Introduction
The B-cell receptor (BCR) signaling pathway plays an
important role in maintaining cellular functions such as
proliferation, selection, maturation, apoptosis and differentiation
of normal B lymphocytes (1,2). Aberrant activation of the BCR signaling
pathway is a common hallmark of several B-lymphoid malignancies.
Bruton's tyrosine kinase (BTK), a non-receptor tyrosine kinase that
belongs to the tyrosine kinase expressed in hepatocellular
carcinoma (TEC) family of kinases, is a crucial terminal kinase
enzyme in the BCR signaling pathway (3). This downstream signal transduction
protein acts as an important effector molecule in governing B-cell
development, differentiation, survival and function. Recently, BTK
has emerged as a novel target in the treatment of B-cell
malignancies (4).
Ibrutinib, a potent, orally administered BTK
inhibitor, has demonstrated promising effectiveness in several
B-cell malignancies and autoimmune diseases in preclinical models
and clinical trials (5). Ibrutinib
has been approved by the Food and Drug Administration for mantle
cell lymphoma (MCL) in November 2013, for chronic lymphocytic
leukemia or small lymphocytic lymphoma (CLL/SLL) in July 2014, and
for Waldenström macroglobulinemia (WM) in January 2015 (6–8). The
present review includes three main topics: i) The BCR/BTK signaling
pathway in B-cell malignancies; ii) the clinical application of
ibrutinib in CLL/SLL; and iii) the side effects of ibrutinib and
their management.
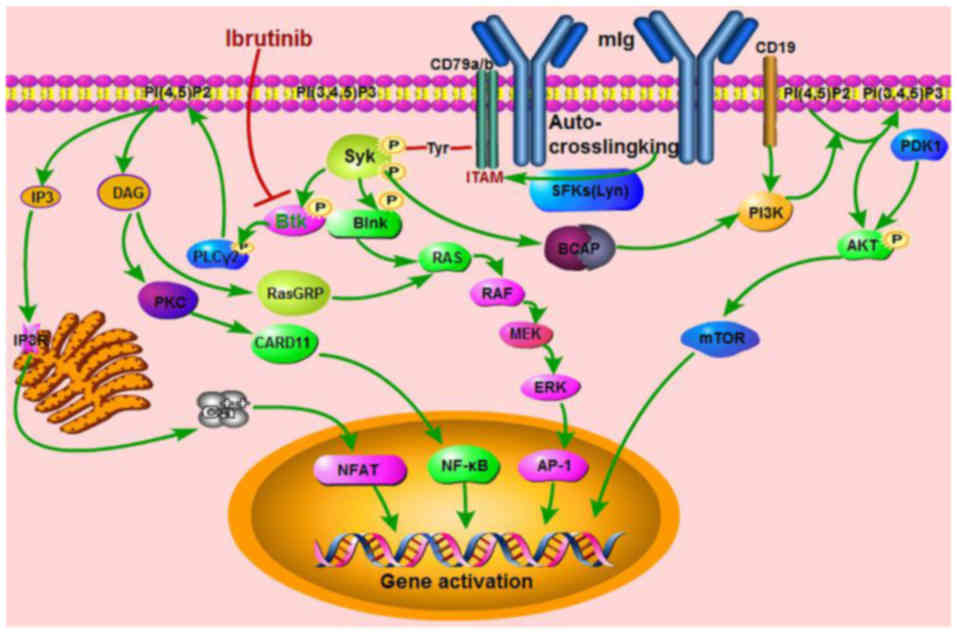
BCR/BTK signaling pathway in B-cell
malignancies
The BCR signaling pathway plays an important role in
the development and maturation of B cells. The BCR consists of
membrane-bound immunoglobulin (mIg) non-covalently associated with
a heterodimer of CD79a (Igα) and CD79b (Igβ), which contains the
immunoreceptor tyrosine-based activation motif (ITAM) (9). Following binding of the mIg, signals are
transduced across the plasma membrane, leading to phosphorylation
of the ITAM primarily by Src-family tyrosine kinases (SFKs), such
as Lyn. Phosphorylated ITAM leads to SFK binding by Src-homology 2
(SH2) domains (9). This binding,
results in the activation and phosphorylation of spleen tyrosine
kinases (SYKs) (10). Once Syk is
activated, the BCR signal is transduced through a group of proteins
associated with the adaptor protein, such as B-cell adaptor for
phosphoinositide-3-kinase (BCAP), B-cell linker (Blnk) and its
downstream signaling components, BTK and phospholipase Cγ2 (PLCγ2)
(2,9).
When phosphorylated by Syk, Blnk recruits BTK and PLCγ2. After
their recruitment, Btk phosphorylates PLCγ2, which then cleaves the
phosphoinositide phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2],
generating inositol triphosphate (IP3) and diacylglycerol (DAG)
(2,9,10). IP3
binds to the IP3 receptor (IP3R) in the membrane of the endoplasmic
reticulum to facilitate the mobilization of calcium ions, which
leads to the activation of nuclear factor for activated T-cells
(NF-AT) (2,9). DAG activates protein kinase C (PKC) and
its downstream substrates caspase recruitment domain 11 protein
(CARD11) and subsequently initiates NF-κB signaling (2,10). In
addition, DAG also binds to Ras guanyl nucleotide-releasing protein
(RasGRP) and activates the RAS/RAF/MEK/ERK signaling pathway
(9–11). With the assistance of PI3K, the
activity of which is localized to the plasma membrane by a
lipid-binding domain or by recruitment to CD19 or BCAP, PI(4,5)P2
generates phosphatidylinositol 3,4,5-triphosphate [(PI(3,4,5)P3)],
which subsequently activates protein kinase B (AKT) and causes
upregulation of mammalian target of the rapamycin (mTOR) pathway
(10,11). PI(3,4,5)P3 in turn recruits BTK,
PLCγ2, and 3-phosphoinositide-dependent protein kinase 1 (PDK1)
(10,12). The pathways of the BCR/BTK signaling
cascade are depicted in Fig. 1.
BTK was first revealed to be defective in the
primary immunodeficiency X-linked agammaglobulinemia (XLA), which
was initially described by Dr Ogden Carr Bruton in 1952 (13,14). It is
encoded by the XLA gene that is located on chromosome Xq21.3–22 and
has a length of 37.5 kb (15,16). BTK is a non-receptor tyrosine kinase
that belongs to the TEC family kinases (TFKs), which has five
members (BTK, TEC, ITK, BMX and RLK/TXK) (17,18). BTK
carries a conserved cysteine residue adjacent to an ATP-binding
site, which is critical for inhibition by tyrosine kinase
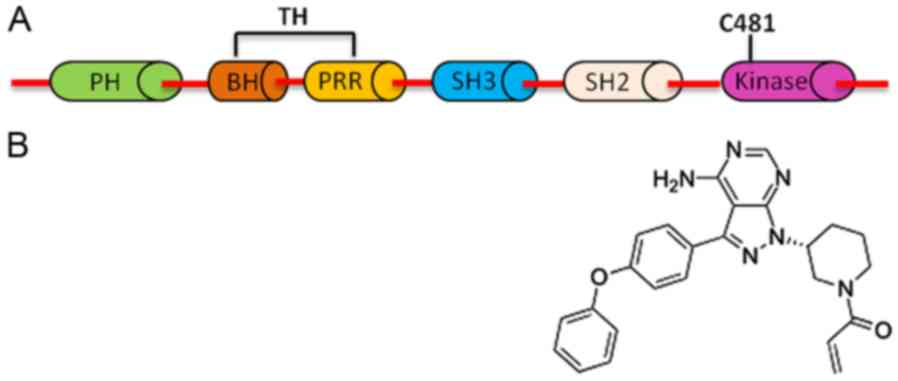
inhibitors (17,19). BTK has five domains: Pleckstrin
homology (PH), Tec homology (TH), two Src homology domains (SH2 and
SH3), and tyrosine kinase or Src homology 1 domain (TK or SH1,
respectively) (18,20). The expression of BTK is restricted to
B lymphocytes, myeloid cells, erythroid cells, platelets, mast
cells, macrophages, natural killer (NK) cells and myeloid-derived
suppressor cells (20–23). BTK is crucial for B-cell development,
function of mature B cells, proliferation and survival of B-cells
(20, 24). Constitutive-activation or mutation of the BTK pathways
has been implicated in maintaining the malignant phenotype in a
wide variety of B-cell malignancies, such as CLL, MCL and WM
(25–27), and acute lymphoblastic leukemia (ALL)
(28). BTK has been recognized as a
therapy target for B-cell malignancies (4,29).
Ibrutinib (formerly referred to as PCI-32765) is a
first-in-class, orally bioavailable, small-molecule covalent
inhibitor of BTK, which selectively and irreversibly inhibits BTK
(19,26,30).
Ibrutinib selectively binds to the cysteine residue at position 481
(Cys-481) in the allosteric inhibitory segment of BTK (TK/SH1
domain), which blocks adenosine triphosphate (ATP) from binding,
thereby preventing Btk phosphorylation and activation (19). The domain structure of BTK, the C481
binding site of ibrutinib and the chemical structure of ibrutinib
are presented in Fig. 2. As a
small-molecule inhibitor, kinase selectivity is a factor important
for the efficacy and safety of ibrutinib. However, other kinases
also have a cysteine residue in the ATP-binding site, such as TFK
members, EGFR family kinases, BLK and JAK3 (31). Therefore, this on-target or off-target
effect of ibrutinib may be used in other diseases or may cause
adverse events (AEs). Recently, ibrutinib has also been used as a
novel anticancer drug for several solid tumors and hematological
malignancies, such as breast, ovarian, gastric and lung cancer,
glioma, glioblastoma, CD117+ acute myeloid leukemia and
BCR+ ALL (32–39).
Drug-drug interactions (DDIs)
Ibrutinib is primarily metabolized in the liver and
is eliminated predominantly by cytochrome P450 enzyme 3A4 (CYP3A4)
and less by CYP2D6 (40,41). Caution must be exercised regarding the
co-administration of ibrutinib with strong or moderate CYP3A4
inhibitors and inducers. Recently, in an analysis of 118
ibrutinib-treated patients with CLL, 75 patients (64%) were on
medications that could increase ibrutinib toxicity and 4 (3%) were
receiving drugs that could decrease ibrutinib efficacy (42). A previous study revealed that
ketoconazole (a strong CYP3A4 inhibitor) increased the ibrutinib
area under the curve (AUC) by 24-fold, while rifampin (a strong
CYP3A4 inducer) decreased the ibrutinib AUC by 10-fold (41). Thus, co-administration of ibrutinib
with CYP3A4 inhibitors or inducers should be avoided. If
co-administration of strong CYP3A4 inhibitors or inducers is
necessary, ibrutinib dosage modifications may be required. The
CPY3A4 inhibitors and inducers commonly used with ibrutinib are
summarized in Table I.
 | Table I.Commonly used CPY3A4 inhibitors and
inducers with ibrutinib. |
Table I.
Commonly used CPY3A4 inhibitors and
inducers with ibrutinib.
| Drug
classification | Drugs |
|---|
| Strong CYP3A4
inhibitors |
|
Antifungal drugs | Ketoconazole,
voriconazole, posaconazole |
|
Antibiotics | Clarithromycin,
telithromycin |
|
Antivirals | Indinavir,
nelfinavir, ritonavir, saquinavir |
|
Antidepressants | Nefazodone |
|
Others | Cobicistat,
buprenorphine/naloxone |
| Moderate CYP3A4
inhibitors |
|
Anti-arrhythmic drugs | Amiodarone,
diltiazem, verapamil, dronedarone |
|
Antifungal drugs | Fluconazole,
itraconazole |
|
Antibiotics | Erythromycin,
ciprofloxacin |
|
Antivirals | Amprenavir,
atazanavir, darunavir, fosamprenavir |
|
Anti-emetics | Aprepitant |
|
Antineoplastic agents | Crizotinib,
imatinib |
| Mild CYP3A4
inhibitors |
|
Antibiotics | Azithromycin |
|
Psychotropic drugs | Fluvoxamine |
|
Other | Grapefruit juice,
Seville oranges |
| Strong CYP3A4
inducers |
|
Antituberculosis drugs | Rifampin |
|
Psychotropic drugs | Carbamazepine,
phenytoin |
| Moderate CYP3A4
inducers |
|
Anti-HIV drugs | Efavirenz |
|
Others | St Johns wort |
There is in vitro evidence to demostrate that
ibrutinib inhibits P-glycoprotein (P-gp) and breast cancer
resistance protein (BCRP), although it is not a P-gp or BCRP
substrate (43). To minimize the risk
of interactions, it is recommended that co-administration of
ibrutinib and P-gp or BCRP substrates with a narrow therapeutic
range should occur at least 6 h before or after ibrutinib (44). In addition, ibrutinib has a
pH-dependent solubility, with lower solubility at higher pH.
Although, co-administration of ibrutinib with omeprazole leads to
the lower Cmax of ibrutinib, medicinal products that
increase gastric pH have been used without restrictions in clinical
trials (44).
Application of ibrutinib in CLL/SLL
A series of in vitro and mouse xenograft model
preclinical studies have investigated the activity of ibrutinib
against CLL (45–47).
Treatment-naïve (TN) CLL/SLL
In an open-label phase 1b/2 trial (NCT01105247 and
PCYC-1102) (48), previously
untreated CLL/SLL patients who required therapy and were aged at
least 65 years were enrolled. A total of 15 patients (48%) had
unmutated immunoglobulin heavy-chain variable region (IGHV), 2 (6%)
had del(17p13.1), 1 (3%) had del(11q22.3), 17 (55%) had del(13q14),
and 8 (26%) had trisomy 12. The patients received 28-day cycles of
once-daily ibrutinib 420 mg (n=27) or 840 mg (n=4). The primary
endpoint was safety and the severity of AEs for all patients.
Toxicity (such as diarrhea, nausea and fatigue) was mainly of
mild-to-moderate severity (grade 1–2). A total of 3 (10%) patients
developed grade 3 infections, 1 patient developed grade 3
neutropenia, and 1 developed grade 4 thrombocytopenia. The median
time to initial response, best response and complete response (CR)
was 1.9, 5.9 and 12.4 months, respectively. After a median
follow-up of 22.1 months, 22 (71%) patients achieved an objective
response (OR), and 4 patients (13%) had a CR. For all 31 patients
at 24 months, the Kaplan-Meier estimate of progression-free
survival (PFS) was 96.3% and the overall survival (OS) was 96.6%.
This phase 1b/2 trial suggested that ibrutinib was well-tolerated
and effective in a previously untreated population of elderly
patients with CLL/SLL (48).
RESONATE-2 was an international, open-label,
randomized phase 3 trial that evaluated the efficacy of ibrutinib
(at a dose of 420 mg once daily) and chlorambucil in previously
untreated patients with CLL or SLL aged ≥65 years and without
chromosome 17p13.1 deletion (49). Of
the patients, 70% (189/269) were aged at least 70 years, 45%
(122/269) had advanced-stage disease (Rai stage III or IV), and 20%
(54/269) had chromosome 11q22.3 deletion. The primary endpoint was
PFS. At a median follow-up of 18.4 months, ibrutinib resulted in a
significantly longer PFS compared with chlorambucil (median, not
reached vs. 18.9 months, respectively) and resulted in a reduction
of 84% in the risk of progression or death (hazard ratio = 0.16;
P<0.001). At 18 months, the PFS was 90% in the ibrutinib group
and 52% in the chlorambucil group. The 2-year OS rate in the
ibrutinib group and chlorambucil groups was 98 and 85%,
respectively. The overall response rate (ORR) was 86 vs. 35%
(P<0.001) in the ibrutinib and chlorambucil groups,
respectively. Approximately 20% of the patients in the ibrutinib
group suffered from AEs of any grade: 4 patients (3%) in the
ibrutinib group had grade 3 hemorrhage and 1 (1%) had grade 4
hemorrhage (49).
Recently, a randomized phase 3 study (NCT02048813)
on ibrutinib-based therapy compared with standard
fludarabine/cyclophosphamide/rituximab (FCR) chemoimmunotherapy
(CIT) in treatment-naïve patients with CLL aged <70 years and
without deletion 17p- was reported (50). The study revealed that the ibrutinib
with rituximab (IR) group was superior to the FCR group
independently of age, sex, performance status, disease stage or the
presence/absence of del11q23, with fewer grade 3 and 4
treatment-related AEs (58 vs. 72%, respectively). IR was also
superior to FCR for IGHV unmutated but not IGHV mutated patients.
Another phase 3 trial (NCT01886872) enrolled 547 untreated CLL
patients aged ≥65 years to evaluate the efficacy of ibrutinib-based
therapy compared with CIT (51). The
2-year PFS was 74% with bendamustine plus rituximab (BR), 87% with
ibrutinib alone and 88% with ibrutinib plus rituximab (IR). There
was no significant difference in OS among the three treatment
groups with a median follow-up of 38 months. The rate of grade ≥3
hematological AEs was 61% in the BR group, 41% in the ibrutinib
group and 39% in the IR group. The grade ≥3 non-hematological AEs
were 63% in the BR group, 74% in the ibrutinib group and 74% in the
IR group (51).
iLLUMINATE (NCT02264574) is a multicentre,
randomized, open-label, phase 3 trial comparing the efficacy of the
combination of ibrutinib plus obinutuzumab (IO) with chlorambucil
plus obinutuzumab (CO) (52). A total
of 229 patients were enrolled (aged ≥65 years or <65 years) with
previously untreated CLL/SLL (52).
The estimated 30-month PFS was 79% in the IO group and 31% in the
CO group. The grade 3–4 AEs in both groups were neutropenia and
thrombocytopenia. Serious AEs occurred in 65 out of 113 patients
(58%) treated with IO and in 40 out of 115 patients (35%) treated
with CO.
Relapsed or refractory CLL/SLL
In a multi-institutional phase 1 dose-escalating
study, two dosing schedules of ibrutinib were analyzed in patients
with relapsed or refractory CLL/SLL (R/R CLL/SLL) and other B-cell
malignancies (FL, MCL, MZL, DLBCL and WM) (53). A total of 56 patients received
ibrutinib at doses of 1.25 (n=7), 2.5 (n=9), 5.0 (n=6), 8.3 (n=8),
or 12.5 (n=7) mg/kg/day on a 28-day on/7-day off schedule (35-day
cycle), or continuous dosing of 8.3 mg/kg/day or 560 mg/day until
development of progressive disease (PD) or unacceptable toxicity.
The results favored 12.5 mg/kg/day without reaching the maximum
tolerated dose (MTD). The majority of AEs were grade 1 and 2 and
self-limited. In the 50 patients evaluated for response, the
objective response rate was 60%, including a CR rate of 16%. The
median PFS in all patients was 13.6 months. Another phase 1b/2
trial (NCT01105247, PCYC-1102) assessed the safety, efficacy,
pharmacokinetics, and pharmacodynamics of ibrutinib in patients
with R/R CLL or SLL (54). A total of
85 patients, 65% of whom were considered to have advanced-stage
disease, were stratified into two treatment groups of once-daily
ibrutinib 420 mg (n=51) or 840 mg (n=34) until development of PD or
unacceptable toxicity. There was no difference in the time to peak
ibrutinib concentration in the blood of the two groups. Toxic
effects included transient diarrhea, fatigue and upper respiratory
tract infection, which were predominantly grade 1 or 2, without the
need for treatment suspension. A total of 2 patients in the 420-mg
cohort (4%) and 4 patients in the 840-mg cohort (12%) suffered from
SAEs that led to discontinuation of treatment. The overall response
rate was the same in the two groups (71%). An additional 20 and 15%
of the patients in the 420 and 840 mg cohorts, respectively, had a
partial response (PR), with lymphocytosis. The response was
irrelevant to the clinical and genomic risk factors present
pre-treatment, such as advanced-stage disease, number of previous
treatments and the 17p13.1 deletion. At 26 months, the PFS was 75%
and the OS was 83% (54).
In the first randomized phase 3 RESONATE study
(PCYC-1112), 391 elderly patients with R/R CLL/SLL were randomized
to receive either ibrutinib (at a dose of 420 mg once daily) or
ofatumumab (55). The primary
endpoint was PFS. During a median follow-up of 9.4 months, the
median PFS was not reached in the ibrutinib cohort compared with
8.1 months in the ofatumumab cohort. Ibrutinib also significantly
improved OS (1-year OS rate 90% vs. 81%, respectively) and overall
response rate (42.6% vs. 4.1%, respectively) (55).
In 2015, the 3-year follow-up data from PCYC-1102
and the ongoing long-term extension study PCYC-1103 in 132 patients
(85 patients with R/R CLL/SLL, 31 patients with TN, and 16 patients
with ≥2 prior therapies) revealed that prolonged treatment with
ibrutinib ameliorated the quality of response over time, with
durable remissions and acceptable toxicity (56). In 2017, the authors reported the
safety and efficacy outcomes with continued follow-up (up to 44
months) of patients in the phase 1b/2 study (PCYC-1102) and
extension study (PCYC-1103) receiving ibrutinib 420 mg once-daily
until progression (57). A total of
94 CLL/SLL patients (27 with TN and 67 with R/R) were treated with
ibrutinib. The best overall response was 85% in TN patients and 94%
in R/R patients. The median PFS was not reached in either group and
the 30-month PFS rate was 96 and 76% for TN and R/R patients,
respectively (57). Recently, OBrien
et al reported 5-year follow-up data from the PCYC-1102 and
PCYC-1103 studies on ibrutinib monotherapy for TN (n=31) and R/R
(n=101) CLL/SLL (58). With a 5-year
follow-up, ibrutinib yielded an overall response rate of 89%, with
CR of 29% in TN and 10% in R/R patients. The 5-year PFS rate was 92
and 44% in TN and R/R patients, respectively. The median PFS in R/R
patients was 51 months. Subgroup analysis demonstrated that the
median PFS in those with del(11q), del(17p), and unmutated IGHV was
51, 26 and 43 months, respectively. A total of 45% of TN patients
and 72% of R/R patients discontinued treatment, with the most
common reasons being AEs (19% TN and 21% R/R) and disease
progression (6% TN and 33% R/R). The most common grade ≥3 AEs in TN
and R/R patients were hypertension (32% TN and 25% R/R), pneumonia
(10% TN and 27% R/R), neutropenia (3% TN and 21% R/R),
thrombocytopenia (3% TN and 11% R/R), and atrial fibrillation (AF;
6% TN and 9% R/R) (58).
CLL patients with 17p deletion (del17p) have poor
responses and survival after CIT. A phase 2, single-arm trial of
ibrutinib for previously untreated and R/R CLL patients with TP53
aberrations demonstrated that 32 (97%) of 33 previously untreated
patients and 12 (80%) of 15 R/R CLL patients achieved an objective
response (59). The study revealed
that the activity and safety profile of ibrutinib in CLL with TP53
aberrations are encouraging. Recently, the 5-year follow-up of this
study was reported (60). The overall
response rate at 6 months was 95.8% (CR, 29.2%) for the TP53 cohort
and 93.9% (CR, 27.3%) for the elderly cohort. The 5-year PFS was
74.4% and 19.4% in TN-CLL and RR-CLL in the TP53 cohort,
respectively, and the OS was 85.3 vs. 53.7%, respectively. The
5-year PFS and OS in RR-CLL were 64.8 and 71.6% in the elderly
cohort, respectively. AF occurred in 18 (20.9%) patients, and there
was no reported serious bleeding. Another phase 2, open-label,
multicentre, single-arm study (RESONATE-17) with R/R del17p CLL/SLL
patients received ibrutinib 420 mg/day until PD or unacceptable
toxicity (61). A total of 83%
patients (119/144) had an overall response according to
investigator assessment. The 2-year PFS and OS were 63% and 75%,
respectively. Grade 3–4 bleeding occurred in 13 patients.
Forty-three patients suffered from grade 3 or worse infections,
including 19 patients with pneumonia (61). Recently, a pooled analysis from 3
clinical trials (PCYC-1102/1103, PCYC-1112 and PCYC-1117) evaluated
230 R/R CLL patients with del17p (62). With a median follow-up of 28 months,
the overall response rate was 85%. The estimated PFS rate at 12, 24
and 30 months was 80, 65 and 57%, respectively. The estimated OS
rate at 12, 24 and 30 months was 85, 77 and 69%, respectively.
Subgroup analysis revealed that the overall response rate was the
same for patients with and without complex karyotype (86% for
both). However, patients with complex karyotype had a worse
estimated median PFS (26 vs. 52 months) and OS (32 months vs. not
reached). Grade ≥3 AEs occurring in ≥5% of patients included
neutropenia, pneumonia, hypertension, thrombocytopenia, anemia and
urinary tract infection.
All these clinical trials suggested that ibrutinib
is well tolerated and effective in the treatment of newly diagnosed
and R/R CLL, including patients with del17p.
Real-world studies
In a real-world study of ibrutinib in R/R CLL
patients (65% with del17p/TP53 mutations) from the Swedish Chronic
Lymphocytic Leukemia Group, the response rate was 84% and the PFS
was 77% at a median follow-up of 10.2 months (63). Patients with del17p/TP53 mutations had
shorter PFS and OS (63).
The data on practice patterns following ibrutinib
discontinuation are limited. Mato et al conducted a
multicenter, retrospective analysis on 178 patients with CLL
(ibrutinib n=143; idelalisib n=35) who discontinued BTK inhibitor
therapy (64). The most common
reasons for BTK inhibitor discontinuation were toxicity (51%),
disease progression (29%), and Richter transformation (RT) (8%).
The reported overall response rate was 58% (15% CR; 22% stable
disease and 20% partial response) in the ibrutinib cohort. The
median PFS and OS for the entire cohort (n=178) from BTK inhibitor
initiation were 10.5 and 29 months, respectively. The median PFS in
BTK inhibitor-intolerant patients treated with an alternative BTK
inhibitor was not reached vs. 7 months for patients with CLL
progression on the first BTK inhibitor, and who were subsequently
treated with an alternative BTK inhibitor. Therefore, BTK
inhibitor-intolerant patients without CLL progression, may be
successfully treated with different BTK inhibitors (64).
The real-world results of ibrutinib in R/R CLL
patients with poor prognosis (92.9% of patients had advanced Binet
stage B/C, 45.1% had del17p and/or TP53 mutation, and 54.2% had at
least one significant comorbidity) previously treated with FCR,
R-bendamustine, chlorambucil ± steroids ± R, ofatumumab,
alemtuzumab ± steroids, R-CHOP and transplantation in France
revealed that the best overall response rate was 88.5% (65). The authors also evaluated the safety
of ibrutinib, with 73.4% of patients experiencing ≥1 AEs of
mild-to-moderate severity and 33.2% experiencing ≥1 SAEs,
confirming the good safety and efficacy of ibrutinib in a
real-world setting. In the largest real-world experience of novel
agents in CLL, ibrutinib appears to be superior to idelalisib as
first kinase inhibitors (KI) (66).
Recently, the largest named patient program (NPP),
which was compared with the phase 3 RESONATE (PCYC-1112) study, was
conducted across 30 countries and 2,908 patients with R/R CLL were
enrolled (67). The estimated
proportion of patients on treatment at 12 months was 77.3 and 81.5%
in the NPP and RESONATE study, respectively (67). In the NPP, 332 patients (11.4%)
discontinued ibrutinib (mostly due to death, n=123; PD, n=55; AEs,
n=50), whereas in RESONATE, the discontinuation rate due to AEs was
4% of the patients (67). In a subset
of the UK/Ireland NPP patients (n=315), the 1-year OS rate was
83.8%, with 73.7% of patients still receiving therapy at 1 year
(68). Using univariate analysis, the
NPP analysis by the UK CLL Forum demonstrated that OS and
discontinuation-free survival (DFS) were not associated with the
number of prior lines of therapy or 17p deletion, while, with
multivariate analysis revealed that older patients with del17p had
inferior survival when treated with ibrutinib (68). With multivariate analysis in the
international NPP study, time-on-treatment was significantly longer
for patients who were younger (67).
This NPP study suggested that ibrutinib is effective and
well-tolerated in the real-world setting, with similar
time-on-treatment to the RESONATE (PCYC-1112) study (67).
What is the relationship of dose intensity (DI) and
response? Barr et al evaluated the effect of ibrutinib dose
adherence on patient outcomes in the phase 3 RESONATE trial
(69). This study demonstrated that
patients with higher ibrutinib DI experienced longer median PFS,
independent of del17p or TP53 mutation. Patients missing ≥8
consecutive days of ibrutinib had a shorter median PFS vs. those
missing <8 days (10.9 months vs. not reached).
Combination therapy of ibrutinib with
other agents
Despite the promising therapeutic efficacy of
ibrutinib in CLL, CR is infrequent, and acquired resistance to
ibrutinib has been reported. Combination therapy may increase the
rate of CR and overcome resistance to ibrutinib.
An in vitro study reporting that ibrutinib
can interfere with the cell-mediated antitumor activities of
therapeutic CD20 antibodies provided grounds for combination
therapy (70). Another in
vitro and mouse model study demonstrated that the XPO1
inhibitor selinexor exerted a synergistic effect with ibrutinib in
primary CLL cells and increased OS compared with ibrutinib alone
(71).
The results of previous clinical studies reported a
good response with good tolerability when ibrutinib was used in
combination with chemoimmunotherapy, anti-CD20 antibodies
(rituximab, ofatumumab, obinutuzumab and TG-1101), or Bcl-2
inhibitors (ABT-199) (72–78).
A single-arm, phase 2 study evaluated the safety and
activity of ibrutinib plus rituximab for patients with high-risk
CLL (73). The 18-month PFS in all
patients was 78.0%, whereas in those with a del17p or TP53
mutation, it was 72.4%. The 18-month OS in all patients was 83.8%,
whereas in those with del17p or TP53 mutation, it was 78.4%.
Toxicity was mainly mild to moderate in severity (grade 1–2), with
5 patients (13%) experiencing grade 3 infections, and there were no
grade 4 or 5 infections (73).
Another phase 1b/2 study assessed the safety and activity of
ibrutinib in combination with the anti-CD20 antibody ofatumumab in
R/R CLL (including prolymphocytic leukemia, or Richters
transformation) (74). A total of 71
patients were treated, including 66 CLL/SLL patients. The trial was
divided into 3 groups: Ibrutinib lead-in (group 1, n=27),
concurrent start (group 2, n=20), or ofatumumab lead-in (group 3,
n=24). Of the 71 patients, 68 (96%) were evaluable for response.
The overall response rates among patients with CLL/SLL patients
were 100%, 78.9% and 70.8% in groups 1, 2 and 3, respectively. The
median PFS had not yet been reached at a median time on study of
12.5 months. The estimated 12-month PFS was 88.7%, 85% and 75%, in
groups 1, 2 and 3, respectively. The estimated 12-month OS was
92.3%, 85% and 87.5% in groups 1, 2 and 3, respectively (74). Ublituximab (TG-1101), a novel
glycoengineered anti-CD20 antibody, was also evaluated in
combination with ibrutinib in R/R CLL in a phase 2 study (76). Combination therapy resulted in an
overall response rate of 88% at 6 months, with 2 subjects (5%)
achieving a CR and 34 subjects (83%) a PR. In the high-risk CLL
population (n=20), the overall response rates were 95%, with 10% CR
and 85% PR. The most common AEs were infusion-related reaction
(IRR), diarrhea, fatigue, nausea and rash (76). All these clinical trials suggested
that ibrutinib in combination with anti-CD20 antibodies can be used
as a treatment option for CLL/SLL.
A phase 1b clinical trial (PCYC-1108) evaluated the
safety and efficacy of ibrutinib in combination with CIT in
patients with R/R CLL (78). The
study enrolled patients into 2 parallel cohorts, bendamustine,
rituximab plus ibrutinib (BR-ibrutinib, n=30) and fludarabine,
cyclophosphamide, rituximab plus ibrutinib (FCR-ibrutinib, n=3).
CIT was up to 6 cycles and ibrutinib was administered daily at a
dose of 420 mg after CIT infusions until disease progression or
unacceptable toxicity. With a median treatment duration of 15.7
months, the estimated 6- and 12-month PFS of the BR-ibrutinib
cohort was 93.1% and 85.9%, respectively. The overall response rate
with BR-ibrutinib was 93.3%, and with FCR-ibrutinib it was 100%.
Safety was as expected with either CIT or single-agent ibrutinib,
with the majority of treatment-related AEs being grade 1 or 2. A
total of 6 SAEs in the BR-ibrutinib cohort (1 event of tumorlysis,
2 events of cellulitis, 1 event of dehydration, 2 events of febrile
neutropenia) and 1 SAE in the FCR-ibrutinib cohort
(gastrointestinal bleeding) were reported. This PCYC-1108 study
demonstrated that ibrutinib may enhance CIT efficacy without
additive toxicities (78).
A randomized, double-blind, placebo-controlled,
phase 3 study (HELIOS) evaluated ibrutinib in combination with
bendamustine plus rituximab compared with placebo, bendamustine,
and rituximab for CLL/SLL patients who had received one or more
prior systemic therapies (79). The
investigator-assessed overall response rates were 86% (249/289) of
patients in the ibrutinib group compared with 69% (199/289) of
patients in the placebo group. At a median follow-up of 17 months,
the PFS was significantly improved (not reached in the ibrutinib
group vs. 13.3 months in the placebo group). PFS at 18 months was
79% in the ibrutinib group and 24% in the placebo group (79). There was no statistically significant
difference in OS between the two groups. However, after adjusting
for crossover (90 out of 289 patients from the placebo group
crossed over to receive ibrutinib after disease progression),
patients in the ibrutinib group had a significantly better OS
compared with those in the placebo group (79). The frequency of infections was similar
between the ibrutinib and placebo groups (all-grade: 70% vs. 70%).
Major hemorrhage was more frequent in the ibrutinib group compared
with the placebo group (4% vs. 2%, respectively) (79).
A phase 1 trial evaluated the safety and efficacy of
the triplet combination of ibrutinib with a novel anti-CD20 mAb
(ublituximab) and a PI3Kδ inhibitor (TGR-1202) in patients with R/R
B-cell malignancies (80). A total of
38 patients were enrolled: 20 CLL/SLL, 6 FL, 6 DLBCL, 4 MCL and 2
MZL cases. The overall response rates of 36 evaluable patients were
CLL/SLL 100% (CR 3, PR 16), MCL 100% (CR 1, PR 3), FL/MZL 86% (CR
2, PR 4) and DLBCL 17% (CR 0, PR 1).
Recently, a phase 1 study of lenalidomide and
ibrutinib in combination with rituximab in R/R CLL was reported
(81). The study demonstrated that
the overall response rate was 67% and the combination of ibrutinib,
lenalidomide and rituximab did not appear to be more effective
compared with the rituximab-lenalidomide doublet or single-agent
ibrutinib with sustained grade 4 neutropenia (81). Table II
summarizes the completed clinical trials of ibrutinib in CLL/SLL
malignancies.
 | Table II.Finished clinical trials of ibrutinib
in CLL/SLL malignancies. |
Table II.
Finished clinical trials of ibrutinib
in CLL/SLL malignancies.
| Patient
population/numbers | Therapeutic
regimen | Phase | Efficacy | ≥Grade 3 adverse
events (AE) | Ref. |
|---|
| R/R B-Cell
malignancies (56) | Ibrutinib | 1 | ORR (60%), CR
(16%) | Hematologic
toxicities, | (53) |
|
|
|
| MCL:ORR (78%), CR
(33%), | Nonhematologic
toxicity (diarrhea, dyspepsia, fatigue, pain) |
|
|
|
|
| PR (44%), SD
(11%) |
|
|
| TN CD20+
B-NHL (33) | Ibrutinib and
R-CHOP | 1b | ORR (94%) | Neutropenia,
thrombocytopenia, anemia | (72) |
| TN CLL (31)
(NCT01105247) | Ibrutinib | 1b/2 | ORR (71%), CR
(13%) | Infections,
neutropenia, thrombocytopenia | (48) |
|
|
|
| 24 months PFS
(96.3%) |
|
|
|
|
|
| 24 months OS
(96.6%) |
|
|
| TN CLL (269)
(RESONATE-2) | Ibrutinib vs. | 3 | ORR (86% vs.
35%) | Hemorrhage,
diarrhea, hypertension, atrial fibrillation | (49) |
|
| chlorambucil |
| 18 months PFS (90%
vs. 52%) |
|
|
|
|
|
| 24 months OS (98%
vs. 85%) |
|
|
| TN CLL (529)
(NCT02048813) | Ibrutinib vs. IR
vs. FCR | 3 | NA | Neutropenia | (50) |
| TN CLL (547)
(NCT01886872) | Ibrutinib (182) vs.
IR (182) vs. BR (183) | 3 | 2 years PFS (87%
vs. 88% vs. 74%) | NA | (51) |
| TN CLL (229)
(NCT02264574) |
Ibrutinib+obinutuzumab (113) vs.
chlorambucil+obinutuzumab (116) | 3 | 30 months PFS (79%
vs. 31%) | Neutropenia,
thrombocytopenia | (52) |
| R/R CLL (85)
(NCT01105247) | Ibrutinib | 1b/2 | ORR (71%), PR
(20%) | Neutropenia,
sinusitis, hypertension, pyrexia, fatigue | (54) |
| R/R CLL (391) | Ibrutinib vs.
ofatumumab | 3 | ORR (42.6% vs.
4.1%) | Neutropenia,
thrombocytopenia, pneumonia | (55) |
| (RESONATE,
PCYC-1112) |
|
| 12 months OS (90%
vs. 81%) |
|
|
| CLL with TP53
aberrations (51) | Ibrutinib | 2 | ORR (TN: 97%; R/R:
80%) | Neutropenia,
anemia, thrombocytopenia, pneumonia, rash | (59) |
| CLL (TP53 cohort
vs. elderly cohort) | Ibrutinib | 2 | ORR (95.8 vs.
93.9%) | Neutropenia,
anemia, thrombocytopenia, infection, atrial | (60) |
|
|
|
| CR (29.2 vs.
27.3%) | fibrillation,
diarrhea, rash, arthritis |
|
|
|
|
| TP53 cohort: |
|
|
|
|
|
| 5-year PFS: TN-CLL,
74.4%; RR-CLL, 19.4% |
|
|
|
|
|
| OS: 85.3 vs.
53.7% |
|
|
|
|
|
| Elderly
cohort: |
|
|
|
|
|
| 5-year PFS: |
|
|
|
|
|
| RR-CLL, 64.8% |
|
|
|
|
|
| OS:71.6% |
|
|
| R/R CLL with 17p
deletion | Ibrutinib | 2 | ORR (83%) | Bleeding,
pneumonia, sepsis, acute myocardial infarction, | (61) |
| (145)
(RESONATE-17) |
|
| 24-months
PFS:63% | abnormal hepatic
function |
|
|
|
|
| 24-months
OS:75% |
|
|
| R/R CLL (40) | Ibrutinib and
rituximab | 2 | 18 months PFS
(78%) | Infections,
subdural hematoma, neutropenia, mucositis | (73) |
| R/R CLL (71) | Ibrutinib and
ofatumumab | 1/1b | ORR (83.3%), | Pneumonia, atrial
fibrillation, bleeding | (74) |
|
|
|
| 12-months PFS
(83.1%), |
|
|
|
|
| 1 | 2-months OS
(88.6%) |
|
|
| R/R CLL (45) | Ibrutinib and
ublituximab | 2 | ORR (88%), | Anaemia,
neutropenia, IRRs, thrombocytopenia | (76) |
| R/R CLL (33)
(PCYC-1108) | Ibrutinib+BR,
Ibrutinib+FCR | 1b | ORR (I+BR: 93.1%;
I+FCR:100%) | Tumorlysis,
cellulitis, dehydration, febrile neutropenia, gastrointestinal
bleeding | (78) |
| R/R CLL (289 in
each group) |
Ibrutinib+bendamustine+ | 3 | ORR (86% vs.
69%) | Neutropenia,
thrombocytopenia | (79) |
| (HELIOS) | rituximab |
| 18-months PFS (79%
vs. 24%) |
|
|
|
| (BR) vs.
placebo+BR |
|
|
|
|
| R/R CLL (12) | Ibrutinib,
lenalidomide, and rituximab | 1 | ORR (67%),
12-months PFS (77.9%) | Neutropenia,
thrombocytopenia, hemolytic anemia, febrile neutropenia, infection,
abdominal pain, myalgia/arthralgia | (81) |
Ibrutinib resistance and management
As described above, ibrutinib alone or as part of
combination therapies has achieved remarkable responses in
treatment-naïve patients or those with R/R B-cell malignancies.
However, several patients developed PD during ibrutinib treatment.
Recently, Hershkovitz-Rokah et al reviewed 5 published
studies including 539 patients in total (82). They found that primary resistance
ranged from 10.2% to 35% and acquired resistance ranged from 17.5%
to 54% (82).
A 49-year-old woman enrolled in a phase 1 study of
ibrutinib exhibited a rapidly rising lymphocyte count and
progressive lymphadenopathy at month 21 (53). RNA sequencing revealed a
thymidine-to-adenine mutation at nucleotide 1634 of the BTK
complementary DNA (83). This
mutation leads to a substitution of serine for cysteine at residue
481 (C481S), which disrupts covalent binding of ibrutinib to the
sulfhydryl group of C481 of BTK in the active site and leads to a
loss of inhibition of BTK enzymatic activity (83).
Another study demonstrated that BTKCys481 and PLCγ2
mutations appear early and have the potential to be used as
biomarkers for future relapse in CLL patients (84). Ahn et al reported a prognostic
scoring system in CLL patients treated with single-agent ibrutinib
on an investigator-initiated phase 2 trial (85). With median follow-up of 34 months,
17.9% patients (15/84) progressed. Most cases of
ibrutinib-resistant CLL were due to mutations in BTK and/or PLCG2.
Using high-sensitivity testing could detect mutations up to 15
months before manifestation of clinical progression (85). Long-term follow-up results and
validation of the prognostic models in a large independent cohort
of patients with CLL was reported (86). They determined that with BTK and/or
PLCG2 mutations heralded ibrutinib resistance.
A study that evaluated prolonged use of ibrutinib in
a real-life setting from the French Innovative Leukemia
Organization (FILO) Group was reported in an ASH meeting (87). This real-life study also highlighted
the onset of BTK and PLCγ2 mutations before any evidence of disease
progression.
Except BTK, PLCγ2 and CARD11 mutation-mediated
ibrutinib resistance, nurse-like cells (NLC) in the tumor
microenvironment (TME) may play a role in ibrutinib resistance
(88). It has been demonstrated that
ibrutinib may impair the phagocytosis of rituximab-coated leukemic
cells from CLL patients by human macrophages and antagonize
rituximab-dependent NK cell-mediated cytotoxicity (89,90). These
results suggested that the sequential administration, but not the
concurrent treatment, with these agents may enhance their antitumor
activity.
A preclinical study demonstrated that HSP90
inhibitors can overcome ibrutinib resistance in vitro and
in vivo by the degradation of both BTK and IκB kinase, with
downstream loss of MAPK and non-classical NF-κB signaling (91). Srour et al reported that a
chemotherapy-free combination that includes dexamethasone,
rituximab, lenalidomide and bortezomib (DR2IVE) can overcome
resistance in heavily treated ibrutinib-resistant MCL patients,
achieving an optimal response (92).
Other resistance mechanisms and how to overcome ibrutinib
resistance was summarized in a review by Hershkovitz-Rokah
(82) and is investigated in ongoing
clinical trials (Clinical trial information: NCT02912754,
NCT03513562, NCT02973399, NCT02914327).
Side effects and management
AF
One study demonstrated that the incidence of AF was
~5% in the ibrutinib group in CLL (55). Another study including 4 RCTs
(RESONATE, RESONATE-2, HELIOS and RAY) reported that the AF
incidence was 6.5% for the ibrutinib group at 16.6 months and 10.4%
at 36 months of follow-up (93).
Univariate analyses identified prior history of AF, ibrutinib
exposure, age ≥65 years, hypertension, and hyperlipidemia as
significant risk factors for developing AF. However, the effect of
prior coronary artery disease, valvular heart disease and diabetes
were not identified as significant risk factors. Multivariate
analyses revealed that prior history of AF, ibrutinib exposure and
age ≥65 years were independent predictors of AF. Gustine et
al identified 112 patients with WM and revealed that 12 (10.7%)
patients were diagnosed with AF, of whom 6 (50%) had a prior
history of AF (94). The median time
to AF was 3.9 months vs. 33.4 months in patients with a prior
history of AF compared with patients without a history of AF,
respectively (94). The incidence may
increase with the duration of the medication (up to 16% in
longer-term follow-up) (95). A
systematic review and meta-analysis suggested that ibrutinib
consistently increases the risk of AF compared with control. The
authors advised that patient exposure to ibrutinib should be
closely monitored (96). Although BTK
and TEC protein kinases are expressed in cardiac tissue and
inhibited by ibrutinib, the mechanism of AF remains elusive and
requires further investigation. A preclinical mouse model suggested
that ibrutinib-related AF may be mediated through inhibition of
PI3K/Akt signaling (97).
In an international retrospective study, Thompson
et al reported that median time to onset AF was 3.8 months.
AF was persistent in 65% (35/56) cases despite treatment and
ibrutinib was permanently discontinued in 46% (26/56) cases
(98).
Since bleeding is another common side effect and
several anticoagulants and anti-arrhythmic drugs (e.g. verapamil,
amiodarone) interact with ibrutinib (99,100),
managing AF is particularly challenging due to the increased
bleeding risk of anticoagulation for AF stroke prophylaxis. Wang
et al reported that MCL patients who suffered from AF were
controlled with β-blockers and/or antiarrhythmics (amiodarone,
dronedarone), and 1 patient underwent ablation therapy. Half of the
patients who suffered from AF were treated with anticoagulants
(warfarin and low-dose heparin), and no patients discontinued
therapy as a result of AF (101).
Brown et al revealed that 49.0% (24/49) of the patients with
AF in the ibrutinib group and 33.3% (4/12) in the comparator were
managed without any interruption or modification of the study drug.
Patients with ibrutinib dose interruption for ≥7 days vs. those for
<7 days exhibited no statistically significant difference in the
18-month PFS (93). Also in this
study, the author summarized that 34.7% (17/49) of patients of the
ibrutinib group who had an AF event were receiving antiplatelet
medications and 8.2% (4/49) were taking an anticoagulant. The most
commonly used antiplatelet and anticoagulant agents were aspirin
and low-molecular-weight heparin, respectively (93). Most scholars advised avoiding
P-glycoprotein substrates (e.g., digoxin) and CYP3A4 inhibitors
(e.g., verapamil, amiodarone, diltiazem) to control the
ibrutinib-related AF (IRAF) and propose deciding whether to
prescribe anticoagulation according to the
CHA2DS2-VASc (the risk of stroke) and
HAS-BLED (the risk of bleeding) scoring methods to decide whether
to give anticoagulation or not (102–104).
If CHA2DS2-VASc ≤ HAS-BLED, there is no need to
prescribe an anticoagulant agent and ibrutinib therapy may continue
(102,104). In a secondary analysis of data from
the phase 2 PCYC-1102 study and phase 3 PCYC-1112 study, the use of
antithrombotic agents was common, with ≥50% of patients on
ibrutinib receiving an anticoagulant, antiplatelet, or a
combination of both (105).
Recently, Gribben et al provided detailed recommendations on
how to prescribe anticoagulation (apixaban and dabigatran) and
antiplatelet (aspirin and clopidogrel) therapy (106).
Bleeding
The other important AE is bleeding (56,101).
Studies demonstrated that ibrutinib selectively inhibits collagen
and von Willebrand factor (vWF)-mediated platelet activation
(107–109). The platelet function assay revealed
impaired aggregation at baseline after ibrutinib treatment
increased from 22/85 to 41/85 (108). Brown et al summarized 4 RCTs
(RESONATE, RESONATE-2, HELIOS and RAY) and revealed that the
incidence of bleeding events was 38.8% (293/756) and 17.2%
(129/749) in the ibrutinib group and comparator groups,
respectively; the majority of events were grade 1 or 2 (91.5 and
88.3%, respectively) (93). In a
phase 3 trial enrolling 280 patients with R/R MCL (ibrutinib group:
n=139; temsirolimus group: n=141), a major bleeding event was
reported in 14 (10%) patients in the ibrutinib group and in 9 (6%)
in the temsirolimus group (110). A
systematic review confirmed an increased incidence of any-grade
bleeding, with a 2.93-fold increase in the ibrutinib group compared
with the control group, with the risk of major bleeding also
increased (111).
Several patients treated with ibrutinib have
comorbidities such as cardiovascular disease, warranting
antiplatelet or anticoagulant therapy for primary or secondary
prevention. Based on the observation of occasional severe
hemorrhage, including subdural hematoma and post-traumatic surgical
hemorrhage, the safety issue of combination therapy with ibrutinib
and antiplatelet/anticoagulant drugs was proposed, but the
information on ibrutinib in combination with anticoagulant or
antiplatelet treatment was not released (101,112).
At present, few data are available on the safety of concomitant
ibrutinib with warfarin/other vitamin K antagonist, as patients
requiring anticoagulant therapy with warfarin/other vitamin K
antagonist were excluded from subsequent trials (55,73). In
the HELIOS study, 6 out of 11 patients with major hemorrhage in the
ibrutinib group were taking a concomitant anticoagulant or
antiplatelet treatment (79). In the
RESONATE study, the authors excluded patients requiring warfarin,
but not those requiring other forms of anticoagulation. The major
hemorrhage incidence was similar in the two study groups (2
patients in the ibrutinib group and 3 patients in the ofatumumab
group), while bleeding-related AEs of any grade were more common in
the ibrutinib group compared with the ofatumumab group (44% vs.
12%, respectively) (55). Data from
the PCYC-1104-CA trial suggest that the combination of antiplatelet
agents and ibrutinib increases the risk of bleeding (101). Another study revealed that grade ≥3
bleeding occurred in ~3% in patients without concomitant
anticoagulant/antiplatelet therapy (105). A real-world study reported no
increased incidence of bleeding in 19 patients who received
concomitant low-molecular-weight heparin and 5 patients who
received aspirin or clopidogrel (63). Similarly, in a report of patients on
concomitant directly acting oral anticoagulant (DOAC) therapy, none
of the 15 patients developed major bleeding (98). Experience with ibrutinib in
combination with both antiplatelet and anticoagulant agents is
limited. A meta-analysis demonstrated that dual antiplatelet
therapy with aspirin and a P2Y12 antagonist (clopidogrel, prasugrel
or ticagrelor) increases the risk of major bleeding by 40–50%
compared with single-agent antiplatelet therapy (113). Adding ibrutinib to dual antiplatelet
therapy (DAPT) is likely to further increase this risk. Based on
the available data, Shatzel et al reviewed patients taking
ibrutinib with concomitant antiplatelet and anticoagulant agents;
more information may be found in that review (114).
Bleeding events in patients receiving ibrutinib are
usually mild, mostly grade 1 and 2, and do not require dose
modification/interruption. In the case of serious bleeding,
platelet transfusion is recommended, regardless of the platelet
count. Antifibrinolytic drugs, such as tranexamic acid, may be
prescribed if bleeding persists after platelet transfusion.
Additionally, in vitro studies demonstrated that platelet
aggregation is fully restored within 5–7 days after ibrutinib
cessation (107,109). It was recommended that ibrutinib
should be withheld for 3–7 days before and after any surgical or
invasive procedures (112).
Infection
B-cell malignancies are characterized by immune
dysregulation, with associated hypogammaglobulinemia and recurrent
infections. Considering that ibrutinib plays important roles in
both normal and malignant B lymphocytes, the effect of ibrutinib on
infection risk and immunoglobulin levels in B-cell malignancies
should be assessed. Apart from BTK, ibrutinib is also an
irreversible inhibitor of interleukin-2-inducible T-cell kinase
(ITK), which plays an important role in inflammatory responses and
T-cell maturation (115). Without
ITK, CD4+ T cells do not effectively differentiate into
Th2 effector cells and cannot elicit protective responses to
pathogens, which increases the risk of severe and opportunistic
infections, such as Candida species, Pneumocystis jirovecii,
Epstein-Barr virus and varicella zoster virus (VZV) (115,116).
Byrd et al had revealed that the most common AEs of grade ≥3
of ibrutinib included pneumonia (12%) (54). OBrien et al also reported that
~10% of the patients developed grade 3 infections (48). Some case reports and retrospective
cohort studies also demonstrated that ibrutinib was associated with
serious pneumonia and opportunistic infections, such as
pneumocystosis, aspergillosis, herpes simplex virus, VZV,
cytomegalovirus, cryptococcal meningitis, candidiasis, fusarium and
other invasive mold infections (117–119). A
previous study demonstrated that 5 cases (5.2%) of Pneumocystis
jirovecii pneumonia (PJP) were identified in 96 patients with
CLL treated with ibrutinib (120).
Furthermore, the authors revealed that all patients had a CD4
T-cell count >500/µl and IgG >500 mg/dl at the time of PJP,
indicating that PJP may be linked to ibrutinib (120). Lionakis et al reported that
39% (7/18) patients with primary CNS lymphoma (PCNSL) who received
ibrutinib combination therapy developed invasive aspergillosis
(121). Diamantopoulos et al
reported a 72-year-old man with stage IV MCL (122). After receiving ibrutinib for 5
months, he developed Staphylococcus aureus meningitis. A
systematic review demonstrated that ~56% of patients on
single-agent ibrutinib and 52% of those on combination therapy
developed infectious complications, and grade 3–4 infectious AEs
occurred in 26 and 20%, respectively (123). Aspergillus, VZV and Pneumocystis
were the most common opportunistic pathogens (123). Recently, Varughese et al
reviewed 378 patients with lymphoid malignancies, and revealed that
43 of the patients suffered from serious infection, of whom 37.2%
(16/43) developed invasive fungal infections (IFIs) (124). Ghez et al reported the
results of a retrospective survey in France, and identified 33
cases of IFIs in patients receiving ibrutinib alone or in
combination (125). Most of IFIs
occurred within a median of 3 months after starting ibrutinib.
Invasive aspergillosis (IA) accounted for the majority of IFIs
(27/33), including 40.7% (11/27) with CNS localizations (125). Tuberculosis infection after
ibrutinib should also be taken into consideration (126).
However, a different sensitivity of malignant and
normal B cells to ibrutinib may potentially restore immune system
function. Sun et al assessed humoral immunity and B-cell
subsets in 86 patients with TN or R/R CLL who had received
ibrutinib for at least 12 months (127). They, consistently with other
researchers, found that there was a decrease in serum IgG and IgM
levels and an increase in serum IgA level (48,54,127). Yin
et al noted elevated peripheral blood CD4+ and
CD8+ T-cell numbers and T-cell-related cytokine levels
(128). Studies have also reported
that the infection rate declined by more than 50% after 6 months of
ibrutinib therapy (56,127). This may be due to the partial
reconstitution of humoral immunity and cellular immunity (127,128).
Secondary primary malignancies (SPMs). Another less
reported but important AE of ibrutinib are SPMs. The overall
incidence of other malignancies was 5% in clinical trials,
including skin cancers (4%) and other carcinomas (1%) (101).
Hepatitis B virus (HBV) reactivation. Certain
therapies for hematological malignancies are associated with
increased risk of HBV reactivation in previously infected patients
(129,130). De Jesus Ngoma et al reported
the first case of HBV reactivation in an 80-year-old CLL patient
receiving ibrutinib, suggesting that such treatment must be
associated with HBV screening (131). In 2017, Herishanu et al
reported a 79-year-old HBsAg-negative, anti-HBs- and
anti-HBc-positive CLL patient who suffered from severe HBV
reactivation 1 year after starting ibrutinib (132). However, Tedeschi et al
revealed that 18% (7/38) of CLL patients were determined to be
HbsAg-negative/anti-Hbc-positive with undetectable HBV DNA before
starting ibrutinib (133). After a
median follow-up of 25 months, none of the patients exhibited HBV
reactivation, suggesting that any recommendation that prophylaxis
during ibrutinib treatment should be administered to
anti-Hbc-positive patients was not enough. Thus, further systematic
studies are warranted to assess the risk of HBV reactivation during
ibrutinib therapy to provide appropriate recommendations.
Other side-effects. In published studies, rash or
pruritic rash occurs at a frequency of 13–27% with single-agent
ibrutinib (48,54,134). The
combination therapy of ibrutinib with rituximab and lenalidomide is
associated with a high incidence of all-grade rash, up to 82%
(grade 3, 36%) (135). Mannis et
al reported the case of a 67-year-old man with del17p CLL who
developed non-pruritic, edematous papules after 2 weeks of
ibrutinib treatment, characterized by peripheral-to-central spread
(136). Jensen et al reported
the case of a 79-year-old man with WM who developed a painless,
slightly pruritic rash starting from the groin and wrists and
subsequently spreading to the trunk and extremities after 12 weeks
of ibrutinib treatment (137).
Iberri et al defined the clinicopathological
characteristics and management of patients with
ibrutinib-associated rash (138).
They observed two distinct rash subtypes: i) A non-palpable,
largely asymptomatic petechial rash and ii) a palpable eruption
characterized by pruritic, non-blanching, violaceous papules. Most
non-palpable rashes were grade 1–2 and required no dose
interruptions or delays of ibrutinib. Patients with palpable rash
all received topical corticosteroids and oral antihistamines, with
(grade 3) or without (grade 1–2) interruption of ibrutinib
(138).
Neffendorf et al had reported that an
80-year-old woman with CLL who had received ibrutinib had a 1-month
history of deteriorating vision and bilateral cataracts (139). As tyrosine kinase receptors, such as
EPHA2, are known to be key regulators in lens clarity and
organization, the authors raised the possibility that a tyrosine
kinase mechanism may be implicated in the cataracts. They also
advised that old patients who receive ibrutinib should have
baseline and repeated visual acuity testing, as well as
ophthalmological assessment in case of visual deterioration
(139). Wallace et al
reported the case of a 78-year-old man with a history of MCL who
experienced new-onset cardiomyopathy and ventricular tachycardia
after 4 months of ibrutinib therapy (140). Finally, a case report indicated that
a CLL patient on ibrutinib suffered from progressive multifocal
leukoencephalopathy (141).
Conclusion
Ibrutinib, alone or as combination therapy, is
effective and well-tolerated in patients with CLL. The safety
profile of ibrutinib is manageable. Single-agent or combination
therapies can significantly improve outcomes in patients with
high-risk disease. In clinical trials and real-world experience,
AF, bleeding and infection have emerged as the most challenging
complications associated with the use of ibrutinib. Collaboration
from multidisciplinary experts (cardiologists, epidemiologists and
hepatologists) should be considered to deal with
ibrutinib-associated AF, bleeding, infections and HBV reactivation,
among others. Candidates for ibrutinib treatment should be provided
with an adequate program of surveillance to avoid SAEs and
unnecessary discontinuation.
Acknowledgements
Not applicable.
Funding
The review was supported by the National Natural
Science Foundation of China (grant no. 81560030), the Natural
Science Foundation of Jiangxi Province (grant no. 20151BAB205021)
and the Natural Science Foundation of Social Development Projects
of Jiangxi Province (grant no. 20151BBG70170).
Availability of data and materials
Not applicable.
Authors contributions
FZ initiated the study and wrote the manuscript. LY
revised it critically for important intellectual content. QS and AT
gave important advice concerning the literature research. JC
approved the version to be published. All authors read and approved
the manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Niiro H and Clark EA: Regulation of B-cell
fate by antigen-receptor signals. Nat Rev Immunol. 2:945–956. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bojarczuk K, Bobrowicz M, Dwojak M, Miazek
N, Zapala P, Bunes A, Siernicka M, Rozanska M and Winiarska M:
B-cell receptor signaling in the pathogenesis of lymphoid
malignancies. Blood Cells Mol Dis. 55:255–265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mohamed AJ, Vargas L, Nore BF, Backesjo
CM, Christensson B and Smith CI: Nucleocytoplasmic shuttling of
Bruton's tyrosine kinase. J Biol Chem. 275:40614–40619. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hendriks RW, Yuvaraj S and Kil LP:
Targeting Bruton's tyrosine kinase in B cell malignancies. Nat Rev
Cancer. 14:219–232. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Honigberg LA, Smith AM, Sirisawad M,
Verner E, Loury D, Chang B, Li S, Pan Z, Thamm DH, Miller RA, et
al: The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell
activation and is efficacious in models of autoimmune disease and
B-cell malignancy. Proc Natl Acad Sci USA. 107:13075–13080. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Small S: Ibrutinib approved for the
treatment of mantle cell lymphoma. Clin Adv Hematol Oncol.
11:8082013.
|
|
7
|
Dangi-Garimella S: FDA grants accelerated
approval for ibrutinib for CLL. Am J Manag Care.
20:E102014.PubMed/NCBI
|
|
8
|
Raedler LA: Imbruvica (Ibrutinib): First
drug approved for the treatment of patients with Waldenströms
macroglobulinemia. Am Health Drug Benefits. 9:(Spec Feature).
89–92. 2016.PubMed/NCBI
|
|
9
|
Packard TA and Cambier JC: B lymphocyte
antigen receptor signaling: Initiation, amplification, and
regulation. F1000Prime Rep. 5:402013. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rickert RC: New insights into pre-BCR and
BCR signalling with relevance to B cell malignancies. Nat Rev
Immunol. 13:578–591. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kaur V and Swami A: Ibrutinib in CLL: A
focus on adverse events, resistance, and novel approaches beyond
ibrutinib. Ann Hematol. 96:1175–1184. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Zhang LL, Champlin RE and Wang ML:
Targeting Bruton's tyrosine kinase with ibrutinib in B-cell
malignancies. Clin Pharmacol Ther. 97:455–468. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vetrie D, Vorechovský I, Sideras P,
Holland J, Davies A, Flinter F, Hammarström L, Kinnon C, Levinsky
R, Bobrow M, et al: The gene involved in X-linked
agammaglobulinaemia is a member of the src family of
protein-tyrosine kinases. Nature. 361:226–233. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bruton OC: Agammaglobulinemia. Pediatrics.
9:722–728. 1952.PubMed/NCBI
|
|
15
|
Hagemann TL, Chen Y, Rosen FS and Kwan SP:
Genomic organization of the Btk gene and exon scanning for
mutations in patients with X-linked agammaglobulinemia. Hum Mol
Genet. 3:1743–1749. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng S, Ma J, Guo A, Lu P, Leonard JP,
Coleman M, Liu M, Buggy JJ, Furman RR and Wang YL: BTK inhibition
targets in vivo CLL proliferation through its effects on B-cell
receptor signaling activity. Leukemia. 28:649–657. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Berglöf A, Hamasy A, Meinke S, Palma M,
Krstic A, Månsson R, Kimby E, Österborg A and Smith CI: Targets for
ibrutinib beyond B cell malignancies. Scand J Immunol. 82:208–217.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bradshaw JM: The Src, Syk, and Tec family
kinases: Distinct types of molecular switches. Cell Signal.
22:1175–1184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pan Z, Scheerens H, Li SJ, Schultz BE,
Sprengeler PA, Burrill LC, Mendonca RV, Sweeney MD, Scott KC,
Grothaus PG, et al: Discovery of selective irreversible inhibitors
for Bruton's tyrosine kinase. ChemMedChem. 2:58–61. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kawakami Y, Kitaura J, Hata D, Yao L and
Kawakami T: Functions of Bruton's tyrosine kinase in mast and B
cells. J Leukoc Biol. 65:286–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khare A, Viswanathan B, Gund R, Jain N,
Ravindran B, George A, Rath S and Bal V: Role of Bruton's tyrosine
kinase in macrophage apoptosis. Apoptosis. 16:334–346. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bao Y, Zheng J, Han C, Jin J, Han H, Liu
Y, Lau YL, Tu W and Cao X: Tyrosine kinase Btk is required for NK
cell activation. J Biol Chem. 287:23769–23778. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stiff A, Trikha P, Wesolowski R, Kendra K,
Hsu V, Uppati S, McMichael E, Duggan M, Campbell A, Keller K, et
al: Myeloid-derived suppressor cells express Bruton's tyrosine
kinase and can be depleted in tumor-bearing hosts by ibrutinib
treatment. Cancer Res. 76:2125–2136. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Satterthwaite AB, Li Z and Witte ON: Btk
function in B cell development and response. Semin Immunol.
10:309–316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Küppers R: Mechanisms of B-cell lymphoma
pathogenesis. Nat Rev Cancer. 5:251–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Buggy JJ and Elias L: Bruton tyrosine
kinase (BTK) and its role in B-cell malignancy. Int Rev Immunol.
31:119–132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cinar M, Hamedani F, Mo Z, Cinar B, Amin
HM and Alkan S: Bruton tyrosine kinase is commonly overexpressed in
mantle cell lymphoma and its attenuation by Ibrutinib induces
apoptosis. Leuk Res. 37:1271–1277. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goodman PA, Wood CM, Vassilev AO, Mao C
and Uckun FM: Defective expression of Bruton's tyrosine kinase in
acute lymphoblastic leukemia. Leuk Lymphoma. 44:1011–1018. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pal Singh S, Dammeijer F and Hendriks RW:
Role of Bruton's tyrosine kinase in B cells and malignancies. Mol
Cancer. 17:572018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brown JR: Ibrutinib (PCI-32765), the first
BTK (Bruton's tyrosine kinase) inhibitor in clinical trials. Curr
Hematol Malig Rep. 8:1–6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Singh J, Petter RC and Kluge AF: Targeted
covalent drugs of the kinase family. Curr Opin Chem Biol.
14:475–480. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Grabinski N and Ewald F: Ibrutinib
(ImbruvicaTM) potently inhibits ErbB receptor phosphorylation and
cell viability of ErbB2-positive breast cancer cells. Invest New
Drugs. 32:1096–1104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zucha MA, Wu AT, Lee WH, Wang LS, Lin WW,
Yuan CC and Yeh CT: Bruton's tyrosine kinase (Btk) inhibitor
ibrutinib suppresses stem-like traits in ovarian cancer.
Oncotarget. 6:13255–13268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang JD, Chen XY, Ji KW and Tao F:
Targeting Btk with ibrutinib inhibit gastric carcinoma cells
growth. Am J Transl Res. 8:3003–3012. 2016.PubMed/NCBI
|
|
35
|
Gao W, Wang M, Wang L, Lu H, Wu S, Dai B,
Ou Z, Zhang L, Heymach JV, Gold KA, et al: Selective antitumor
activity of ibrutinib in EGFR-mutant non-small cell lung cancer
cells. J Natl Cancer Inst. 106:dju2042014.doi: 10.1093/jnci/dju204.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wei L, Su YK, Lin CM, Chao TY, Huang SP,
Huynh TT, Jan HJ, Whang-Peng J, Chiou JF, Wu AT, et al: Preclinical
investigation of ibrutinib, a Bruton's kinase tyrosine (Btk)
inhibitor, in suppressing glioma tumorigenesis and stem cell
phenotypes. Oncotarget. 7:69961–69975. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang J, Liu X, Hong Y, Wang S, Chen P, Gu
A, Guo X and Zhao P: Ibrutinib, a Bruton's tyrosine kinase
inhibitor, exhibits antitumoral activity and induces autophagy in
glioblastoma. J Exp Clin Cancer Res. 36:962017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rushworth SA, Pillinger G, Abdul-Aziz A,
Piddock R, Shafat MS, Murray MY, Zaitseva L, Lawes MJ, MacEwan DJ
and Bowles KM: Activity of Bruton's tyrosine-kinase inhibitor
ibrutinib in patients with CD117-positive acute myeloid leukaemia:
A mechanistic study using patient-derived blast cells. Lancet
Haematol. 2:e204–e211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim E, Hurtz C, Koehrer S, Wang Z,
Balasubramanian S, Chang BY, Müschen M, Davis RE and Burger JA:
Ibrutinib inhibits pre-BCR+ B-cell acute lymphoblastic
leukemia progression by targeting BTK and BLK. Blood.
129:1155–1165. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Deeks ED: Ibrutinib: A Review in chronic
lymphocytic leukaemia. Drugs. 77:225–236. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
de Zwart L, Snoeys J, De Jong J,
Sukbuntherng J, Mannaert E and Monshouwer M: Ibrutinib dosing
strategies based on interaction potential of CYP3A4 perpetrators
using physiologically based pharmacokinetic modeling. Clin
Pharmacol Ther. 100:548–557. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Finnes HD, Chaffee KG, Call TG, Ding W,
Kenderian SS, Bowen DA, Conte M, McCullough KB, Merten JA, Bartoo
GT, et al: Pharmacovigilance during ibrutinib therapy for chronic
lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) in
routine clinical practice. Leuk Lymphoma. 58:1376–1383. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
FDA U: Imbruvica (ibrutinib) capsules: US
prescribing information. http://www.fda.gov.2016
|
|
44
|
Ltd J-C: Imbruvica 140 mg hard capsules.
https://www.medicines.org.uk/emc/medicine/293832018
|
|
45
|
Herman SEM, Gordon AL, Hertlein E,
Ramanunni A, Zhang X, Jaglowski S, Flynn J, Jones J, Blum KA, Buggy
JJ, et al: Bruton tyrosine kinase represents a promising
therapeutic target for treatment of chronic lymphocytic leukemia
and is effectively targeted by PCI-32765. Blood. 117:6287–6296.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Woyach JA, Bojnik E, Ruppert AS,
Stefanovski MR, Goettl VM, Smucker KA, Smith LL, Dubovsky JA, Towns
WH, MacMurray J, et al: Bruton's tyrosine kinase (BTK) function is
important to the development and expansion of chronic lymphocytic
leukemia (CLL). Blood. 123:1207–1213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Herman SE, Sun X, McAuley EM, Hsieh MM,
Pittaluga S, Raffeld M, Liu D, Keyvanfar K, Chapman CM, Chen J, et
al: Modeling tumor-host interactions of chronic lymphocytic
leukemia in xenografted mice to study tumor biology and evaluate
targeted therapy. Leukemia. 27:2311–2321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
OBrien S, Furman RR, Coutre SE, Sharman
JP, Burger JA, Blum KA, Grant B, Richards DA, Coleman M, Wierda WG,
et al: Ibrutinib as initial therapy for elderly patients with
chronic lymphocytic leukaemia or small lymphocytic lymphoma: An
open-label, multicentre, phase 1b/2 trial. Lancet Oncol. 15:48–58.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Burger JA, Tedeschi A, Barr PM, Robak T,
Owen C, Ghia P, Bairey O, Hillmen P, Bartlett NL, Li J, et al
RESONATE-2 Investigators, : Ibrutinib as initial yherapy for
patients with chronic lymphocytic leukemia. N Engl J Med.
373:2425–2437. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shanafelt TD, Wang V, Kay NE, Hanson CA,
OBrien SM, Barrientos JC, Erba HP, Stone RM, Litzow MR and Tallman
MS: A Randomized phase III study of ibrutinib (PCI-32765)-based
therapy vs. standard fludarabine, cyclophosphamide, and rituximab
(FCR) chemoimmunotherapy in untreated younger patients with chronic
lymphocytic leukemia (CLL): A trial of the ECOG-ACRIN Cancer
Research Group (E1912). Blood. 132:LBA–4-LBA-4. 2018. View Article : Google Scholar
|
|
51
|
Woyach JA, Ruppert AS, Heerema NA, Zhao W,
Booth AM, Ding W, Bartlett NL, Brander DM, Barr PM, Rogers KA, et
al: Ibrutinib regimens versus chemoimmunotherapy in older patients
with untreated CLL. N Engl J Med. 379:2517–2528. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Moreno C, Greil R, Demirkan F, Tedeschi A,
Anz B, Larratt L, Simkovic M, Samoilova O, Novak J, Ben-Yehuda D,
et al: Ibrutinib plus obinutuzumab versus chlorambucil plus
obinutuzumab in first-line treatment of chronic lymphocytic
leukaemia (iLLUMINATE): A multicentre, randomised, open-label,
phase 3 trial. Lancet Oncol. 20:43–56. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Advani RH, Buggy JJ, Sharman JP, Smith SM,
Boyd TE, Grant B, Kolibaba KS, Furman RR, Rodriguez S, Chang BY, et
al: Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has
significant activity in patients with relapsed/refractory B-cell
malignancies. J Clin Oncol. 31:88–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Byrd JC, Furman RR, Coutre SE, Flinn IW,
Burger JA, Blum KA, Grant B, Sharman JP, Coleman M, Wierda WG, et
al: Targeting BTK with ibrutinib in relapsed chronic lymphocytic
leukemia. N Engl J Med. 369:32–42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Byrd JC, Brown JR, OBrien S, Barrientos
JC, Kay NE, Reddy NM, Coutre S, Tam CS, Mulligan SP, Jaeger U, et
al RESONATE Investigators, : Ibrutinib versus ofatumumab in
previously treated chronic lymphoid leukemia. N Engl J Med.
371:213–223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Byrd JC, Furman RR, Coutre SE, Burger JA,
Blum KA, Coleman M, Wierda WG, Jones JA, Zhao W, Heerema NA, et al:
Three-year follow-up of treatment-naïve and previously treated
patients with CLL and SLL receiving single-agent ibrutinib. Blood.
125:2497–2506. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Coutré SE, Furman RR, Flinn IW, Burger JA,
Blum K, Sharman J, Jones J, Wierda W, Zhao W, Heerema NA, et al:
Extended treatment with single-agent ibrutinib at the 420 mg dose
leads to durable responses in chronic lymphocytic leukemia/small
lymphocytic lymphoma. Clin Cancer Res. 23:1149–1155. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
OBrien S, Furman RR, Coutre S, Flinn IW,
Burger JA, Blum K, Sharman J, Wierda W, Jones J, Zhao W, et al:
Single-agent ibrutinib in treatment-naïve and relapsed/refractory
chronic lymphocytic leukemia: A 5-year experience. Blood.
131:1910–1919. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Farooqui MZ, Valdez J, Martyr S, Aue G,
Saba N, Niemann CU, Herman SE, Tian X, Marti G, Soto S, et al:
Ibrutinib for previously untreated and relapsed or refractory
chronic lymphocytic leukaemia with TP53 aberrations: A phase 2,
single-arm trial. Lancet Oncol. 16:169–176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ahn IE, Farooqui MZH, Tian X, Valdez J,
Sun C, Soto S, Lotter J, Housel S, Stetler-Stevenson M, Yuan CM, et
al: Depth and durability of response to ibrutinib in CLL: 5-year
follow-up of a phase 2 study. Blood. 131:2357–2366. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
OBrien S, Jones JA, Coutre SE, Mato AR,
Hillmen P, Tam C, Österborg A, Siddiqi T, Thirman MJ, Furman RR, et
al: Ibrutinib for patients with relapsed or refractory chronic
lymphocytic leukaemia with 17p deletion (RESONATE-17): A phase 2,
open-label, multicentre study. Lancet Oncol. 17:1409–1418. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Jones J, Mato A, Coutre S, Byrd JC, Furman
RR, Hillmen P, Osterborg A, Tam C, Stilgenbauer S, Wierda WG, et
al: Evaluation of 230 patients with relapsed/refractory deletion
17p chronic lymphocytic leukaemia treated with ibrutinib from 3
clinical trials. Br J Haematol. 182:504–512. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Winqvist M, Asklid A, Andersson PO,
Karlsson K, Karlsson C, Lauri B, Lundin J, Mattsson M, Norin S,
Sandstedt A, et al: Real-world results of ibrutinib in patients
with relapsed or refractory chronic lymphocytic leukemia: Data from
95 consecutive patients treated in a compassionate use program. A
study from the Swedish Chronic Lymphocytic Leukemia Group.
Haematologica. 101:1573–1580. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Mato AR, Nabhan C, Barr PM, Ujjani CS,
Hill BT, Lamanna N, Skarbnik AP, Howlett C, Pu JJ, Sehgal AR, et
al: Outcomes of CLL patients treated with sequential kinase
inhibitor therapy: A real world experience. Blood. 128:2199–2205.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ysebaert L, Aurran-Schleinitz T, Dartigeas
C, Dilhuydy MS, Feugier P, Michallet AS, Tournilhac O, Dupuis J,
Sinet P, Albrecht C, et al: Real-world results of ibrutinib in
relapsed/refractory CLL in France: Early results on a large series
of 428 patients. Am J Hematol. 92:E166–E168. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Mato AR, Hill BT, Lamanna N, Barr PM,
Ujjani CS, Brander DM, Howlett C, Skarbnik AP, Cheson BD, Zent CS,
et al: Optimal sequencing of ibrutinib, idelalisib, and venetoclax
in chronic lymphocytic leukemia: Results from a multicenter study
of 683 patients. Ann Oncol. 28:1050–1056. 2017.PubMed/NCBI
|
|
67
|
Hillmen P, Diels J, Healy N, Iraqi W,
Aschan J and Wildgust M: Ibrutinib for chronic lymphocytic
leukemia: International experience from a named patient program.
Haematologica. 103:e204–e206. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
UK CLL Forum, . Ibrutinib for
relapsed/refractory chronic lymphocytic leukemia: A UK and Ireland
analysis of outcomes in 315 patients. Haematologica. 101:1563–1572.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Barr PM, Brown JR, Hillmen P, OBrien S,
Barrientos JC, Reddy NM, Coutre S, Mulligan SP, Jaeger U, Furman
RR, et al: Impact of ibrutinib dose adherence on therapeutic
efficacy in patients with previously treated CLL/SLL. Blood.
129:2612–2615. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Da Roit F, Engelberts PJ, Taylor RP, Breij
EC, Gritti G, Rambaldi A, Introna M, Parren PW, Beurskens FJ and
Golay J: Ibrutinib interferes with the cell-mediated anti-tumor
activities of therapeutic CD20 antibodies: Implications for
combination therapy. Haematologica. 100:77–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hing ZA, Mantel R, Beckwith KA, Guinn D,
Williams E, Smith LL, Williams K, Johnson AJ, Lehman AM, Byrd JC,
et al: Selinexor is effective in acquired resistance to ibrutinib
and synergizes with ibrutinib in chronic lymphocytic leukemia.
Blood. 125:3128–3132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Younes A, Thieblemont C, Morschhauser F,
Flinn I, Friedberg JW, Amorim S, Hivert B, Westin J, Vermeulen J,
Bandyopadhyay N, et al: Combination of ibrutinib with rituximab,
cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP)
for treatment-naive patients with CD20-positive B-cell non-Hodgkin
lymphoma: A non-randomised, phase 1b study. Lancet Oncol.
15:1019–1026. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Burger JA, Keating MJ, Wierda WG, Hartmann
E, Hoellenriegel J, Rosin NY, de Weerdt I, Jeyakumar G, Ferrajoli
A, Cardenas-Turanzas M, et al: Safety and activity of ibrutinib
plus rituximab for patients with high-risk chronic lymphocytic
leukaemia: A single-arm, phase 2 study. Lancet Oncol. 15:1090–1099.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Jaglowski SM, Jones JA, Nagar V, Flynn JM,
Andritsos LA, Maddocks KJ, Woyach JA, Blum KA, Grever MR, Smucker
K, et al: Safety and activity of BTK inhibitor ibrutinib combined
with ofatumumab in chronic lymphocytic leukemia: A phase 1b/2
study. Blood. 126:842–850. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Maddocks K, Christian B, Jaglowski S,
Flynn J, Jones JA, Porcu P, Wei L, Jenkins C, Lozanski G, Byrd JC,
et al: A phase 1/1b study of rituximab, bendamustine, and ibrutinib
in patients with untreated and relapsed/refractory non-Hodgkin
lymphoma. Blood. 125:242–248. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Sharman JP, Farber CM, Mahadevan D,
Schreeder MT, Brooks HD, Kolibaba KS, Fanning S, Klein L, Greenwald
DR, Sportelli P, et al: Ublituximab (TG-1101), a novel
glycoengineered anti-CD20 antibody, in combination with ibrutinib
is safe and highly active in patients with relapsed and/or
refractory chronic lymphocytic leukaemia: Results of a phase 2
trial. Br J Haematol. 176:412–420. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Cervantes-Gomez F, Lamothe B, Woyach JA,
Wierda WG, Keating MJ, Balakrishnan K and Gandhi V: Pharmacological
and protein profiling suggests venetoclax (ABT-199) as optimal
partner with ibrutinib in chronic lymphocytic leukemia. Clin Cancer
Res. 21:3705–3715. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Brown JR, Barrientos JC, Barr PM, Flinn
IW, Burger JA, Tran A, Clow F, James DF, Graef T, Friedberg JW, et
al: The Bruton tyrosine kinase inhibitor ibrutinib with
chemoimmunotherapy in patients with chronic lymphocytic leukemia.
Blood. 125:2915–2922. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chanan-Khan A, Cramer P, Demirkan F,
Fraser G, Silva RS, Grosicki S, Pristupa A, Janssens A, Mayer J,
Bartlett NL, et al HELIOS investigators, : Ibrutinib combined with
bendamustine and rituximab compared with placebo, bendamustine, and
rituximab for previously treated chronic lymphocytic leukaemia or
small lymphocytic lymphoma (HELIOS): A randomised, double-blind,
phase 3 study. Lancet Oncol. 17:200–211. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Nastoupil L, Lunning MA, Vose JM,
Schreeder MT, Siddiqi T, Flowers CR, Cohen JB, Burger JA, Wierda
WG, OBrien S, et al: Chemo-free triplet combination of TGR-1202,
ublituximab and ibrutinib is well tolerated and highly active in
patients with advanced CLL and NHL. Hematol Oncol. 35:112–113.
2017. View Article : Google Scholar
|
|
81
|
Ujjani C, Wang H, Skarbnik A, Trivedi N,
Ramzi P, Khan N and Cheson BD: A phase 1 study of lenalidomide and
ibrutinib in combination with rituximab in relapsed and refractory
CLL. Blood Adv. 2:762–768. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Hershkovitz-Rokah O, Pulver D, Lenz G and
Shpilberg O: Ibrutinib resistance in mantle cell lymphoma:
Clinical, molecular and treatment aspects. Br J Haematol.
181:306–319. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Furman RR, Cheng S, Lu P, Setty M, Perez
AR, Guo A, Racchumi J, Xu G, Wu H, Ma J, et al: Ibrutinib
resistance in chronic lymphocytic leukemia. N Engl J Med.
370:2352–2354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Woyach JA, Ruppert AS, Guinn D, Lehman A,
Blachly JS, Lozanski A, Heerema NA, Zhao W, Coleman J, Jones D, et
al: BTKC481S-mediated resistance to ibrutinib in chronic
lymphocytic leukemia. J Clin Oncol. 35:1437–1443. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Ahn IE, Underbayev C, Albitar A, Herman
SE, Tian X, Maric I, Arthur DC, Wake L, Pittaluga S, Yuan CM, et
al: Clonal evolution leading to ibrutinib resistance in chronic
lymphocytic leukemia. Blood. 129:1469–1479. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Ahn IE, Tian X, Albitar M, Herman SEM,
Cook EM, Soto S, Ma W, Ipe D, Tsao LC, Cheng M, et al: Validation
of clinical prognostic models and integration of genetic biomarkers
of drug resistance in CLL patients treated with ibrutinib. Blood.
132:186. 2018. View Article : Google Scholar
|
|
87
|
Quinquenel A, Fornecker LM, Letestu R,
Ysebaert L, Fleury C, Lazarian G, Dilhuydy MS, Nollet D, Guieze R,
Feugier P, et al: High prevalence of BTK mutations on ibrutinib
therapy after 3 years of treatment in a real-life cohort of CLL
patients: A study from the French Innovative Leukemia Organization
(FILO) Group. Blood. 132:584. 2018. View Article : Google Scholar
|
|
88
|
Boissard F, Fournié JJ, Quillet-Mary A,
Ysebaert L and Poupot M: Nurse-like cells mediate ibrutinib
resistance in chronic lymphocytic leukemia patients. Blood Cancer
J. 5:e3552015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Borge M, Belén Almejún M, Podaza E, Colado
A, Fernández Grecco H, Cabrejo M, Bezares RF, Giordano M and
Gamberale R: Ibrutinib impairs the phagocytosis of rituximab-coated
leukemic cells from chronic lymphocytic leukemia patients by human
macrophages. Haematologica. 100:e140–e142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Kohrt HE, Sagiv-Barfi I, Rafiq S, Herman
SE, Butchar JP, Cheney C, Zhang X, Buggy JJ, Muthusamy N, Levy R,
et al: Ibrutinib antagonizes rituximab-dependent NK cell-mediated
cytotoxicity. Blood. 123:1957–1960. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Jacobson C, Kopp N, Layer JV, Redd RA,
Tschuri S, Haebe S, van Bodegom D, Bird L, Christie AL,
Christodoulou A, et al: HSP90 inhibition overcomes ibrutinib
resistance in mantle cell lymphoma. Blood. 128:2517–2526. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Srour SA, Lee HJ, Nomie K, Ye H, Chen W,
Oriabure O, Romaguera J and Wang ML: Novel chemotherapy-free
combination regimen for ibrutinib-resistant mantle cell lymphoma.
Br J Haematol. 181:561–564. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Brown JR, Moslehi J, OBrien S, Ghia P,
Hillmen P, Cymbalista F, Shanafelt TD, Fraser G, Rule S, Kipps TJ,
et al: Characterization of atrial fibrillation adverse events
reported in ibrutinib randomized controlled registration trials.
Haematologica. 102:1796–1805. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Gustine JN, Meid K, Dubeau TE, Treon SP
and Castillo JJ: Atrial fibrillation associated with ibrutinib in
Waldenström macroglobulinemia. Am J Hematol. 91:E312–E313. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Farooqui M, Valdez J, Soto S, Bray A, Tian
X and Wiestner A: Atrial fibrillation in CLL/SLL patients on
ibrutinib. Blood. 126:2933. 2015. View Article : Google Scholar
|
|
96
|
Leong DP, Caron F, Hillis C, Duan A,
Healey JS, Fraser G and Siegal D: The risk of atrial fibrillation
with ibrutinib use: A systematic review and meta-analysis. Blood.
128:138–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
McMullen JR, Boey EJ, Ooi JY, Seymour JF,
Keating MJ and Tam CS: Ibrutinib increases the risk of atrial
fibrillation, potentially through inhibition of cardiac PI3K-Akt
signaling. Blood. 124:3829–3830. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Thompson PA, Lévy V, Tam CS, Al Nawakil C,
Goudot FX, Quinquenel A, Ysebaert L, Michallet AS, Dilhuydy MS, Van
Den Neste E, et al: Atrial fibrillation in CLL patients treated
with ibrutinib. An international retrospective study. Br J
Haematol. 175:462–466. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Lambert Kuhn E, Levêque D, Lioure B,
Gourieux B and Bilbault P: Adverse event potentially due to an
interaction between ibrutinib and verapamil: A case report. J Clin
Pharm Ther. 41:104–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Mulligan SP, Ward CM, Whalley D and Hilmer
SN: Atrial fibrillation, anticoagulant stroke prophylaxis and
bleeding risk with ibrutinib therapy for chronic lymphocytic
leukaemia and lymphoproliferative disorders. Br J Haematol.
175:359–364. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Wang ML, Blum KA, Martin P, Goy A, Auer R,
Kahl BS, Jurczak W, Advani RH, Romaguera JE, Williams ME, et al:
Long-term follow-up of MCL patients treated with single-agent
ibrutinib: Updated safety and efficacy results. Blood. 126:739–745.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Vrontikis A, Carey J, Gilreath JA, Halwani
A, Stephens DM and Sweetenham JW: Proposed algorithm for managing
ibrutinib-related atrial fibrillation. Oncology (Williston Park).
30:970–974, 980-971, C973. 2016.PubMed/NCBI
|
|
103
|
Thorp BC and Badoux X: Atrial fibrillation
as a complication of ibrutinib therapy: Clinical features and
challenges of management. Leuk Lymphoma. 59:311–320. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
de Weerdt I, Koopmans SM, Kater AP and van
Gelder M: Incidence and management of toxicity associated with
ibrutinib and idelalisib: A practical approach. Haematologica.
102:1629–1639. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Jones JA, Hillmen P, Coutre S, Tam C,
Furman RR, Barr PM, Schuster SJ, Kipps TJ, Flinn IW, Jaeger U, et
al: Use of anticoagulants and antiplatelet in patients with chronic
lymphocytic leukaemia treated with single-agent ibrutinib. Br J
Haematol. 178:286–291. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Gribben JG, Bosch F, Cymbalista F, Geisler
CH, Ghia P, Hillmen P, Moreno C and Stilgenbauer S: Optimising
outcomes for patients with chronic lymphocytic leukaemia on
ibrutinib therapy: European recommendations for clinical practice.
Br J Haematol. 180:666–679. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Levade M, David E, Garcia C, Laurent PA,
Cadot S, Michallet AS, Bordet JC, Tam C, Sié P, Ysebaert L, et al:
Ibrutinib treatment affects collagen and von Willebrand
factor-dependent platelet functions. Blood. 124:3991–3995. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Lipsky AH, Farooqui MZ, Tian X, Martyr S,
Cullinane AM, Nghiem K, Sun C, Valdez J, Niemann CU, Herman SE, et
al: Incidence and risk factors of bleeding-related adverse events
in patients with chronic lymphocytic leukemia treated with
ibrutinib. Haematologica. 100:1571–1578. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Kamel S, Horton L, Ysebaert L, Levade M,
Burbury K, Tan S, Cole-Sinclair M, Reynolds J, Filshie R, Schischka
S, et al: Ibrutinib inhibits collagen-mediated but not ADP-mediated
platelet aggregation. Leukemia. 29:783–787. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Dreyling M, Jurczak W, Jerkeman M, Silva
RS, Rusconi C, Trneny M, Offner F, Caballero D, Joao C,
Witzens-Harig M, et al: Ibrutinib versus temsirolimus in patients
with relapsed or refractory mantle-cell lymphoma: An international,
randomised, open-label, phase 3 study. Lancet. 387:770–778. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Yun S, Vincelette ND, Acharya U and
Abraham I: Risk of atrial fibrillation and bleeding diathesis
associated with ibrutinib treatment: A systematic review and pooled
analysis of four randomized controlled trials. Clin Lymphoma
Myeloma Leuk. 17:31–37.e13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Treon SP, Tripsas CK, Meid K, Warren D,
Varma G, Green R, Argyropoulos KV, Yang G, Cao Y, Xu L, et al:
Ibrutinib in previously treated Waldenströms macroglobulinemia. N
Engl J Med. 372:1430–1440. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Serebruany VL, Malinin AI, Ferguson JJ,
Vahabi J, Atar D and Hennekens CH: Bleeding risks of combination
vs. single antiplatelet therapy: a meta-analysis of 18 randomized
trials comprising 129314 patients. Fundam Clin Pharmacol.
22:315–321. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Shatzel JJ, Olson SR, Tao DL, McCarty OJT,
Danilov AV and DeLoughery TG: Ibrutinib-associated bleeding:
Pathogenesis, management and risk reduction strategies. J Thromb
Haemost. 15:835–847. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Dubovsky JA, Beckwith KA, Natarajan G,
Woyach JA, Jaglowski S, Zhong Y, Hessler JD, Liu TM, Chang BY,
Larkin KM, et al: Ibrutinib is an irreversible molecular inhibitor
of ITK driving a Th1-selective pressure in T lymphocytes. Blood.
122:2539–2549. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Ghosh S, Bienemann K, Boztug K and
Borkhardt A: Interleukin-2-inducible T-cell kinase (ITK) deficiency
- clinical and molecular aspects. J Clin Immunol. 34:892–899. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Kreiniz N, Bejar J, Polliack A and Tadmor
T: Severe pneumonia associated with ibrutinib monotherapy for CLL
and lymphoma. Hematol Oncol. 2017.PubMed/NCBI
|
|
118
|
Issa N, Arbona-Haddad E, Nevett-Fernandez
A, Prestes D, Liakos A, Woolley A, Hammond S, Brown J, Baden L and
Marty F: Opportunistic infections (OIs) in patients with
hematologic malignancies (HM) treated with Bruton's tyrosine kinase
(BTK) and phosphoinositide 3 kinase (PI3K) inhibitors: An 8-year
retrospective cohort study. Open Forum Infect Dis. 4
(Suppl_1):S6992017. View Article : Google Scholar :
|
|
119
|
Chan TS, Au-Yeung R, Chim CS, Wong SC and
Kwong YL: Disseminated fusarium infection after ibrutinib therapy
in chronic lymphocytic leukaemia. Ann Hematol. 96:871–872. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Ahn IE, Jerussi T, Farooqui M, Tian X,
Wiestner A and Gea-Banacloche J: Atypical Pneumocystis
jirovecii pneumonia in previously untreated patients with CLL
on single-agent ibrutinib. Blood. 128:1940–1943. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Lionakis MS, Dunleavy K, Roschewski M,
Widemann BC, Butman JA, Schmitz R, Yang Y, Cole DE, Melani C,
Higham CS, et al: Inhibition of B cell receptor signaling by
ibrutinib in orimary CNS lymphoma. Cancer Cell. 31:833–843.e835.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Diamantopoulos PT, Psichogiou M,
Pantazatou A, Zervakis K, Rougala N, Giannakopoulou N, Daikos G and
Viniou NA: Staphylococcus aureus meningitis in a patient
with mantle cell lymphoma under treatment with ibrutinib. Ann
Hematol. 96:1049–1050. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Tillman BF, Pauff JM, Satyanarayana G,
Talbott M and Warner JL: Systematic review of infectious events
with the Bruton tyrosine kinase inhibitor ibrutinib in the
treatment of hematologic malignancies. Eur J Haematol. 2017.
|
|
124
|
Varughese T, Taur Y, Cohen N, Palomba ML,
Seo SK, Hohl TM and Redelman-Sidi G: Serious infections in patients
receiving ibrutinib for treatment of lymphoid cancer. Clin Infect
Dis. 67:687–692. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Ghez D, Calleja A, Protin C, Baron M,
Ledoux MP, Damaj G, Dupont M, Dreyfus B, Ferrant E, Herbaux C, et
al French Innovative Leukemia Organization (FILO) CLL group, :
Early-onset invasive aspergillosis and other fungal infections in
patients treated with ibrutinib. Blood. 131:1955–1959. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Wang SY, Ebert T, Jaekel N, Schubert S,
Niederwieser D and Al-Ali HK: Miliary tuberculosis after initiation
of ibrutinib in chronic lymphocytic leukemia. Ann Hematol.
94:1419–1420. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Sun C, Tian X, Lee YS, Gunti S, Lipsky A,
Herman SE, Salem D, Stetler-Stevenson M, Yuan C, Kardava L, et al:
Partial reconstitution of humoral immunity and fewer infections in
patients with chronic lymphocytic leukemia treated with ibrutinib.
Blood. 126:2213–2219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Yin Q, Sivina M, Robins H, Yusko E,
Vignali M, OBrien S, Keating MJ, Ferrajoli A, Estrov Z, Jain N, et
al: Ibrutinib therapy Increases T cell repertoire diversity in
patients with chronic lymphocytic leukemia. J Immunol.
198:1740–1747. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Yeo W, Chan TC, Leung NW, Lam WY, Mo FK,
Chu MT, Chan HL, Hui EP, Lei KI, Mok TS, et al: Hepatitis B virus
reactivation in lymphoma patients with prior resolved hepatitis B
undergoing anticancer therapy with or without rituximab. J Clin
Oncol. 27:605–611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Yeo W and Johnson PJ: Diagnosis,
prevention and management of hepatitis B virus reactivation during
anticancer therapy. Hepatology. 43:209–220. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
de Jésus Ngoma P, Kabamba B, Dahlqvist G,
Sempoux C, Lanthier N, Shindano T, Van Den Neste E and Horsmans Y:
Occult HBV reactivation induced by ibrutinib treatment: A case
report. Acta Gastroenterol Belg. 78:424–426. 2015.PubMed/NCBI
|
|
132
|
Herishanu Y, Katchman H and Polliack A:
Severe hepatitis B virus reactivation related to ibrutinib
monotherapy. Ann Hematol. 96:689–690. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Tedeschi A, Frustaci AM, Mazzucchelli M,
Cairoli R and Montillo M: Is HBV prophylaxis required during CLL
treatment with ibrutinib? Leuk Lymphoma. 58:2966–2968. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Wang ML, Rule S, Martin P, Goy A, Auer R,
Kahl BS, Jurczak W, Advani RH, Romaguera JE, Williams ME, et al:
Targeting BTK with ibrutinib in relapsed or refractory mantle-cell
lymphoma. N Engl J Med. 369:507–516. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Ujjani CS, Jung SH, Pitcher B, Martin P,
Park SI, Blum KA, Smith SM, Czuczman M, Davids MS, Levine E, et al:
Phase 1 trial of rituximab, lenalidomide, and ibrutinib in
previously untreated follicular lymphoma: Alliance A051103. Blood.
128:2510–2516. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Mannis G, Wu D, Dea T, Mauro T and Hsu G:
Ibrutinib rash in a patient with 17p del chronic lymphocytic
leukemia. Am J Hematol. 90:1792015. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Jensen AB, Stausbøl-Grøn B, Riber-Hansen R
and dAmore F: Ibrutinib-associated skin toxicity: A case of
maculopapular rash in a 79-year old Caucasian male patient with
relapsed Waldenstroms macroglobulinemia and review of the
literature. Dermatol Rep. 9:69762017. View Article : Google Scholar
|
|
138
|
Iberri DJ, Kwong BY, Stevens LA, Coutre
SE, Kim J, Sabile JM and Advani RH: Ibrutinib-associated rash: A
single-centre experience of clinicopathological features and
management. Br J Haematol. 2016.PubMed/NCBI
|
|
139
|
Neffendorf JE, Gout I and Hildebrand GD:
Ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med.
369:1277–1279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Wallace N, Wong E, Cooper D and Chao H: A
case of new-onset cardiomyopathy and ventricular tachycardia in a
patient receiving ibrutinib for relapsed mantle cell lymphoma. Clin
Case Rep. 4:1120–1121. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Lutz M, Schulze AB, Rebber E, Wiebe S,
Zoubi T, Grauer OM, Keßler T, Kerkhoff A, Lenz G and Berdel WE:
Progressive multifocal leukoencephalopathy after ibrutinib therapy
for chronic lymphocytic leukemia. Cancer Res Treat. 49:548–552.
2017. View Article : Google Scholar : PubMed/NCBI
|