Introduction
The onset of colorectal cancer (CRC) is relatively
insidious, with a tendency for metastasis; a number of patients
with CRC also have a poor prognosis, which has contributed to the
increase in the CRC mortality rates to the second highest globally
among all cancer types (1).
Currently, the treatment of advanced-stage CRC is mainly limited to
chemotherapy with cytotoxic agents, including oxaliplatin (Oxa)
(2), irinotecan and 5-fluorouracil.
Oxa has a high affinity for DNA and promotes cell apoptosis through
the formation of platinum-DNA adducts (3,4). OXA
has been proven to be the first metal coordination complex for the
treatment of CRC (5) and is one of
the most effective chemotherapeutic drugs. However, the long-term
use of Oxa can lead to drug resistance (6), as well as to severe side-effects, such
as neurotoxicity and hepatotoxicity (7,8).
Therefore, the identification of novel compounds to meet the needs
of OXA-resistant or OXA-intolerant patients is of utmost importance
(6).
In recent years, a number of copper complexes have
been developed, whose mechanisms of action are distinct from
current platinum-based drugs (9,10),
including the induction of reactive oxygen species (ROS) (11–13),
DNA cleavage (10,12,14),
ferroptosis (15), cell cycle
blockage (11) and
ubiquitin-proteasome system inhibition (16). Among these mechanisms, intracellular
ROS generation has been reported most frequently. ROS comprises a
large class of unstable molecules that contain oxygen often being
generated as natural by-products, with the mitochondria being
considered as the prime source of endogenous ROS (17,18).
ROS play a pivotal role in regulating and triggering apoptosis,
thereby modulating cancer cell proliferation, survival and drug
resistance (17). Tumor cells can
activate the ROS scavenging system to counteract ROS damage and
further resist cell apoptosis, which contributes to malignant
transformation, metastasis and resistance to anticancer drugs.
Therefore, it is crucial to understand the complex
(oxidation-reduction) REDOX process in cancer cells and its
underlying mechanisms, which can help identify more effective
strategies with which to eliminate cancer cells and overcome the
limitations of Oxa (19,20).
Cancer treatment can also be achieved by controlling
the growth of cancer cells or by promoting cell death (21) and common therapeutic strategies
include the activation of pro-apoptotic molecules or the inhibition
of anti-apoptotic molecules. Bcl-2 protein family proteins are
pivotal regulators of cell survival (22,23).
Survivin, a member of the family of inhibitory apoptosis proteins
(IAPs), is tumor-specific and is not expressed in normal tissues or
is expressed at low levels. In addition, Bcl-2 and survivin are
critical anti-apoptotic proteins that promote cell survival through
multiple pathways, including maintaining the integrity of the
mitochondria (22) and inhibiting
the activity of the terminal effector enzymes, caspase-3 and
caspase-7 (24). Previous studies
have attempted to target Bcl-2 (25) and survivin (26) to promote cancer cell apoptosis.
Among the copper complexes that have been shown to
exert significant inhibitory effects on tumor cells, copper (II)
complexes containing 1,10-phenanthroline ligand (27) are among the extensively studied. In
a previous study, it was demonstrated that copper (II) complex of
salicylate phenanthroline [Cu(sal)(phen)] has prominent antitumor
potential against triple-negative breast cancer in vitro and
in vivo (28). Few studies
(9,19) involving copper (II) complexes
against CRC have been reported in the literature. Therefore, the
effects of Cu(sal)(phen) on CRC cells were investigated in the
present study, demonstrating the excellent efficacy of this complex
against CRC in vivo.
Materials and methods
Cells, cell culture and reagents
To validate the effects of Cu(sal)(phen) on CRC, two
human primary adenocarcinoma colon cancer cell lines, SW480 and
HCT116, whose tumor biology has been extensively studied in the
literature (29), were selected for
use in the present study. Since these cells are derived from two
different patients with CRC, the two investigated cell lines
exhibit some differences at the genetic level (30), which made the results more reliable
to a certain extent. The HCT116 (CCL-247EMT) and SW480 (CCL-228)
human CRC cell lines were obtained from The Cell Bank of Type
Culture Collection of the Chinese Academy of Sciences and cultured
in DMEM containing 10% FBS and 1% penicillin/streptomycin (all
purchased from Hyclone; Cytiva) at 37°C in an atmosphere of 5%
CO2. Cu(sal)(phen) and Oxa were provided by Hubei
Dinglong Chemical Co., Ltd. and Selleck Chemicals,
respectively.
Cell viability and colony formation
assay
Cell viability was determined using
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) assay with CellTiter 96® AQueous One Solution Cell
Proliferation Assay (Promega Corporation). Accordingly, the cells
were grown in 96-well plates overnight and then treated with
Cu(sal)(phen) and Oxa at various concentrations (1, 2.5, 5, 10, 25
µM) with dimethyl sulfoxide (DMSO) used as the control. Following
48 or 72 h of treatment, MTS was added to the plates and incubated
for 3 h at 37°C. Subsequently, the optical density was recorded at
492 nm using a Synergy 2 plate reader (BioTek Instruments, Inc.).
The half-maximal inhibitory concentration (IC50) was
calculated based on the percentage decrease in the optical density
at 492 nm relative to the control group using GraphPad prism 7.0
(Dotmatics).
A colony formation assay was conducted in order to
evaluate cell proliferation. Briefly, the cells were seeded in
six-well plates at a density of 500 cell/well for 48 h, followed by
treatment with 1 and 2.5 µM Cu(sal)(phen) and Oxa for 14 days. The
medium was changed every 3 days, and the colonies were fixed with
4% paraformaldehyde for 50 min at room temperature. Finally, 0.5%
crystal violet dye (Sinopharm Chemical Reagent Co., Ltd.) for 50
min was used to stain cell colonies, and the colonies were counted
using the Fiji (ImageJ 2.1.0) software (National Institutes of
Health).
Apoptosis assay
An Annexin V-FITC/PI Apoptosis Assay kit (Beijing
Zoman Biotechnology Co., Ltd.) was used to examine cell apoptosis
after HCT116 and SW480 cells were treated with Cu(sal)(phen) (25
µM) or Oxa (25 µM) with or without 20 µM
carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone
(Z-VAD-FMK; GlpBio) solution in DMSO at 37°C. In brief, the cells
were collected (200 × g, 5 min, 4°C) following treatment and
staining with Annexin V-FITC and propidium iodide (PI) for 15 min
in the dark. The samples were then measured using a BD Accuri™ C6
Flow Cytometer (BD Biosciences), and the data were analyzed using
FlowJo 10.6.2 software (BD Biosciences).
ROS accumulation assay
A Reactive Oxygen Species Assay kit (Beyotime
Institute of Biotechnology) was used to detect ROS accumulation.
According to the manufacturer's instructions, the cells were
pre-treated with or without N-acetylcysteine (NAC; 5 mM,
Selleck Chemicals) or glutathione (GSH; 1 mM, GlpBio) for 1 h at
37°C prior to the addition of Cu(sal)(phen) and harvested (200 × g,
5 min, 4°C), followed by staining with 2′,7′-dichlorofluorescein
diacetate (DCFH-DA) for 20 min at 37°C. Samples were detected, and
the data were analyzed using a BD Accuri™ C6 Flow Cytometer (BD
Biosciences) and FlowJo 10.6.2 software (BD Biosciences),
respectively.
Mitochondrial membrane potential (Δψm)
assay
The depolarization of Δψm was measured using a
Mitochondrial Membrane Potential Assay kit with JC-1 (Bestbio).
According to the manufacturer's instructions, the treated and
collected cells were stained with JC-1 for 30 min at 37°C and
observed using a flow cytometer (BD Biosciences). Finally, the data
were gathered and analyzed using FlowJo 10.6.2 software (BD
Biosciences).
Western blot analysis
Cells treated with Cu(sal)(phen) and Oxa were lysed
with RIPA lysis buffer (Beyotime Institute of Biotechnology)
supplemented with complete protease inhibitor cocktail (Roche
Applied Science) and phenylmethane sulfonyl fluoride. The protein
concentration was determined using the BCA method. Following
heating for 10 min; at 95°C in loading buffer, 25 µg proteins were
separated by 10% SDS-PAGE and transferred onto a PVDF membrane
blocked with a blocking buffer [Odyssey Blocking Buffer (PBS);
LI-COR Biosciences] for 1 h at room temperature. Western blot
analysis was performed using primary and secondary antibody
incubation. Subsequently, blots were acquired using Odyssey SA
(LI-COR Biosciences) and images analyzed using Image Studio ver 5.2
software (LI-COR Biosciences). Primary antibody incubation was
carried out overnight at 4°C. The following primary antibodies were
used: Anti-Bcl-2 (1:1,000, cat. no. ab32124; Abcam), anti-survivin
(1:500, cat. no. 2808S; Cell Signaling Technology, Inc.),
anti-cleaved poly (ADP-ribose) polymerase (PARP; 1:300, cat. no.
9541S; Cell Signaling Technology, Inc.), anti-γ-H2A histone family
member X (γ-H2AX; 1:5,000, cat. no. ab81299; Abcam), anti-caspase-3
(1:1,000, cat. no. 9662s; Cell Signaling Technology, Inc.),
anti-cleaved casepase-3 (1:1,000, cat. no. 9664; Cell Signaling
Technology, Inc.), anti-p-JAK2 (1:1,000, cat. no. 3771s; Cell
Signaling Technology, Inc.), anti-p-STAT3 (1:500, cat. no. ab76315;
Abcam), anti-p-STAT5 (1:1,000, cat. no. 68000-1-Ig; ProteinTech
Group, Inc.) and anti-actin (1:2,000, cat. no. EM21002; Huabio).
The following secondary antibodies were used (incubation for 1 h at
room temperature): Odyssey Blocking Buffer (PBS),
fluorescently-labeled goat anti-rabbit (cat. no. 926-32211) or goat
anti-mouse antibody (cat. no. 926-32210) (both 1:10,000; LI-COR
Biosciences).
Xenograft tumor experiment
All animal experiments were carried out in
accordance with the protocols of the Institutional Animal Care and
Use Committee of Jianghan University. The animal experiments
followed the rules of the Animal Ethics Committee of Jianghan
University (Wuhan, China) and were performed in accordance with
relevant guidelines and regulations, including the ARRIVE
guidelines (ethics permission no. JHDXLL:2019-001). BALB/c-nu
female mice [5 weeks old; specific pathogen-free (SPF); Beijing,
n=25) Biotechnology Co., Ltd.] were domesticated for 1 week and
then used to construct a xenograft tumor model. All mice were
maintained in a specific pathogen-free state, with a regulated 12-h
light/dark cycle, and constant temperature (22±2°C) and relative
humidity (50±10%). HCT116 cells (5×106) were suspended
in 100 µl medium containing 50% Matrigel and injected
subcutaneously into the right flanks of the mice. After the tumors
grew to ~100 mm3, the mice selected according to the
criteria (n=21, tumor volume ~100 mm3; 4 mice were
excluded as their tumor size exceeded the standard deviation of
mouse tumor volume by 3-fold) were randomly divided into three
groups (n=7) and intraperitoneally administered either 70% DMSO
solution in saline (control solution) as the vehicle control,
Cu(sal)(phen) (5 mg/kg in control solution) or Oxa (5 mg, in
saline) every other day. The mouse tumor volumes and body weights
were monitored every other day for 18 days. Tumor volumes were
calculated based on the following formula: V=0.5 × l ×
w2, where l is the length (mm), w is the width (mm), and
V is the volume of tumor. The mice were sacrificed with isoflurane.
Briefly, the mice were placed in a plexiglass chamber with 5%
isoflurane until respiration ceased. The death of the mice was
confirmed by a lack of active paw reflex and no heartbeat. The
tumor weights were recorded and the tumor tissues were fixed in
Bouin's (100%) fixative solution (Phygene) for 48 h at room
temperature. The tumor tissues were then embedded into paraffin
blocks. After sectioning the tissue blocks into 4-µm-thick slices,
the sections were stained with a hematoxylin and eosin (H&E)
staining solution (Wuhan POWERFUL Biotechnology Co., Ltd.;
http://boerfu.net/) for 4 min 15 sec at room
temperature. An upright microscope (ECLIPSE Ni-U, Nikon
Corporation) was used to observed the sections and the images were
captured and analyzed at ×200 magnification using NIS-Elements D
software (5.3.00, Nikon Corporation).
For immunohistochemistry (IHC), the aforementioned
tissue sections (4-µm-thick) were deparaffinized with xylene,
rehydrated with a decreasing ethanol series, and then washed three
times with distilled water for 5 min at room temperature.
Subsequently, these sections were blocked using 3% BSA (cat. no.
A8010, Beijing Solarbio Science & Technology Co., Ltd.) for 30
min at room temperature. The slides were then incubated with
anti-Ki67 (1:1,000, cat. no. ab32124; Abcam), anti-Bcl-2 (1:100,
cat. no. ab32124; Abcam) and anti-survivin (1:400, cat. no. 2808S;
Cell Signaling Technology, Inc.) antibodies overnight at 4°C. The
sections were then stained with DAB (cat. no. K3468, Dako; Agilent
Technologies, Inc.) at room temperature and the color development
time was controlled using a microscope by the positive brownish
yellow color and the section was washed with tap water to terminate
the color development. Subsequently, the nuclei were counterstained
with hematoxylin (cat. no. BH0001, Wuhan POWERFUL Biotechnology
Co., Ltd.; http://boerfu.net/) at room temperature
for 3 min. Finally, the sections were dehydrated with increasing
concentrations of ethanol and xylene. A PANNORAMIC Digital Slide
Scanner (3D Histech) were used to scan the sections. The images
were analyzed using the Fiji (ImageJ 2.1.0) (National Institutes of
Health) and CaseViewer (2.2, 3D Histech) software.
Furthermore, humane endpoints were set when the
tumor diameter >20 mm, tumor necrosis, ulceration, or tumor
infection. Mice that reached the humane endpoint were euthanized.
No mice were sacrificed due to reaching the humane endpoints.
Statistical analysis
Results from three independent experiments were
presented as the mean ± SEM. One- or two-way ANOVA (Tukey's
multiple comparisons test) were used to compare the differences
among groups. Statistical differences were evaluated using GraphPad
prism 7.0 (GraphPad Software, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Cu(sal)(phen) inhibits CRC cell
viability and proliferation
To evaluate the potential effect of Cu(sal)(phen) on
CRC cells, an MTS assay was conducted with various concentrations
of Cu(sal)(phen). Oxa was used as a positive control. Cu(sal)(phen)
displayed a potent ability to inhibit cell viability in a
concentration- and time-dependent manner in the HCT116 and SW480
cell lines (Fig. 1A). Oxa exhibited
a moderate inhibitory effect on the viability of HCT116 cells, even
though it is one of the most commonly used chemotherapeutic drugs
for the clinical treatment of CRC (3,7).
Furthermore, Oxa had a minimal inhibitory effect on the SW480 cell
line, confirming that it was indeed an Oxa-resistant cell line, as
previously reported (31). However,
the SW480 cells were sensitive to Cu(sal)(phen) treatment (Fig. 1A). The IC50 values of
Cu(sal)(phen) at 48 and 72 h were 4.28 and 3.48 µM, respectively,
while the IC50 values of Oxa at 48 and 72 h were >100
and 66.55 µM, respectively. These findings suggested that
Cu(sal)(phen) was more effective than Oxa in inhibiting CRC cell
viability.
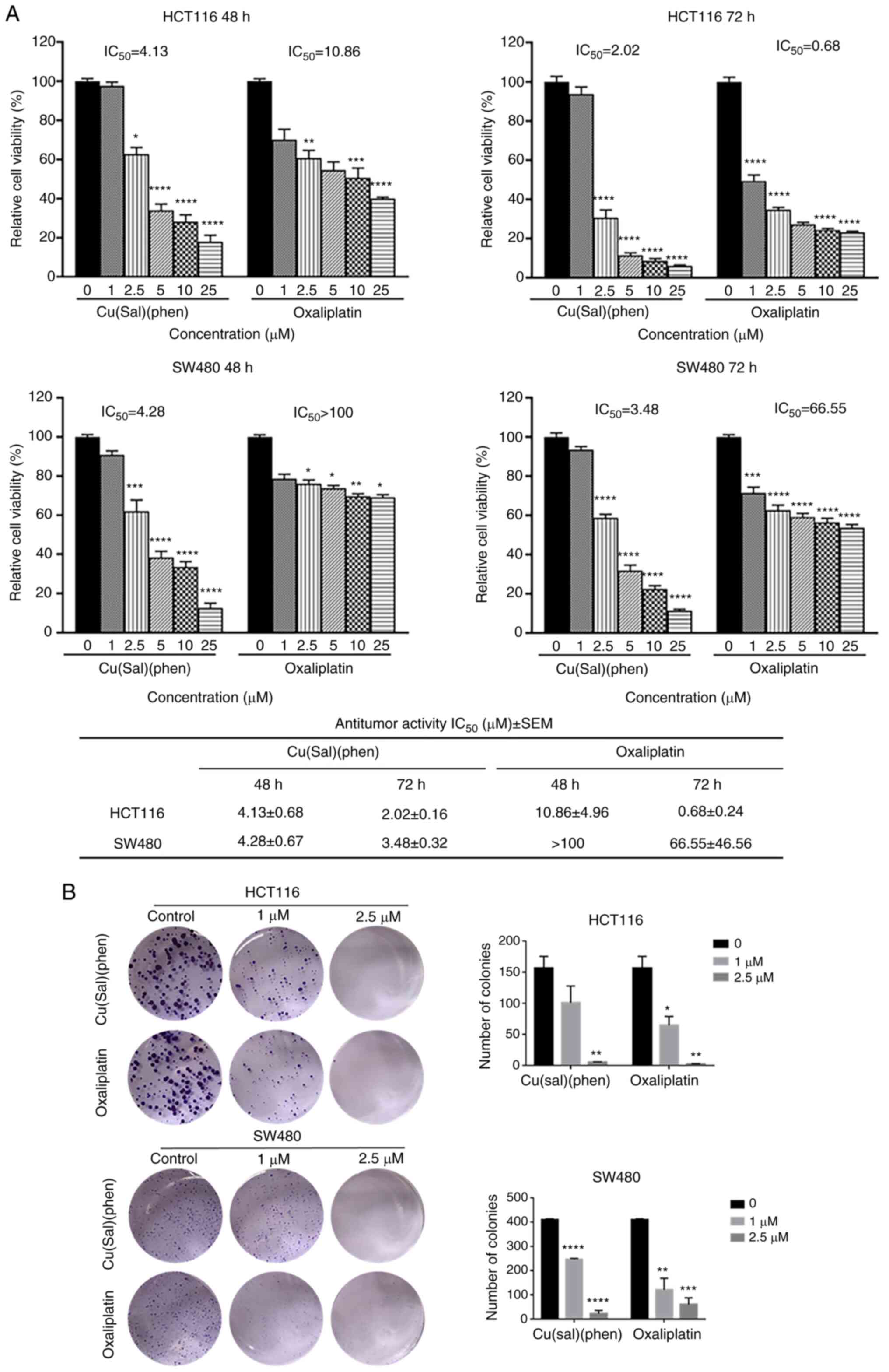 | Figure 1.Cu(sal)(phen) inhibits CRC cell
growth. (A) HCT116 and SW480 cells were treated with 0–25 µM
Cu(sal)(phen), and cell viability was measured using an MTS assay
at the indicated time points. (B) Cell proliferation was analyzed
using a colony formation assay. Cells were seeded in six-well
plates at a density of 500 cells/well for 48 h followed by
treatment with Cu(sal)(phen) and Oxa for 14 days. The medium was
changed every 3 days and the colonies were fixed with 4%
paraformaldehyde and then stained with 0.5% crystal violet dye. At
the end of the experiment, the colonies were counted using Fiji
(ImageJ 2.1.0) software. These results are presented as the mean of
three independent experiments performed in triplicate. The bars
represent the mean ± SD. *P<0.05, **P<0.01, ***P<0.001 and
****P<0.0001, compared to the control (no treatment).
Cu(sal)(phen), copper (II) complex of salicylate phenanthroline;
CRC, colorectal cancer; MTS,
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium;
Oxa, oxaliplatin. |
To further validate the inhibitory effects of
Cu(sal)(phen) on CRC cell viability, a colony formation assay was
performed. As shown in Fig. 1B, the
proliferation was markedly lower than that of the control groups in
the Cu(sal)(phen)-treated HCT116 and SW480 cells. Oxa treatment
exerted similar effects as Cu(sal)(phen) treatment. The
aforementioned results demonstrated that Cu(sal)(phen) effectively
inhibited CRC cell growth in vitro.
Cu(sal)(phen) induces CRC cell
apoptosis
Since Cu(sal)(phen) was more effective than Oxa in
decreasing cell viability, the role of Cu(sal)(phen) in inducing
cell apoptosis was then investigated. The HCT116 and SW480 cells
were treated with various concentrations of Cu(sal)(phen) or Oxa
for 24 h, and cell apoptosis was then measured using an apoptosis
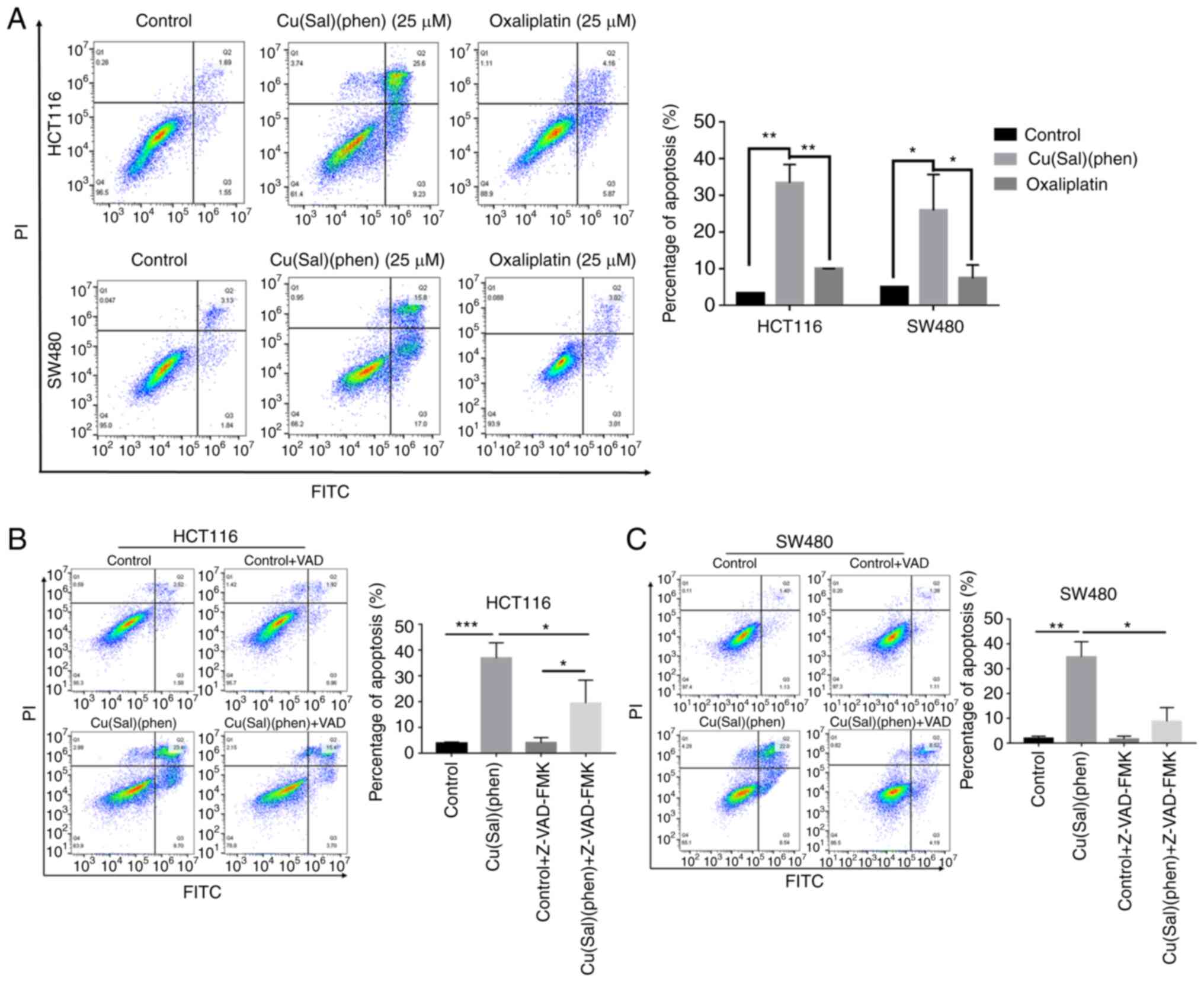
assay. As shown in Fig. S1,
Cu(sal)(phen) effectively induced the apoptosis of the two cell
lines in a concentration-dependent manner. At a concentration of 25
µM, the apoptotic rate was as high as 26.4% (HCT116 cells) and
25.8% (SW480 cells). However, the apoptotic rate of the two cell
lines only remained at ~10% following treatment with 25 µM Oxa
(Fig. 2A). Using an apoptosis
inhibitor, Z-VAD-FMK, it was found that pre-treatment of the CRC
cells with 20 µM Z-VAD-FMK for 1 h prior to the addition of
Cu(sal)(phen) significantly attenuated the apoptosis of both the
HCT116 and SW480 cells (Fig. 2B and
C). These results indicated that Cu(sal)(phen) was more
effective than Oxa in promoting the apoptosis of the two selected
CRC cell lines at a concentration of 25 µM.
Cu(sal)(phen) promotes apoptosis
through ROS generation
A number of reports on copper (II) complexes have
revealed the crucial role of ROS in promoting cell apoptosis
(13,20,27,32).
In order to elucidate the mechanisms through which Cu(sal)(phen)
induces higher levels of apoptosis than Oxa, a ROS generation assay
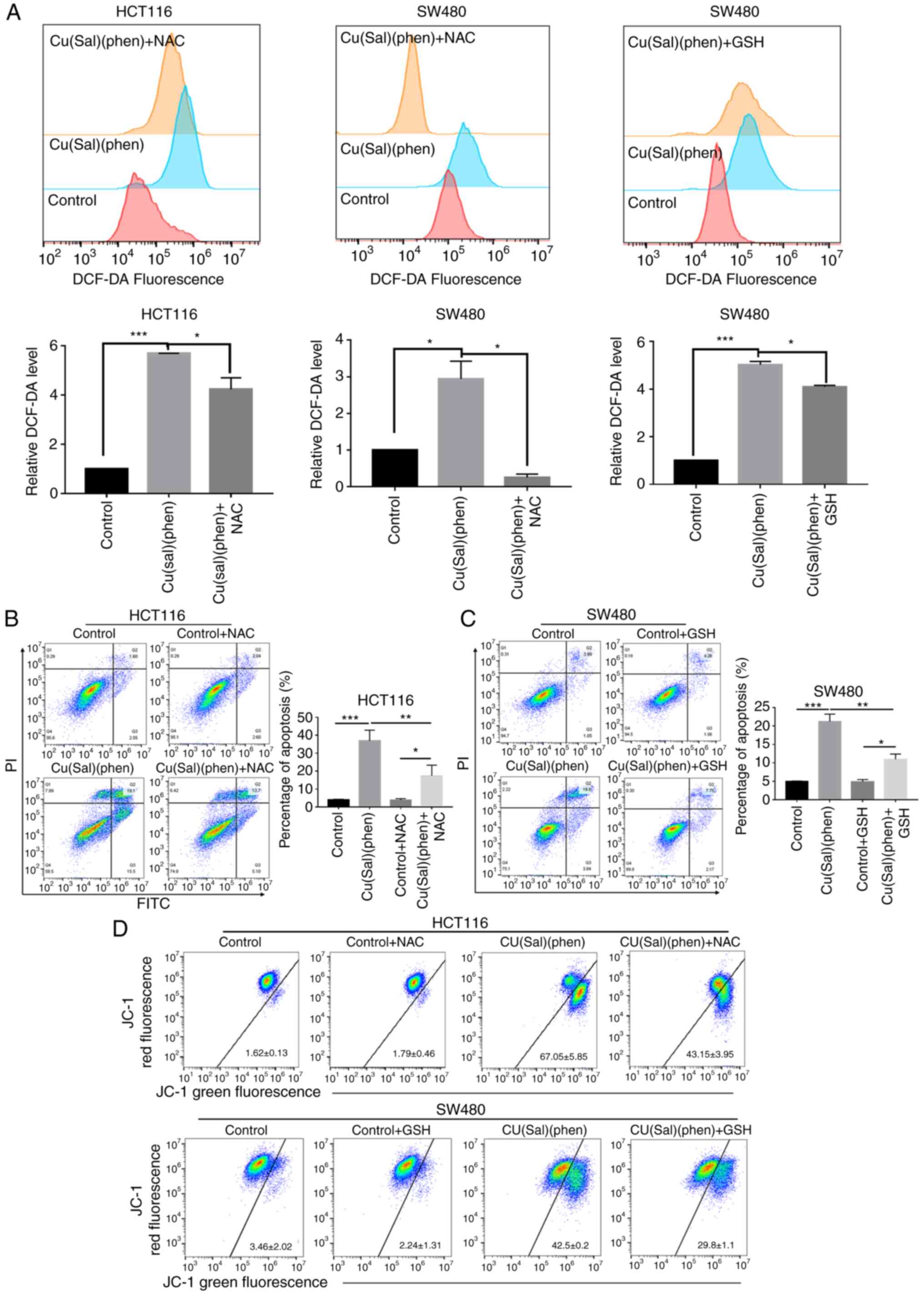
was conducted. The results shown in Fig. 3A revealed a significantly higher
generation of ROS in both CRC cell lines treated with
Cu(sal)(phen), as compared with the control group. The changes in
ROS generation were 5.5- and 2.7-fold in the HCT116 and SW480
cells, respectively. Pre-treatment with the ROS scavenger, NAC
(33), significantly reduced ROS
generation in both the HCT116 and SW480 cell lines (Fig. 3A). By comparison, ROS production did
not differ significantly compared to the control following
treatment of the two CRC cell lines with Oxa (Fig. S2A). To determine the association
between ROS production and cell apoptosis, NAC was added to the CRC
cells to reassess Cu(sal)(phen)-mediated apoptosis. As was
expected, the addition of NAC to the Cu(sal)(phen)-treated HCT116
cells led to a decreased level of apoptosis, as compared with the
Cu(sal)(phen) group (Fig. 3B).
Unexpectedly, the combination of NAC and Cu(sal)(phen) markedly
increased SW480 cell apoptosis (Fig.
S2B). Another antioxidant, GSH, was used to determine
Cu(sal)(phen)-induced ROS production and apoptosis. As shown in
Fig. 3A and C, GSH attenuated
Cu(sal)(phen)-induced ROS generation and SW480 cell apoptosis. It
was thus found that Cu(sal)(phen) initiated apoptosis through ROS
production.
Cu(sal)(phen) induces the
depolarization of Δψm in CRC cells
Mitochondria are considered to be a major source of
ROS (17,18,34),
which is closely associated with cell apoptosis. In addition, cell
apoptosis is often accompanied by Δψm depolarization. For that
reason, in the present study, a Δψm assay was performed, and there
was a notable decrease in Δψm in the HCT116 and SW480 cells
following treatment of the two cell lines with 25 µM Cu(sal)(phen)
(Fig. 3D). Pretreatment with NAC
partially reversed Δψm reduction in Cu(sal)(phen)-treated HCT116
cells. In addition, GSH treatment also reversed the reduction in
Δψm in the Cu(sal)(phen)-treated SW480 cells (Fig. 3D). In line with the results of ROS
assay, the Δψm of the two cell lines treated with Oxa was not
markedly altered compared with the controls (Fig. S3). Thus, as demonstrated by the
results, the Cu(sal)(phen)-induced apoptosis was closely associated
with the release of ROS by the mitochondria.
Cu(sal)(phen)-induced cell death is
dependent on the regulation of apoptosis-related proteins
In order to further elucidate the mechanisms of
Cu(sal)(phen)-induced cell death, a variety of proteins involved in
apoptosis were detected using western blot analysis. Bcl-2 is a
member of the Bcl-2 family which confers a survival advantage to
cancer cells by maintaining mitochondrial integrity (22,35).
Bcl-2 expression in the HCT116 and SW480 cells was downregulated
following treatment with Cu(sal)(phen) at 25 µM compared with the
control group, as shown in Fig. 4A.
However, Bcl-2 expression in the HCT116 cells was not decreased in
the Oxa group. In the SW480 cells treated with Oxa, Bcl-2
expression only decreased to a level comparable to that of
Cu(sal)(phen). Unlike Bcl-2, survivin belongs to the IAP family; it
is overexpressed in cancer cells, but rarely in normal adult
tissues and plays a crucial anti-apoptotic role in cancer cells
(24,36). Survivin expression was downregulated
by Cu(sal)(phen) at a concentration of 25 µM, but not by Oxa in the
two CRC cell lines, as compared with the control (Fig. 4A and B).
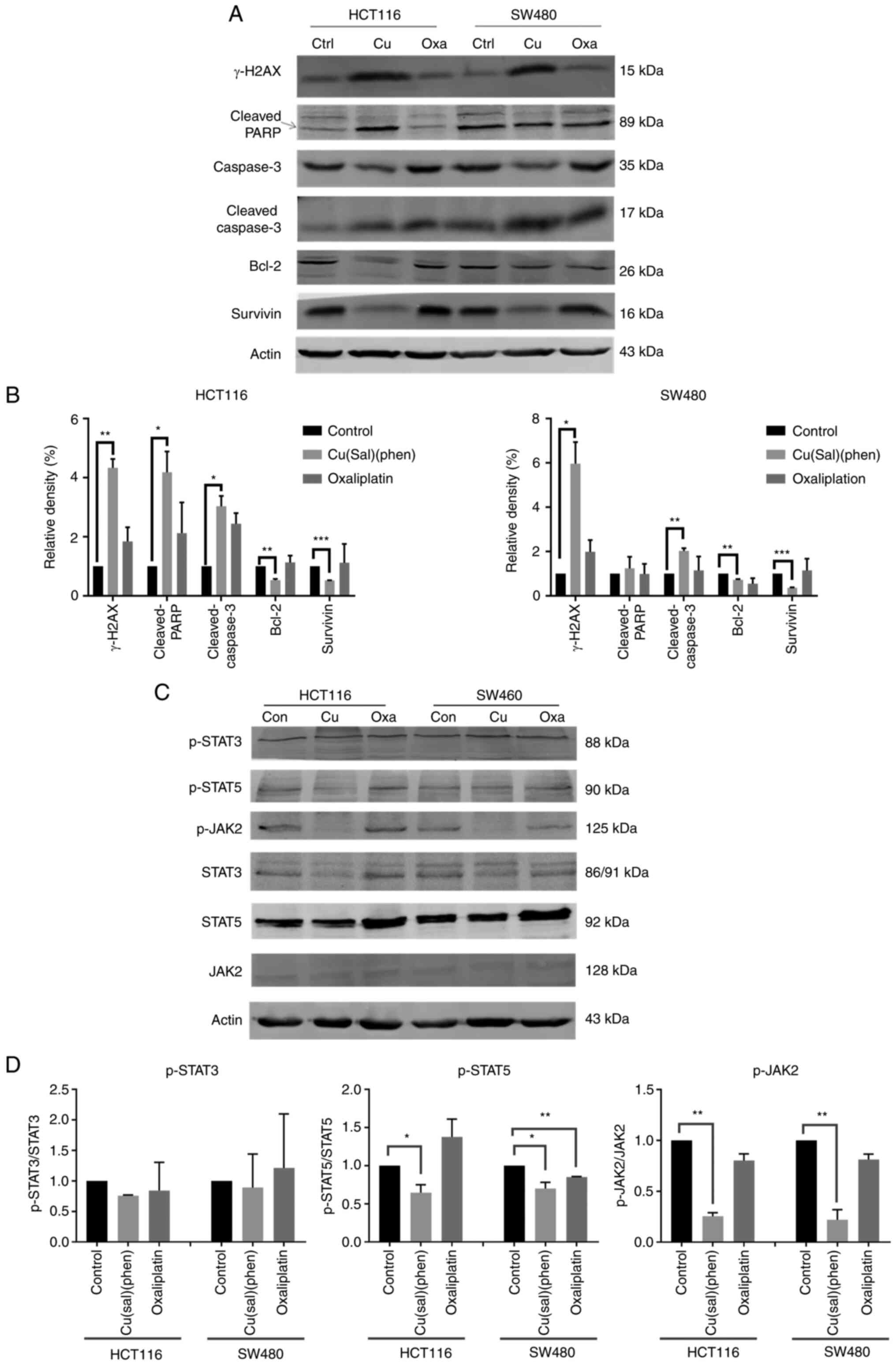 | Figure 4.Cu(sal)(phen) induces CRC cell
apoptosis by downregulating the JAK2/STAT5 pathway. Protein
expression was examined using western blot analysis following
treatment of the HCT116 or SW480 cells with 25 µM Cu(sal)(phen) or
oxaliplatin. DMSO was used as a control. Actin was used as a
loading control. (A) Representative western blots of γ-H2AX,
c-PARP, caspase-3, c-caspase-3, Bcl-2 and survivin in HCT116 and
SW480 cells. (B) Quantitative analysis of the western blots of
HCT116 and SW480 cells. The densities of the target bands were
scanned, and values were normalized to those of actin. (C)
Representative western blots of p-STAT3, p-STAT5 and p-JAK2 in
HCT116 and SW480 cells. (D) The quantitative analysis of the
western blots in (C) was performed as described in (B). The results
are presented as the mean of two independent experiments. Bars
represent the mean ± SD. *P<0.05, **P<0.01 and ***P<0.001.
Cu(sal)(phen), copper (II) complex of salicylate phenanthroline;
CRC, colorectal cancer; DMSO, dimethyl sulfoxide; γ-H2AX, H2A
histone family member X; c-PARP, cleaved poly(ADP-ribose)
polymerase. |
PARP is a substrate of caspases, and its product,
cleaved PARP, is generally used to detect cancer cell apoptosis
(37). The levels of cleaved PARP
were increased in both CRC cell lines in the Cu(sal)(phen) group
(Fig. 4A and B). Consistent with
this observation, the levels of cleaved caspase-3 were increased in
both cell lines following treatment with Cu(sal)(phen). In order to
elucidate the association between Cu(sal)(phen) and apoptosis, the
level of γ-H2AX, a biomarker of DNA double-strand breaks (DSBs)
often caused by ROS (38), was
analyzed. γ-H2AX expression was significantly increased in both the
HCT116 and SW480 cells following treatment with Cu(sal)(phen)
compared with the control. As a drug which directly acts on DNA,
Oxa was found to upregulate γ-H2AX expression in the two CRC cell
lines; however, its effect was less prominent compared with that of
Cu(sal)(phen).
The JAK2/STATs signaling pathway (33) is involved in the regulation of
apoptosis through Bcl-2 in cancer cells. Therefore, the present
study examined whether Cu(sal)(phen) can affect the JAK2/STAT5
pathway in CRC cells. As shown in Fig.
4C and D, the expression of p-STAT5 and p-JAK2 was
significantly inhibited following treatment of the HCT116 and SW480
cells with 25 µM Cu(sal)(phen), while the expression of p-STAT5 and
p-JAK2 remained unaltered following treatment with 25 µM Oxa. The
level of p-STAT3 remained unaltered following treatment of the two
CRC cell lines with Cu(sal)(phen) or Oxa. These results suggested
that Cu(sal)(phen) induces CRC cell apoptosis by downregulating the
JAK2/STAT5 pathway.
Cu(sal)(phen) suppresses tumor growth
in the HCT116 cell xenograft model
Due to the results obtained from the in vitro
experiments, the antitumor effect of Cu(sal)(phen) was investigated
in vivo using a HCT116 cell xenograft model. The tumor
volumes and body weights of the mice were recorded following the
first administration of the drugs and until the mice were
euthanized. The tumor growth curves illustrated in Fig. 5A suggested that Cu(sal)(phen) and
Oxa markedly attenuated tumor growth in the mice following 18 days
of treatment, as compared with the control group. Of note, the
antitumor effects of Cu(sal)(phen) were almost comparable to those
of Oxa. The results were further supported by the images and
weights of the tumors (Fig. 5C and
D). There was no statistically significant difference in body
weight among the three groups of mice (Fig. 5B). This suggested that Cu(sal)(phen)
can effectively suppress HCT116 cell xenograft tumor growth without
any evident toxicity in vivo.
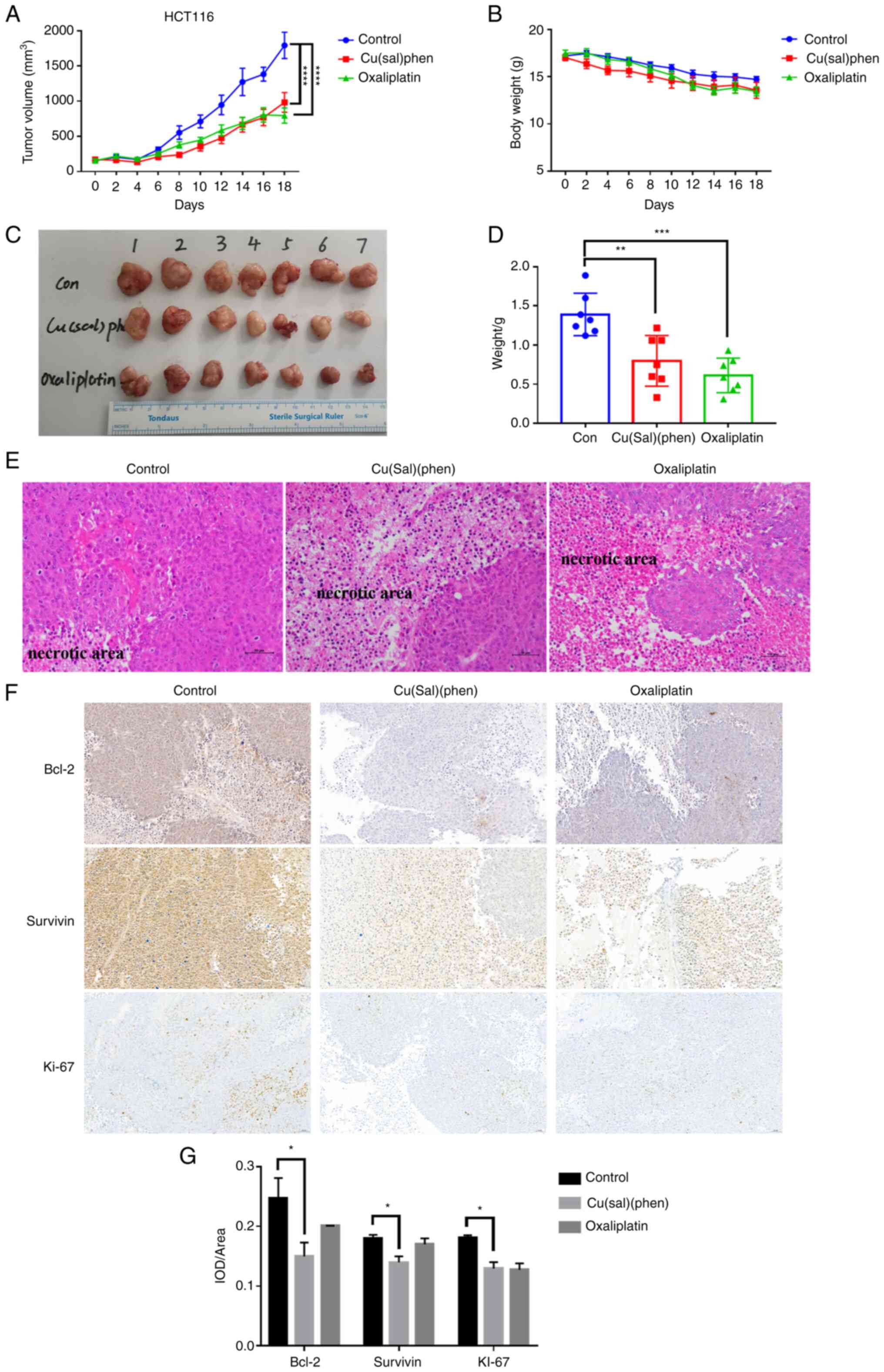 | Figure 5.Cu(sal)(phen) suppresses tumor growth
in the HCT116 cell xenograft model. The mice were injected
intraperitoneally every 2 days with 0.1 ml DMSO or 5 mg/kg
Cu(sal)(phen) or 5 mg/kg Oxa. Tumor size and body weight were
measured every other day throughout the experiments. After 18 days
of treatment, the mice were euthanized, and the tumors were fixed,
sectioned and stained with H&E. (A) Tumor volume for the
tumor-bearing mice is presented as the mean per group (n=7).
****P<0.0001, between the treated and control groups. (B) There
was no significant difference in the mean body weight between the
mice in the treatment and control groups. (C) Images of the tumors
at the end of the study period in each group. (D) Tumor weights in
each group were measured at the end of the treatment. (E) H&E
staining of tumor specimens from the mice injected with DMSO or
Cu(sal)(phen) or Oxa. Magnification, ×200. (F) Cu(sal)(phen)
downregulated the levels of anti-apoptotic and
proliferation-related proteins in xenograft tumors. Formalin-fixed,
paraffin-embedded sections were stained with antibodies against
Bcl-2, survivin and Ki-67. Magnification, ×200. (G) Results of
semi-quantitative analysis of immunohistochemistry images. The IOD
(n=24) was measured using Image-pro plus 6.0 software (Media
Cybernetics). Expression levels of all three proteins in the
treated and control groups. *P<0.05, **P<0.01 and
***P<0.001. Cu(sal)(phen), copper (II) complex of salicylate
phenanthroline; H&E, hematoxylin and eosin; DMSO, dimethyl
sulfoxide; IOD, integrated optical density; Oxa, oxaliplatin. |
To investigate the possible mechanisms of the
antitumor effects of Cu(sal)(phen) in vivo, H&E staining
and IHC of the tumors were performed. H&E staining revealed
evident necrotic areas in the tumors from the drug-treated group
that were not observed in the control tumors (Fig. 5E). Consistent with the results
obtained in vitro, the levels of the two apoptotic proteins,
Bcl-2 and survivin, were decreased, and the level of the
proliferation marker, Ki-67, was decreased (Fig. 5F and G). Collectively, Cu(sal)(phen)
inhibited tumor growth in the HCT116 cell xenograft model, and the
mechanism of the antitumor activity appeared to involve the
downregulation of Bcl-2 and survivin.
Discussion
Platinum-based metal complexes are widely used in
the treatment of various solid tumors (39). However, their use is limited by drug
resistance and significant side effects (40). Therefore, several non-platinum metal
complexes have been investigated due to their novel antitumor
mechanisms of action (40). The
present study reported a copper (II) complex bearing salicylate and
phenanthroline as ligands, abbreviated as Cu(sal)(phen), which
could effectively mediate the apoptosis of HCT116 and SW480 CRC
cell lines through the induction of ROS, mitochondrial
depolarization and downregulation of the anti-apoptotic pathway of
JAK2/STAT5. Cu(sal)(phen) exhibited the ability to induce the
apoptosis of Oxa-sensitive cell line HCT116 and Oxa-resistant cell
line SW480.
Triggering cell apoptosis through different
mechanisms is a crucial strategy in the development of
anti-neoplastics (41).
Mitochondria are a major source of endogenous ROS (17,18,34)
and play a central role in both extrinsic and intrinsic apoptotic
pathways (42,43). The enhancement of ROS generation has
been shown to promote apoptosis and mitochondrial depolarization in
CRC cell lines (44). Furthermore,
ROS production by the copper (II) complex has been demonstrated to
play a pivotal role in cell death (9,32,45).
Based on the aforementioned information, ROS production and
mitochondrial depolarization mediated by Cu(sal)(phen) may be
closely associated with Cu(sal)(phen)-induced apoptosis. The
finding that Cu(sal)(phen)-mediated apoptosis was attenuated by NAC
and Z-VAD-FMK provided further evidence of the association between
apoptosis and ROS production. Given that ROS can lead to
mitochondrial dysfunction in CRC cells (46), it was hypothesized that
Cu(sal)(phen) may initiate apoptosis through the induction of ROS
production that results in mitochondrial depolarization.
Cancer cells have a broad association with ROS, and
ROS generated by metabolic abnormalities and carcinogenic signals
can lead to an increased production of natural antioxidants
(47). As a result, cancer cells
are more dependent on the antioxidant system and more sensitive to
exogenous ROS or antioxidant inhibitors. Adding exogenous ROS to
further increase ROS production in cancer cells can cause ROS
levels to increase to unacceptable levels, resulting in cell death.
This provides a biochemical basis for designing therapeutic
strategies that selectively kill cancer cells using ROS-mediated
mechanisms (48). Of note, it was
found that combined treatment with antioxidant NAC and
Cu(sal)(phen) markedly enhanced SW480 cell apoptosis (data not
shown). However, GSH attenuated the Cu(sal)(phen)-induced apoptosis
of SW480 cells. Although the exact mechanisms involved remains
unclear, the intracellular REDOX system is much more complex than
was previously considered. Further studies are warranted to
determine whether Cu(sal)(phen) can mediate the recently defined
cuproptosis (49), that is not
triggered by caspases and not attenuated by NAC.
Bcl-2 family proteins are pivotal regulators of cell
apoptosis (22,23). In addition, Bcl-2 and survivin are
critical anti-apoptotic members that promote cell survival through
a number of mechanisms, such as maintaining the integrity of the
mitochondria (22) and inhibiting
the activity of terminal effector enzymes, caspase-3 and caspase-7
(24). Previous studies have tried
to promote apoptosis by targeting Bcl-2 (25,50)
and survivin (26,51). In line with these reports, the
present study found that the Bcl-2 and survivin levels were
downregulated by Cu(sal)(phen) in the HCT116 and SW480 cells in
vitro and in the HCT116 cell xenograft model in vivo.
Furthermore, the p-JAK2 and p-STAT5 levels were also downregulated
following treatment of the two CRC cell lines with Cu(sal)(phen).
STAT5 is a transcription factor that activates the transcription of
Bcl-2 (52). Thus, it was inferred
that Cu(sal)(phen) mediated CRC cell apoptosis not only by inducing
ROS generation, but also by downregulating survivin, Bcl-2 and its
upstream proteins, including p-JAK2 and p-STAT5.
In order to further confirm the antitumor efficacy
of Cu(sal)(phen), the levels of the apoptotic marker, cleaved PARP,
and the DSB marker, γ-H2AX, were examined in the HCT116 and SW480
cell lines. In addition, the proliferation marker, Ki-67, was
examined in the HCT116 cell xenograft model. As was expected,
treatment with Cu(sal)(phen) increased the levels of cleaved PARP
and γ-H2AX that was triggered by DSBs in vitro, and further
decreased the level of Ki-67 in vivo, suggesting that
Cu(sal)(phen) exhibited good antitumor efficacy both in
vitro and in vivo.
In conclusion, the findings of the present study
suggest that Cu(sal)(phen) inhibits the proliferation and induces
the apoptosis of CRC cells in vitro, while also inhibiting
tumor growth in vivo in nude mice. In addition, Cu (sal)
(phen) was shown to have a good safety profile in previous in
vivo experiments on Balb/c mice (53). The underlying mechanisms of
Cu(sal)(phen) treatment involve the induction of ROS generation,
the inhibition of the JAK2/STAT5 signaling pathway and the
downregulation of the expression of anti-apoptotic proteins, such
as Bcl-2 and survivin. In the present study, a subcutaneous mouse
model was used to detect the antitumor efficacy of Cu(sal)(phen),
which poses some limitations in evaluating antitumor efficacy. For
example, the subcutaneous mouse model cannot fully simulate the
progression and pathological changes of tumors in the colon, as
tumor cells are inoculated under the skin, rather than in an
orthotopic environment. In order to demonstrate the antitumor
efficacy of Cu (sal) (phen) to a greater extent, we also attempted
to conduct in vivo experiments using the SW480 cell
xenograft model. However, the model of SW480 was not constructed
well due to slow growth. Therefore, the authors aim to explore its
potential in the treatment of CRC in more depth in future
studies.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr David Xu from
UCLA Health, California, CA, USA for critically reading the
manuscript.
Funding
The present study was financially supported by the Huanghe
Talented Scholar Grant of Wuhan City (no. 1010/06850002), the
Jianghan University collaborative Innovation Grant (no.
3010/03100070), the Jianghan University Special Research Grant (no.
3015/08210002). J. Xu is a Chutian Scholar of the Department of
Education, Jianghan University, Wuhan, China.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DW, WZ, YL and JX conceived and designed the
experiments. ZL, LF, DN, MC and DW performed the experiments. ZL,
DW, WZ, YL and JX analyzed the data. DW and JX wrote the
manuscript. DW and JX confirm the authenticity of all the raw data.
All authors reviewed the manuscript, and all authors have read and
approved the final manuscript.
Ethics approvals and consent to
participate
The animal experiments followed the rules of the
Animal Ethics Committee of Jianghan University (Wuhan, China) and
were performed in accordance with relevant guidelines and
regulations, including the ARRIVE guidelines (ethics permission no.
JHDXLL:2019-001).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lu WQ, Hu YY, Lin XP and Fan W: Knockdown
of PKM2 and GLS1 expression can significantly reverse
oxaliplatin-resistance in colorectal cancer cells. Oncotarget.
8:44171–44185. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Asadzadeh Z, Mansoori B, Mohammadi A,
Kazemi T, Mokhtarzadeh A, Shanehbandi D, Hemmat N, Derakhshani A,
Brunetti O, Safaei S, et al: The combination effect of Prominin1
(CD133) suppression and Oxaliplatin treatment in colorectal cancer
therapy. Biomed Pharmacother. 137:1113642021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Y, Sun Z, Cui Y, Zhang H, Zhang S, Wang
X, Liu S and Gao Q: Oxaliplatin derived monofunctional
triazole-containing platinum(II) complex counteracts
oxaliplatin-induced drug resistance in colorectal cancer. Bioorg
Chem. 107:1046362021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen W, Lian W, Yuan Y and Li M: The
synergistic effects of oxaliplatin and piperlongumine on colorectal
cancer are mediated by oxidative stress. Cell Death Dis.
10:6002019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martinez-Balibrea E, Martínez-Cardús A,
Ginés A, Ruiz de Porras V, Moutinho C, Layos L, Manzano JL, Bugés
C, Bystrup S, Esteller M and Abad A: Tumor-related molecular
mechanisms of oxaliplatin resistance. Mol Cancer Ther.
14:1767–1776. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Buchholz A, Sahmoun AE and Kurniali PC:
Characteristics of colorectal patients who discontinued oxaliplatin
therapy. J Clin Oncol. 37 (Suppl):e151552019. View Article : Google Scholar
|
|
8
|
Zedan AH, Hansen TF, Svenningsen ÅF and
Vilholm OJ: Oxaliplatin-induced neuropathy in colorectal cancer:
Many questions with few answers. Clin Colorectal Cancer. 13:73–80.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ali A, Mishra S, Kamaal S, Alarifi A,
Afzal M, Saha KD and Ahmad M: Evaluation of catacholase mimicking
activity and apoptosis in human colorectal carcinoma cell line by
activating mitochondrial pathway of copper(II) complex coupled with
2-(quinolin-8-yloxy)(methyl)benzonitrile and 8-hydroxyquinoline.
Bioorg Chem. 106:1044792021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zehra S, Tabassum S and Arjmand F:
Biochemical pathways of copper complexes: Progress over the past 5
years. Drug Discov Today. 26:1086–1096. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song W, Xu P, Zhi S, Zhu S, Guo Y and Yang
H: Integrated transcriptome and in vitro analysis revealed
antiproliferative effects on human gastric cancer cells by a
benzimidazole-quinoline copper(II) complex. Process Biochem.
102:286–295. 2021. View Article : Google Scholar
|
|
12
|
Sequeira D, Baptista PV, Valente R,
Piedade MFM, Garcia MH, Morais TS and Fernandes AR: Cu(I) complexes
as new antiproliferative agents against sensitive and doxorubicin
resistant colorectal cancer cells: Synthesis, characterization, and
mechanisms of action. Dalton Trans. 50:1845–1865. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Radhakrishnan K, Khamrang T, Sambantham K,
Sali VK, Chitgupi U, Lovell JF, Mohammad AA and Venugopal R:
Identification of cytotoxic copper(II) complexes with
phenanthroline and quinoline, quinoxaline or quinazoline-derived
mixed ligands. Polyhedron. 194:1148862021. View Article : Google Scholar
|
|
14
|
Mahendiran D, Kumar RS, Viswanathan V,
Velmurugan D and Rahiman AK: Targeting of DNA molecules, BSA/c-Met
tyrosine kinase receptors and anti-proliferative activity of
bis(terpyridine)copper(ii) complexes. Dalton Trans. 45:7794–7814.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gou Y, Chen M, Li S, Deng J, Li J, Fang G,
Yang F and Huang G: Dithiocarbazate-copper complexes for bioimaging
and treatment of pancreatic cancer. J Med Chem. 64:5485–5499. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen X, Dou QP, Liu J and Tang D:
Targeting ubiquitin- proteasome system with copper complexes for
cancer therapy. Front Mol Biosci. 8:6491512021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cui Q, Wang JQ, Assaraf YG, Ren L, Gupta
P, Wei L, Ashby CR Jr, Yang DH and Chen ZS: Modulating ROS to
overcome multidrug resistance in cancer. Drug Resist Updat.
41:1–25. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Snezhkina AV, Kudryavtseva AV, Kardymon
OL, Savvateeva MV, Melnikova NV, Krasnov GS and Dmitriev AA: ROS
generation and antioxidant defense systems in normal and malignant
cells. Oxid Med Cell Longev. 2019:61758042019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ng CH, Kong SM, Tiong YL, Maah MJ, Sukram
N, Ahmad M and Khoo ASB: Selective anticancer copper(II)-mixed
ligand complexes: Targeting of ROS and proteasomes. Metallomics.
6:892–906. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Polloni L, Seni Silva AC, Teixeira SC,
Azevedo FVPV, Zóia MAP, da Silva MS, Lima PMAP, Correia LIV, do
Couto Almeida J, da Silva CV, et al: Action of copper(II) complex
with β-diketone and 1,10-phenanthroline (CBP-01) on sarcoma cells
and biological effects under cell death. Biomed Pharmacother.
112:1085862019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pfeffer CM and Singh ATK: Apoptosis: A
target for anticancer therapy. Int J Mol Sci. 19:4482018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Banjara S, Suraweera CD, Hinds MG and
Kvansakul M: The Bcl-2 family: Ancient origins, conserved
structures, and divergent mechanisms. Biomolecules. 10:1282020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun BB, Fu LN, Wang YQ, Gao QY, Xu J, Cao
ZJ, Chen YX and Fang JY: Silencing of JMJD2B induces cell apoptosis
via mitochondria-mediated and death receptor-mediated pathway
activation in colorectal cancer. J Dig Dis. 15:491–500. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li D, Hu C and Li H: Survivin as a novel
target protein for reducing the proliferation of cancer cells.
Biomed Rep. 8:399–406. 2018.PubMed/NCBI
|
|
25
|
de Ridder I, Kerkhofs M, Veettil SP,
Dehaen W and Bultynck G: Cancer cell death strategies by targeting
Bcl-2′s BH4 domain. Biochim Biophys Acta Mol Cell Res.
1868:1189832021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guvenc H, Pavlyukov MS, Joshi K, Kurt H,
Banasavadi-Siddegowda YK, Mao P, Hong C, Yamada R, Kwon CH, Bhasin
D, et al: Impairment of glioma stem cell survival and growth by a
novel inhibitor for Survivin-Ran protein complex. Clin Cancer Res.
19:631–642. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lopes JC, Botelho FV, Barbosa Silva MJ,
Silva SF, Polloni L, Alves Machado PH, Rodrigues de Souza T,
Goulart LR, Silva Caldeira PP, Pereira Maia EC, et al: In vitro and
in vivo antitumoral activity of a ternary copper (II) complex.
Biochem Biophys Res Commun. 533:1021–1026. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fan L, Tian M, Liu Y, Deng Y, Liao Z and
Xu J: Salicylate •phenanthroline copper (II) complex induces
apoptosis in triple-negative breast cancer cells. Oncotarget.
8:29823–29832. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sukhdeo K, Paramban RI, Vidal JG, Elia J,
Martin J, Rivera M, Carrasco DR, Jarrar A, Kalady MF, Carson CT, et
al: Multiplex flow cytometry barcoding and antibody arrays identify
surface antigen profiles of primary and metastatic colon cancer
cell lines. PLoS One. 8:e530152013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kreutz D, Bileck A, Plessl K, Wolrab D,
Groessl M, Keppler BK, Meier SM and Gerner C: Response profiling
using shotgun proteomics enables global metallodrug mechanisms of
action to be established. Chemistry. 23:1881–1890. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun W, Ge Y, Cui JP, Yu YF and Liu BL:
Scutellarin resensitizes oxaliplatin-resistant colorectal cancer
cells to oxaliplatin treatment through inhibition of PKM2. Mol Ther
Oncolytics. 21:87–97. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo WJ, Ye SS, Cao N, Huang JA, Gao J and
Chen QY: ROS-mediated autophagy was involved in cancer cell death
induced by novel copper(II) complex. Exp Toxicol Pathol.
62:577–582. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cao Y, Wang J, Tian H and Fu GH:
Mitochondrial ROS accumulation inhibiting JAK2/STAT3 pathway is a
critical modulator of CYT997-induced autophagy and apoptosis in
gastric cancer. J Exp Clin Cancer Res. 39:1192020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Murphy MP: How mitochondria produce
reactive oxygen species. Biochem J. 417:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Warren CFA, Wong-Brown MW and Bowden NA:
BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis.
10:1772019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Peery RC, Liu JY and Zhang JT: Targeting
survivin for therapeutic discovery: Past, present, and future
promises. Drug Discov Today. 22:1466–1477. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiang F, Zhou JY, Zhang D, Liu MH and Chen
YG: Artesunate induces apoptosis and autophagy in HCT116 colon
cancer cells, and autophagy inhibition enhances the
artesunate-induced apoptosis. Int J Mol Med. 42:1295–1304.
2018.PubMed/NCBI
|
|
38
|
Schütz CS, Stope MB and Bekeschus S: H2A.X
phosphorylation in oxidative stress and risk assessment in plasma
medicine. Oxid Med Cell Longev. 2021:20609862021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Johnstone TC, Suntharalingam K and Lippard
SJ: The next generation of platinum drugs: Targeted Pt(II) agents,
nanoparticle delivery, and Pt(IV) prodrugs. Chem Rev.
116:3436–3486. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gałczyńska K, Drulis-Kawa Z and Arabski M:
Antitumor activity of Pt(II), Ru(III) and Cu(II) complexes.
Molecules. 25:34922020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang NN, Zhang PZ, Zhang J, Wang HN, Li L,
Ren F, Dai PF, Li H and Lv XF: Penfluridol triggers
mitochondrial-mediated apoptosis and suppresses glycolysis in
colorectal cancer cells through down-regulating hexokinase-2. Anat
Rec (Hoboken). 304:520–530. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cui Q, Wen S and Huang P: Targeting cancer
cell mitochondria as a therapeutic approach: Recent updates. Future
Med Chem. 9:929–949. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kleih M, Böpple K, Dong M, Gaißler A,
Heine S, Olayioye MA, Aulitzky WE and Essmann F: Direct impact of
cisplatin on mitochondria induces ROS production that dictates cell
fate of ovarian cancer cells. Cell Death Dis. 10:8512019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xia S, Miao Y and Liu S: Withaferin A
induces apoptosis by ROS-dependent mitochondrial dysfunction in
human colorectal cancer cells. Biochem Biophys Res Commun.
503:2363–2369. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kowol CR, Heffeter P, Miklos W, Gille L,
Trondl R, Cappellacci L, Berger W and Keppler BK: Mechanisms
underlying reductant-induced reactive oxygen species formation by
anticancer copper(II) compounds. J Biol Inorg Chem. 17:409–423.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Basak D, Uddin MN and Hancock J: The role
of oxidative stress and its counteractive utility in colorectal
cancer (CRC). Cancers (Basel). 12:33362020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Moloney JN and Cotter TG: ROS signalling
in the biology of cancer. Semin Cell Dev Biol. 80:50–64. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang F, Pei R, Zhang Z, Liao J, Yu W, Qiao
N, Han Q, Li Y, Hu L, Guo J, et al: Copper induces oxidative stress
and apoptosis through mitochondria-mediated pathway in chicken
hepatocytes. Toxicol In vitro. 54:310–316. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tsvetkov P, Coy S, Petrova B, Dreishpoon
M, Verma A, Abdusamad M, Rossen J, Joesch-Cohen L, Humeidi R,
Spangler RD, et al: Copper induces cell death by targeting
lipoylated TCA cycle proteins. Science. 375:1254–1261. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Piché A, Grim J, Rancourt C, Gómez-Navarro
J, Reed JC and Curiel DT: Modulation of Bcl-2 protein levels by an
intracellular anti-Bcl-2 single-chain antibody increases
drug-induced cytotoxicity in the breast cancer cell line MCF-7.
Cancer Res. 58:2134–2140. 1998.PubMed/NCBI
|
|
51
|
Tanioka M, Nokihara H, Yamamoto N, Yamada
Y, Yamada K, Goto Y, Fujimoto T, Sekiguchi R, Uenaka K, Callies S
and Tamura T: Phase I study of LY2181308, an antisense
oligonucleotide against survivin, in patients with advanced solid
tumors. Cancer Chemother Pharmacol. 68:505–511. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen Y, Zhou Q, Zhang L, Zhong Y, Fan G,
Zhang Z, Wang R, Jin M, Qiu Y and Kong D: Stellettin B induces
apoptosis in human chronic myeloid leukemia cells via targeting
PI3K and Stat5. Oncotarget. 8:28906–28921. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Niu D, Wang D, Fan L, Liu Z, Chen M, Zhang
W and Liu Y, Xu J and Liu Y: The copper (II) complex of salicylate
phenanthroline inhibits proliferation and induces apoptosis of
hepatocellular carcinoma cells. Environ Toxicol. 38:1384–1394.
2023. View Article : Google Scholar : PubMed/NCBI
|