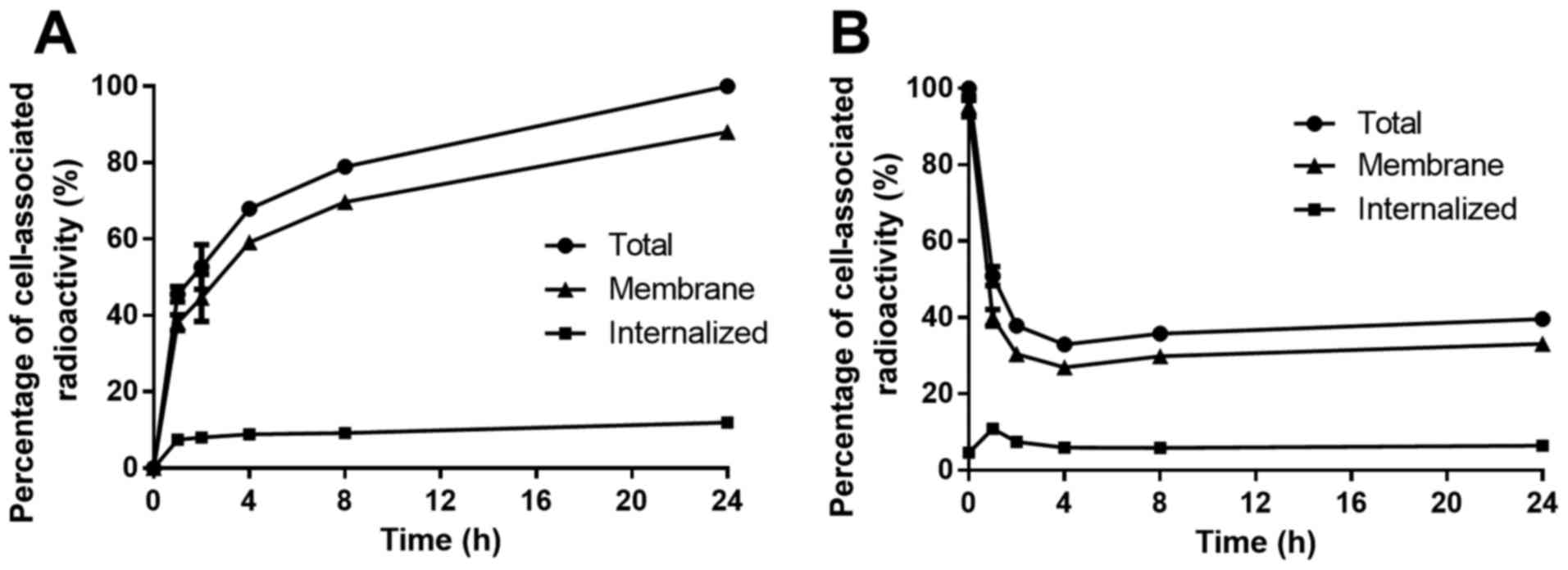
Data concerning cellular processing of
57Co-DOTA-ZEGFR:2377 are presented in
Fig. 2A. Cell-associated
radioactivity increased continuously over time, while the
contribution of internalized radioactivity was low, with <12% of
total cell-associated radioactivity internalized after 24 h. The
cellular retention of 57Co-DOTA-ZEGFR:2377
was also studied at various time-points (Fig. 2B). After an initial rapid
dissociation phase (up to 2 h), the cell-associated radioactivity
plateaued at 40% of initially bound radioactivity. The fraction of
internalized radioactivity was low, similar to the previous
experiment.
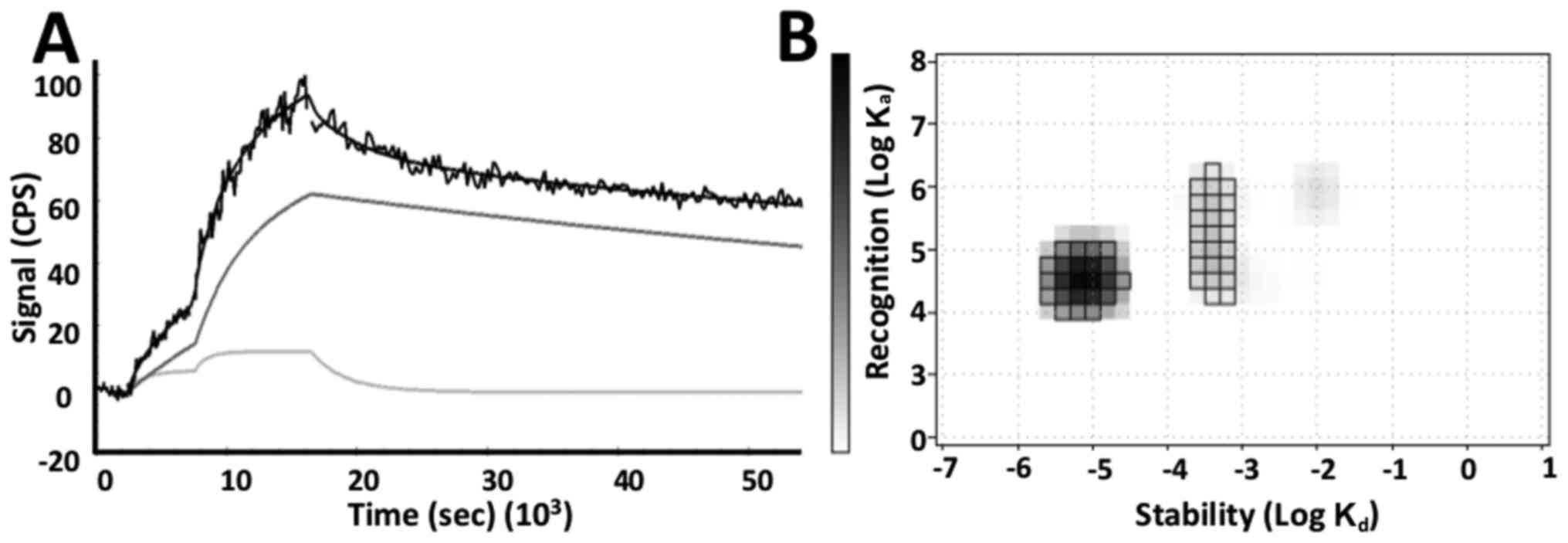
The binding affinity of
57Co-DOTA-ZEGFR:2377 to DU-145 cells was
assessed in real-time. The evaluation of the binding kinetics
revealed that the interaction did not follow a 1:1 Langmuir
adsorption model. A superior fitting was obtained for the 1:2
model, suggesting two independent interactions with different
kinetic parameters (Fig. 3A). The
two processes occured simultaneously and consisted of a dominating
high affinity interaction (79 pM, 66%) and a minor lower affinity
interaction (3.7 nM, 17%) (Fig.
3B).
In vivo studies
Data concerning the biodistribution of
57Co-DOTA-ZEGFR:2377 in BALB/c nu/nu mice
bearing DU-145 PCa xenografts are presented in Table I. In the first experiment, the
biodistribution was compared at 3 h after injection of 35 and 10 µg
of radiolabeled protein, respectively. A significant,
dose-dependent difference in the accumulation of radioactivity in
tumors and normal organs was observed. The concentration of
radioactivity in blood and most normal organs (except for the
spleen and muscles) was significantly higher in the 10-µg group
(n=4, P<0.05). Tumor uptake was also 2-fold higher in the group
of mice injected with 10 µg of
57Co-DOTA-ZEGFR:2377 (2.1±0.1% IA/g),
compared to the 35-µg group (1.1±0.1% IA/g). Tumor-to-non-tumor
ratios were similar in both groups. The high uptake in kidneys
indicated that 57Co-DOTA-ZEGFR:2377 was
cleared by glomerular filtration with subsequent tubular
re-absorption.
 | Table I.Biodistribution of
57Co-DOTA-ZEGFR:2377 after injection in
BALB/c nu/nu mice bearing DU-145 ×enografts. |
Table I.
Biodistribution of
57Co-DOTA-ZEGFR:2377 after injection in
BALB/c nu/nu mice bearing DU-145 ×enografts.
|
| 35 µg | 10 µg |
|---|
|
|
|
|
|---|
|
| 3 h | 3 h | 7 h | 24 h |
|---|
| Blood |
0.73±0.08a | 1.8±0.6
(2.0±0.8) | 1.3±0.2 |
0.30±0.04b,c |
| Saliv. glands | 0.62±0.04 | 1.23±0.08 | 1.1±0.2 |
0.44±0.05b,c |
| Lung | 0.77±0.06 | 1.9±0.2 |
1.3±0.1b |
0.44±0.05b,c |
| Liver | 4.8±0.5 | 7±2 | 5.3±1.7 |
3.1±0.5b |
| Spleen | 1.1±0.2 | 1.0±0.1 |
0.69±0.05b | 0.8±0.1 |
| Small int. |
0.69±0.04a | 1.3±0.2 | 1.0±0.2 |
0.45±0.02b,c |
| Colon |
0.64±0.09a | 1.34±0.09 |
1.10±0.09b |
0.48±0.09b,c |
| Kidney | 319±17 | 300±30 | 278±27 | 247±29 |
| Tumor |
1.1±0.1a |
2.1±0.5d
(1.3±0.2) | 1.8±0.3 |
1.0±0.2b |
| Muscle | 0.4±0.4 | 0.35±0.06 | 0.26±0.05 |
0.15±0.04b |
| Bone |
0.40±0.07a | 0.7±0.2 | 0.57±0.09 |
0.26±0.03b,c |
Specificity of EGFR-targeting in vivo was
assessed by pre-saturation of receptors with non-labeled monoclonal
antibody cetuximab. The tumor uptake in the blocked group of mice
was significantly reduced (P<0.05) compared to the control
group. No significant difference was found in the radioactivity
uptake in blood (Table I).
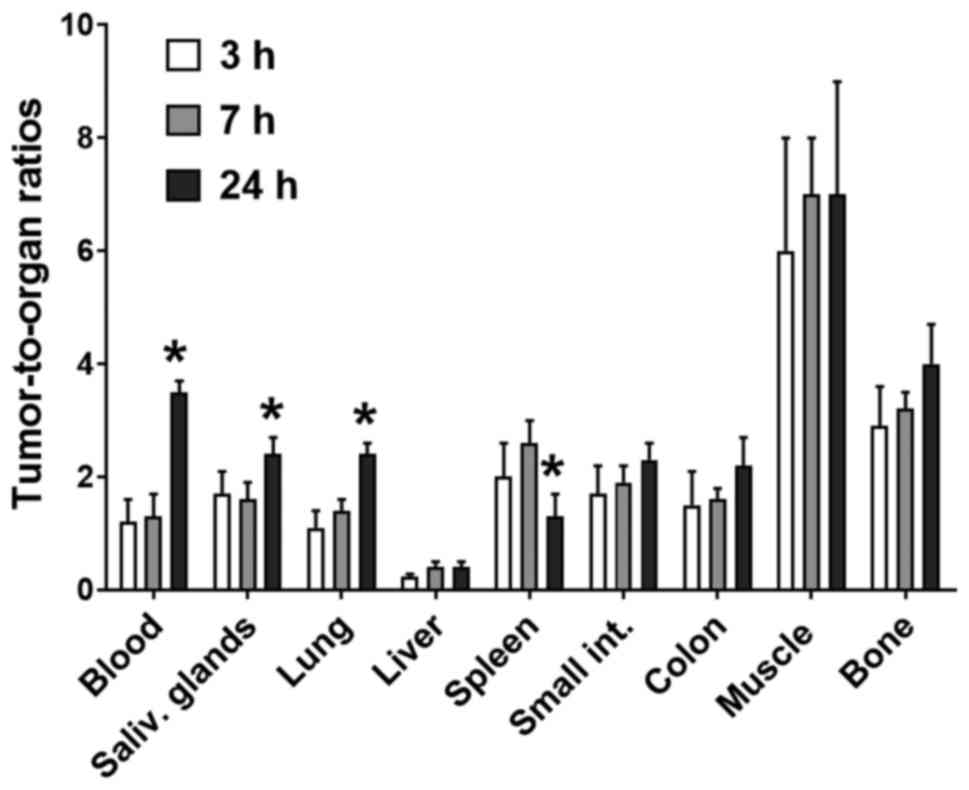
Data on the biodistribution of
57Co-DOTA-ZEGFR:2377 over time are presented
in Table I. In this experiment, two
additional groups of mice were injected with 10 µg and the animals
were sacrificed at 7 and 24 h pi. There was no significant release
of radioactivity from the tumors between 3 and 7 h pi, but
radioactivity uptake in tumors dropped 2-fold at 24 h pi. The
clearance from blood and normal organs was also relatively slow,
and with the exception of lungs, spleen and colon, there was no
noticeable decrease in the radioactivity concentration between 3
and 7 h pi. However, radioactivity uptake in normal organs
decreased 3-fold at 24 h pi which was rapider than in tumors. As a
result, tumor-to-non-tumor ratios were not significantly improved
between 3 and 7 h pi, but significantly increased at 24 h pi (n=4,
P<0.05) (Fig. 4).
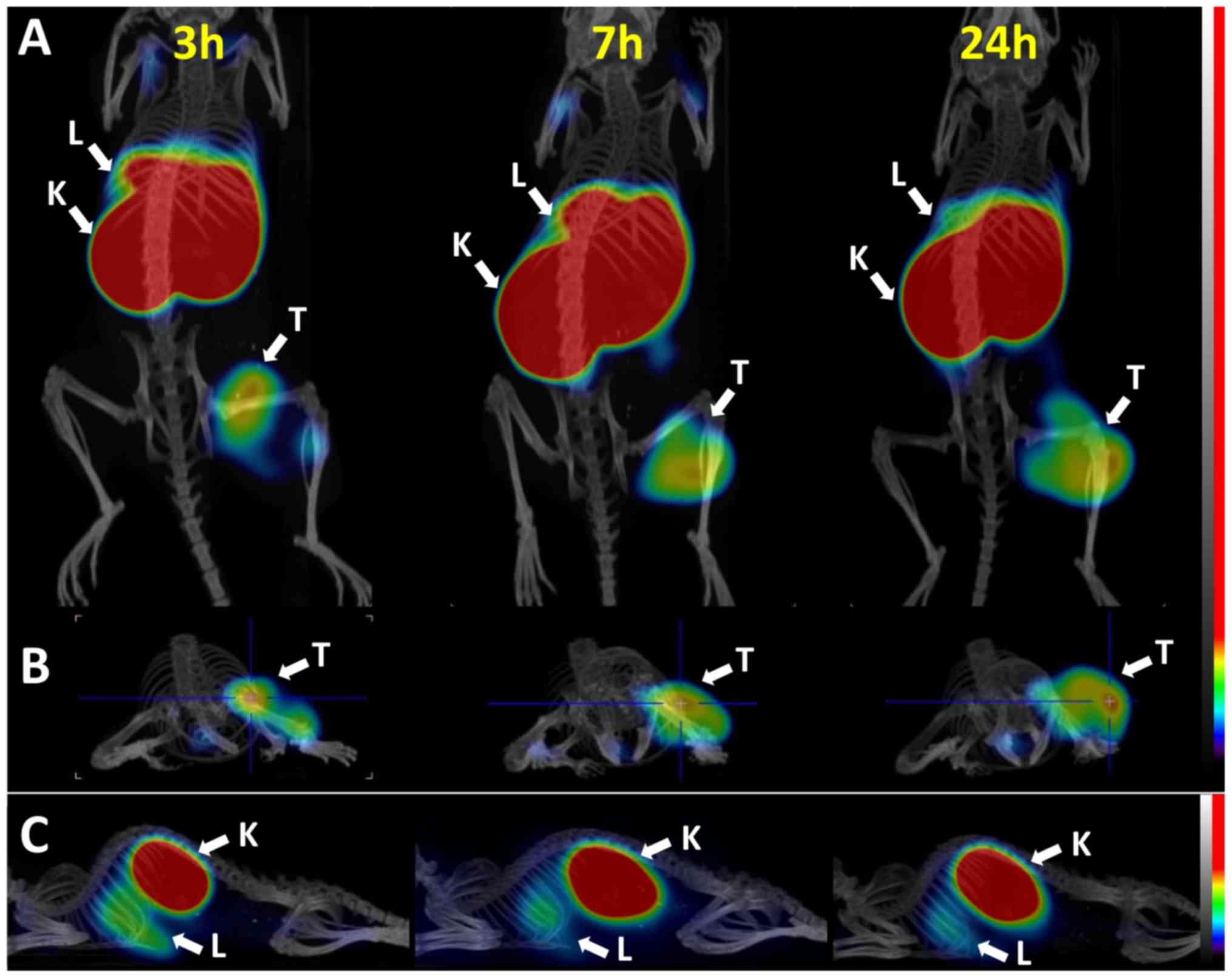
MicroSPECT scans were acquired at 3, 7, and 24 h
after the intravenous administration of 10 µg (4 MBq)
57Co-DOTA-ZEGFR:2377 to BALB/c nu/nu mice
bearing subcutaneous DU-145 ×enografts (Fig. 5). SPECT/CT images confirmed the
capacity of 57Co-DOTA-ZEGFR:2377 to visualize
EGFR expression. The highest uptake of radioactivity was observed
in the kidneys, followed by the liver, concordant with the
biodistribution results. The microSPECT experiment confirmed that
imaging of EGFR expression in PCa is possible.
Discussion
EGFR is significantly upregulated in advanced stages
of PCa and is associated with a high risk of recurrence,
progression towards androgen independence and metastasis (8). Therefore, EGFR has emerged as a
promising target for second-line therapy of disseminated PCa. Such
therapies include neutralization of EGFR by mAb that prevent
binding to its ligands and the subsequent activation of downstream
signaling pathways and TKI that compete with the ATP binding site
of the catalytic domain of tyrosine kinases (39,40).
However, the use of anti-EGFR mAb cetuximab for treatment of
unselected patients exhibited a limited success if any (41,42).
Moreover, adding cetuximab to other therapeutics often aggravated
the side-effects. Both studies claimed the necessity of predictive
biomarkers for EGFR-targeted therapy of PCa. Fleming et al
demonstrated in a post hoc analysis that appearance of a rash could
be a pharmacodynamics biomarker for response enabling selection of
tailored dosing of cetuximab (42).
A further study demonstrated that EGFR overexpression was
correlated with the response of PCa to cetuximab treatment
(43). A probe enabling imaging of
EGFR expression in PCa metastases before treatment and data
concerning receptor occupancy during treatment would provide
important predictive and pharmacodynamic information and would
facilitate the use of cetuximab and TKI for the treatment of
PCa.
Comparison of data from our previous studies
(32–34) demonstrated that
57Co-DOTA-ZEGFR:2377 provided better contrast
of EGFR imaging than 89Zr-cetuximab and
ZEGFR:2377 variants labeled with 111In,
68Ga or 89Zr. Notably,
57Co-ZEGFR:2377 binds to the same epitope as
cetuximab as seen in the in vitro binding specificity assay.
In this experiment, the uptake of
57Co-ZEGFR:2377 on EGFR-expressing DU-145
cells was successfully blocked by the addition of excess cetuximab
(Fig. 1). Binding specificity was
further confirmed in vivo in mice bearing DU-145 ×enografts.
The uptake of 57Co-ZEGFR:2377 in tumors was
significantly lower in mice pre-injected with an excess amount of
cetuximab, while the blood-born radioactivity was similar (Table I). These experiments indicated that
radiocobalt-labeled ZEGFR:2377 could be used for
monitoring receptor occupancy during cetuximab therapy. The
significant reduction of 57Co-DOTA-ZEGFR:2377
tumor uptake in a post-treatment scan compared to a baseline image
would indicate that EGFR in the lesion was blocked
sufficiently.
Data concerning cellular (Fig. 2) processing of 57Co-DOTA-
ZEGFR:2377 were in agreement with the data for A431
cells (37). In both cases, the
internalized fraction was unexpectedly low for a radiometal label
(below 15% after 24 h). For comparison, the internalized fraction
reached 30% for the same conjugate radiolabeled with indium-111
(37). The study of radioactivity
retention after interrupted incubation also revealed a low
internalized fraction that did not increase with time (Fig. 2). Collectively, this may indicate
lower residualizing properties of cobalt-containing
radiocatabolites that could lead to lower retention of labels in
tumors in vivo over time.
The presence of two types of binding sites
characterized by different affinities was found when evaluating the
binding kinetics of 57Co-DOTA-ZEGFR:2377 to
EGFR-expressing DU-145 cells. The data indicated the existence of
two populations of EGFR in DU-145 cells: The major one with
picomolar affinity and another one with low nanomolar affinity.
This finding was consistent with previous results when binding of
natural ligand EGF to EGFR was studied (38) and was revealed to vary depending on
culturing conditions (44,45). A possible explanation for the two
types of interactions is receptor homo- and heterodimerization,
which can result in different accessibility of binding sites. It
should to be noted that both interactions were strong (0.08 and 3.7
nM), with the strongest interaction predominant.
One of the main obstacles for imaging of EGFR
expression in tumors is an appreciable EGFR expression in a number
of normal tissues, particularly in liver hepatocytes. Liver is a
large well-perfused organ. The injection of an insufficient amount
of e.g. anti-EGFR antibody may result in its nearly complete
trapping in liver (46). Clinical
studies have demonstrated, however, that it is possible to adjust
the dose of the anti-EGFR antibody to saturate EGFR in liver and
provide release of radiolabeled antibody into circulation (23). We have also shown in preclinical
studies that it is possible to partially saturate murine EGFR in
liver with 111In-DOTA-ZEGFR:2377 without
saturating receptors in xenografts (32). The optimal protein dose, 35 µg, was
also used in the previous study with
57Co-DOTA-ZEGFR:2377 (33).
However, these initial studies utilized the A431
cell line, having EGFR expression of ~2×106
receptors/cell. The expression level of EGFR in PCa is appreciably
lower (Human Protein Atlas http://www.proteinatlas.org/). For example, the DU-145
cell line has an EGFR expression of 2×105
receptors/cell, i.e. one order of magnitude lower than A431 cells.
The average tumor weight at the time of the experiment was
0.13±0.08 g corresponding to lesions of 4 mm in diameter. The
spatial resolution of modern clinical PET cameras is 6–10 mm and
radioactivity concentration in any objects below this size will be
underestimated due to partial volume effect. Taking this in
account, the tumors in our study were relatively small from a
clinical perspective. The in vivo data revealed a pronounced
influence of injected dose on the biodistribution profile of
57Co-DOTA-ZEGFR:2377. We compared the
biodistribution and tumor uptake after the injection of 35 µg [dose
found optimal for high EGFR expression xenografts A431 (32,33)]
and 10 µg of radiolabeled conjugate at 3 h pi. A detailed
dose-dependent uptake of anti-EGFR affibody molecules in normal
organs was previously studied in detail by Tolmachev et al
(32), creating the rationale for
the choice of protein doses tested in the present study.
Our reasoning was that a high protein dose could
lead to saturation of EGFR in tumors with low expression level in
the same manner as in normal organs with EGFR expression, such as
salivary glands and intestine walls. Decreasing the protein dose
resulted in a significant increase of uptake in tumors as well as
in most studied organs including blood. The same phenomenon was
also observed for 111In-DOTA-ZEGFR:2377 and
111In-DOTA-ZEGFR:1907 studied in high
EGFR-expressing A431 ×enografts (32,46).
The elevated blood radioactivity may be attributed to the
dissociation of non-internalized radioconjugates from
EGFR-expressing organs followed by re-entrance in blood
circulation. In the group of mice injected with 35 µg
57Co-DOTA-ZEGFR:2377, the binding sites were
partially saturated with non-labeled compound, which lead to a
reduction of uptake in tumors and EGFR-expressing organs (liver,
salivary glands, lungs) and lower blood-born radioactivity.
However, in the low EGFR-expressing xenografts used in the present
study, the decrease in protein dose resulted in a significant
2-fold increase in radioactivity uptake in tumors (n=4,
P<0.05).
The higher tumor uptake would increase the
sensitivity of imaging of lesions with low receptor expression,
such as EGFR in PCa. Therefore, the injected dose of 10 µg was used
for measuring the biodistribution over time. The time-points
assessed in the present study were chosen to be clinically
relevant: 3 and 7 h pi correspond to same-day imaging while 24 h pi
corresponds to next-day imaging.
Due to the slow clearance of radioactivity from
blood and normal organs, the tumor-to-organ ratios were not
significantly improved between 3 and 7 h pi. At the later
time-point of 24 h pi, the faster clearance from normal tissues
compared to tumors resulted in significantly higher tumor-to-organ
ratios that enhanced imaging contrast. In contrast to the results
obtained for 57Co-DOTA-ZEGFR:2377 in a high
EGFR-expressing model (33), the
conjugate did not provide positive contrast towards the liver.
However, this is not critical for PCa where liver metastases are
not common. Still, the uptake in tumors after the injection of 10
µg 57Co-DOTA-ZEGFR:2377 exceeded the uptake
in all other studied organs with the exception of the liver and
kidneys. For organs clinically relevant for the detection of PCa
lesions, tumor-to-organ ratios at 24 h pi were 2.2±0.5 for the
colon, 7±2 for muscle, and 4.0±0.7 for bones. Therefore
57Co-DOTA-ZEGFR:2377 was successful in
visualizing the EGFR-expression in PCa as early as 3 h pi and
imaging contrast improved with time (Fig. 4). Previously it was demonstrated
that a single-dose injection of anti-EGFR affibody molecule
produced no pathological evidence of toxicity in rats at a dose
level of 24,490 µg/kg (corresponding to a 1,000× equivalent human
microdose level) (47). Dosimetry
estimated using clinical data for the anti-HER2 affibody molecule,
a tracer with a comparable kidney uptake, revealed that the
absorbed dose to kidneys and liver, after a 100-MBq administration
(Ga-68), would be ~40 and 15 mGy, respectively, which is less than
the maximum allowed absorbed dose of 50 mGy to a single organ in a
healthy adult research subject. The administration of ~200 MBq
would give an effective dose in the range of 5–6 mSv (lower than
for typical 18F-FDG PET examinations, which often give
effective doses of around 7 mSv (48). We may expect that for the anti-EGFR
imaging probe the absorbed doses for kidneys should be similar and
the absorbed dose for liver should be somewhat higher than that for
the anti-HER2 imaging probe.
In conclusion, radiocobalt-labeled
DOTA-ZEGFR:2377 allowed the successful visualization of
EGFR in vivo in a PCa pre-clinical model and could be used
for monitoring of receptor occupancy during cetuximab therapy. The
present study also emphasized the importance of finding optimal
protein dosing for tumors with low EGFR expression in order to
ensure a higher sensitivity in clinical imaging.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Swedish
Cancer Society [grants CAN 2017/649 (JL), CAN2013/586 and CAN
2016/463 (SS), CAN2014/474, CAN 2017/425 (AO), and CAN2015/350
(VT)], the Swedish Research Council [grants 621-2012-5236 (SS),
2015-02509 (AO), and 2015-02353 (VT)], the Swedish Agency for
Innovation VINNOVA [grants 2016-04060 (AO) and 2017-02015 (SS, JL)]
and the Wallenberg Center for Protein Technology (SS, JL). The
molecular imaging work in this publication was supported by the
Wallenberg infrastructure for PET-MRI (WIPPET) at SciLifeLab Pilot
Facility for Preclinical PET-MRI, a Swedish nationally available
imaging platform at Uppsala University, Sweden, financed by the
Knut and Alice Wallenberg Foundation (SPECT/CT).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
BM participated in the labeling of proteins, the
in vitro and in vivo characterization, the data
acquisition, data interpretation and drafted the first version of
manuscript; KGA and JG performed the production and the following
biochemical, biophysical and in vitro characterization of
conjugate; EL participated in the labeling of proteins, the in
vitro and in vivo characterization, the data acquisition
and data interpretation; MR participated in the in vivo
characterization and the data acquisition; VT and AO participated
in the molecular and study design, in the in vivo
characterization, the data acquisition, data interpretation,
drafted the relevant parts of the manuscript and critically revised
the manuscript; SS and JL participated in the molecular and study
design, the data acquisition, data interpretation and critically
revised the manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All animal experiments were planned and performed
in accordance with the Swedish national legislation on the
protection of laboratory animals and the study plans were approved
by the Ethics Committee for Animal Research in Uppsala.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Huggins C and Hodges CV: Studies on
prostate cancer. I. The effect of castration, of estrogen and of
androgen injection on serum phosphatases in metastatic carcinoma of
the prostate. CA Cancer J Clin. 22:232–240. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pienta KJ and Bradley F: Mechanisms
underlying the development of androgen-independent PC. Clin Cancer
Res. 12:1665–1671. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hoffman P and Djavan B: Androgen
deprivation therapy. Rev Urol. 10:305–306. 2008.PubMed/NCBI
|
|
4
|
Yagoda A and Petrylak D: Cytotoxic
chemotherapy for advanced hormone-resistant prostate cancer.
Cancer. 71((Suppl 3)): S1098–S1109. 1993. View Article : Google Scholar
|
|
5
|
Raghavan D, Koczwara B and Javle M:
Evolving strategies of cytotoxic chemotherapy for advanced prostate
cancer. Eur J Cancer. 33:566–574. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mimeault M and Batra SK: Recent advances
on multiple tumorigenic cascades involved in prostatic cancer
progression and targeting therapies. Carcinogenesis. 27:1–22. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Djakiew D: Dysregulated expression of
growth factors and their receptors in the development of prostate
cancer. Prostate. 42:150–160. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hernes E, Fosså SD, Berner AA, Otnes B and
Nesland JM: Expression of the epidermal growth factor receptor
family in prostate carcinoma before and during
androgen-independence. Br J Cancer. 90:449–454. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schlomm T, Kirstein P, Iwers L, Daniel B,
Steuber T, Walz J, Chun FH, Haese A, Kollermann J, Graefen M, et
al: Clinical significance of epidermal growth factor receptor
protein overexpression and gene copy number gains in prostate
cancer. Clin Cancer Res. 13:6579–6584. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shaw G and Prowse DM: Inhibition of
androgen-independent PC cell growth is enhanced by combination
therapy targeting Hedgehog and ErbB signaling. Cancer Cell Int.
8:32008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marmor MD, Skaria KB and Yarden Y: Signal
transduction and oncogenesis by ErbB/HER receptors. Int J Radiat
Oncol Biol Phys. 58:903–913. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oda K, Matsuoka Y, Funahashi A and Kitano
H: A comprehensive pathway map of epidermal growth factor receptor
signalling. Mol Syst Biol. 1(2005.0010)2005.PubMed/NCBI
|
|
13
|
Baselga J: The EGFR as a target for
anticancer therapy-focus on cetuximab. Eur J Cancer. 37((Suppl 4)):
S16–S22. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Herbst RS, Kim ES and Harari PM: IMC-
C225, an anti-epidermal growth factor receptor monoclonal antibody,
for treatment of head and neck cancer. Expert Opin Biol Ther.
1:719–732. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bonomi P: Erlotinib: A new therapeutic
approach for non-small cell lung cancer. Expert Opin Investig
Drugs. 12:1395–1401. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Herbst RS: Erlotinib (Tarceva): An update
on the clinical trial program. Semin Oncol. 30((3 Suppl 7)):
S34–S46. 2003. View Article : Google Scholar
|
|
17
|
Fukuoka M, Yano S, Giaccone G, Tamura T,
Nakagawa K, Douillard JY, Nishiwaki Y, Vansteenkiste J, Kudoh S,
Rischin D, et al: Multi-institutional randomized phase II trial of
gefitinib for previously treated patients with advanced
non-small-cell lung cancer (The IDEAL 1 Trial). J Clin Oncol.
21:2237–2246. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ranson M, Hammond LA, Ferry D, Kris M,
Tullo A, Murray PI, Miller V, Averbuch S, Ochs J, Morris C, et al:
A selective oral epidermal growth factor receptor-tyrosine kinase
inhibitor, is well tolerated and active in patients with solid,
malignant tumors: Results of a phase I trial. J Clin Oncol.
2:2240–2250. 2002. View Article : Google Scholar
|
|
19
|
Guérin O, Fischel JL, Ferrero JM, Bozec A
and Milano G: EGFR targeting in hormone-refractory prostate cancer:
Current appraisal and prospects for treatment. Pharmaceuticals
(Basel). 3:2238–2247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vignot S, Besse B, André F, Spano JP and
Soria JC: Discrepancies between primary tumor and metastasis: A
literature review on clinically established biomarkers. Crit Rev
Oncol Hematol. 84:301–313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cuartero-Plaza A, Martínez-Miralles E,
Rosell R, Vadell-Nadal C, Farré M and Real FX: Radiolocalization of
squamous lung carcinoma with 131I-labeled epidermal growth factor.
Clin Cancer Res. 2:13–20. 1996.PubMed/NCBI
|
|
22
|
Goldenberg A, Masui H, Divgi C, Kamrath H,
Pentlow K and Mendelsohn J: Imaging of human tumor xenografts with
an indium-111-labeled anti-epidermal growth factor receptor
monoclonal antibody. J Natl Cancer Inst. 81:1616–1625. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Divgi CR, Welt S, Kris M, Real FX, Yeh SD,
Gralla R, Merchant B, Schweighart S, Unger M, Larson SM, et al:
Phase I and imaging trial of indium 111-labeled anti-epidermal
growth factor receptor monoclonal antibody 225 in patients with
squamous cell lung carcinoma. J Natl Cancer Inst. 83:97–104. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cai W, Chen K, He L, Cao Q, Koong A and
Chen X: Quantitative PET of EGFR expression in xenograft-bearing
mice using 64Cu-labeled cetuximab, a chimeric anti-EGFR
monoclonal antibody. Eur J Nucl Med Mol Imaging. 34:850–858. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ping Li W, Meyer LA, Capretto DA, Sherman
CD and Anderson CJ: Receptor-binding, biodistribution, and
metabolism studies of 64Cu-DOTA-cetuximab, a PET-imaging
agent for epidermal growth-factor receptor-positive tumors. Cancer
Biother Radiopharm. 23:158–171. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nayak TK, Garmestani K, Baidoo KE, Milenic
DE and Brechbiel MW: Preparation, biological evaluation, and
pharmacokinetics of the human anti-HER1 monoclonal antibody
panitumumab labeled with 86Y for quantitative PET of carcinoma. J
Nucl Med. 51:942–950. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nayak TK, Garmestani K, Milenic DE and
Brechbiel MW: PET and MRI of metastatic peritoneal and pulmonary
colorectal cancer in mice with human epidermal growth factor
receptor 1-targeted 89Zr-labeled panitumumab. J Nucl
Med. 53:113–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chang AJ, De Silva RA and Lapi SE:
Development and characterization of 89Zr-labeled
panitumumab for immuno-positron emission tomographic imaging of the
epidermal growth factor receptor. Mol Imaging. 12:17–27.
2013.PubMed/NCBI
|
|
29
|
Ahlgren S and Tolmachev V: Radionuclide
molecular imaging using Affibody molecules. Curr Pharm Biotechnol.
11:581–589. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sörensen J, Sandberg D, Sandström M,
Wennborg A, Feldwisch J, Tolmachev V, Åström G, Lubberink M,
Garske-Román U, Carlsson J and Lindman H: First-in-human molecular
imaging of HER2 expression in breast cancer metastases using the
111In-ABY-025 affibody molecule. J Nucl Med. 55:730–735.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sörensen J, Velikyan I, Sandberg D,
Wennborg A, Feldwisch J, Tolmachev V, Orlova A, Sandström M,
Lubberink M, Olofsson H, et al: Measuring HER2-receptor expression
in metastatic breast cancer using [68Ga]ABY-025 affibody
PET/CT. Theranostics. 6:262–271. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tolmachev V, Rosik D, Wållberg H, Sjöberg
A, Sandström M, Hansson M, Wennborg A and Orlova A: Imaging of EGFR
expression in murine xenografts using site-specifically labelled
anti-EGFR 111In-DOTA-ZEGFR:2377 Affibody molecule:
Aspect of the injected tracer amount. Eur J Nucl Med Mol Imaging.
37:613–622. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Garousi J, Andersson KG, Dam JH, Olsen BB,
Orlova A, Buijs J, Ståhl S, Löfblom J, Thisgaard H and Tolmachev V:
The use of radiocobalt as a label improves imaging of EGFR using
DOTA-conjugated Affibody molecule. Sci Rep. 7:59612017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Garousi J, Andersson KG, Mitran B, Pichl
ML, Ståhl S, Orlova A, Löfblom J and Tolmachev V: PET imaging of
epidermal growth factor receptor expression in tumours using
89Zr-labelled ZEGFR:2377 affibody molecules. Int J
Oncol. 48:1325–1332. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang D, Kuan CT, Payne J, Kihara A, Murray
A, Wang LM, Alimandi M, Pierce JH, Pastan I and Lippman ME:
Recombinant heregulin-Pseudomonas exotoxin fusion proteins:
Interactions with the heregulin receptors and antitumor activity in
vivo. Clin Cancer Res. 4:993–1004. 1998.PubMed/NCBI
|
|
36
|
Mitran B, Thisgaard H, Rosenström U, Dam
JH, Larhed M, Tolmachev V and Orlova A: High contrast PET imaging
of GRPR expression in prostate cancer using cobalt-labeled Bombesin
antagonist RM26. Contrast Media Mol Imaging 2017. 68736842017.
|
|
37
|
Malmberg J, Tolmachev V and Orlova A:
Imaging agents for in vivo molecular profiling of disseminated
prostate cancer-targeting EGFR receptors in prostate cancer:
Comparison of cellular processing of [111In]-labeled
affibody molecule Z(EGFR:2377) and cetuximab. Int J Oncol.
38:1137–1143. 2011.PubMed/NCBI
|
|
38
|
Björkelund H, Gedda L and Andersson K:
Comparing the epidermal growth factor interaction with four
different cell lines: Intriguing effects imply strong dependency of
cellular context. PLoS One. 6:e165362011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rocha-Lima CM, Soares HP, Raez LE and
Singal R: EGFR targeting of solid tumors. Cancer Control.
14:295–304. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Henson E, Chen Y and Gibson S: EGFR family
members regulation of autophagy is at a crossroads of cell survival
and death in cancer. Cancers (Basel). 9:E272017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Slovin SF, Kelly WK, Wilton A, Kattan M,
Myskowski P, Mendelsohn J and Scher HI: Anti-epidermal growth
factor receptor monoclonal antibody cetuximab plus Doxorubicin in
the treatment of metastatic castration-resistant prostate cancer.
Clin Genitourin Cancer. 7:E77–E82. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fleming MT, Sonpavde G, Kolodziej M,
Awasthi S, Hutson TE, Martincic D, Rastogi A, Rousey SR, Weinstein
RE, Galsky MD, et al: Association of rash with outcomes in a
randomized phase II trial evaluating cetuximab in combination with
mitoxantrone plus prednisone after docetaxel for metastatic
castration-resistant prostate cancer. Clin Genitourin Cancer.
10:6–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cathomas R, Rothermundt C, Klingbiel D,
Bubendorf L, Jaggi R, Betticher DC, Brauchli P, Cotting D, Droege
C, Winterhalder R, et al: Efficacy of cetuximab in metastatic
castration-resistant prostate cancer might depend on EGFR and PTEN
expression: Results from a phase II trial (SAKK 08/07). Clin Cancer
Res. 18:6049–6057. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Özcan F, Klein P, Lemmon MA, Lax I and
Schlessinger J: On the nature of low- and high-affinity EGF
receptors on living cells. Proc Natl Acad Sci USA. 103:5735–5740.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Björkelund H, Gedda L, Malmqvist M and
Andersson K: Resolving the EGF-EGFR interaction characteristics
through a multiple-temperature, multiple-inhibitor, real-time
interaction analysis approach. Mol Clin Oncol. 1:343–352. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tolmachev V, Friedman M, Sandström M,
Eriksson TL, Rosik D, Hodik M, Ståhl S, Frejd FY and Orlova A:
Affibody molecules for epidermal growth factor receptor targeting
in vivo: Aspects of dimerization and labeling chemistry. J Nucl
Med. 50:274–283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Samkoe KS, Gunn JR, Marra K, Hull SM,
Moodie KL, Feldwisch J, Strong TV, Draney DR, Hoopes PJ, Roberts
DW, et al: Toxicity and pharmacokinetic profile for single-dose
injection of ABY-029: A fluorescent anti-EGFR synthetic affibody
molecule for human use. Mol Imaging Biol. 19:512–521. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sandström M, Lindskog K, Velikyan I,
Wennborg A, Feldwisch J, Sandberg D, Tolmachev V, Orlova A,
Sörensen J, Carlsson J, et al: Biodistribution and radiation
dosimetry of the Anti-HER2 affibody molecule
68Ga-ABY-025 in breast cancer patients. J Nucl Med.
57:867–871. 2016. View Article : Google Scholar : PubMed/NCBI
|