Introduction
Essential hypertension (EH) is the most common
cardiovascular disease, and its incidence is increasing yearly with
a trend apparent in younger patients. Previously, a large number of
studies have shown that hyperhomocysteinemia (HHcy) is an
independent risk factor of cardiovascular and cerebrovascular
diseases, and increased hypertension and plasma homocysteine (Hcy)
in causing these diseases have a synergistic effect (1).
Hcy is a body of sulfur-containing amino acids, and
an important intermediate product in the metabolism of methionine
and cysteine. There are four key metabolic enzymes in the metabolic
pathways of Hcy, and CBS and MTHFR are the key common enzymes. The
thermolabile MTHFR C677T gene mutation is the most common
genetic factor to cause mild to moderate HHcy change. C/T mutation
resulting in encoded alanine replaced by valine, decreased its
activity and produced heat, which affected the most important
metabolic pathways of Hcy-remethylation (2). Studies have shown that the T allele
frequencies in different ethnicities and countries vary widely. The
Guangxi Yao population rate was 22.6% (3) and in Australia it was 32.2% (4). The Yang et al (5) study of 15,357 Han patients found that
the mutation frequency in Hainan was the lowest with a T allele of
24.0%, and in Shandong it was the highest (63.1%). This study
regarding the association between the MTHFR C677T gene
polymorphism and EH is not consistent. The study by Fowdar et
al (4) of a population of
Caucasian Australians showed that EH was not associated with the
polymorphism of MTHFR C677T. Alghasham et al
(6) studied an Arab population and
found that the mutation frequency in groups of hypertension and
obesity, and hypertension and diabetes were significantly higher
compared to the healthy group (P<0.05), indicating that the T
allele may be a risk factor for EH.
CBS844ins68 is the 8th exon insertion
mutation, with a size of 68 base pairs (bp). The mutation causes
CBS metabolic activity to decrease and affects the metabolism of
Hcy. Previous studies have shown that the CBS844ins68 gene
polymorphisms exist in different populations; the frequency of the
insertion (I) allele was 10% in Britian, 7% in Pakistan and 1.4% in
the Henan Han population (7–9). Thus
far, the correlation of the CBS844ins68 gene polymorphism
and EH is rarely reported in domestic and foreign countries. Lucock
et al (7) studied a British
population and found that the CBS844ins68 gene polymorphism
may not be an independent risk factor of EH.
The prevalence of hypertension in Chinese provinces
is different. Northeast and North of China is higher compared to
the region of Southwest and Southeast, and the Eastern region is
higher compared to the Western. A number of reasons for the
differences may be associated with the population salt intake,
variation in the proportion of obese subjects, ethnicity and
climate. Standardized prevalence of hypertension in the same area
between various ethnic groups and regions of the same nation were
different. In 1991, the Third National Sample Survey of
hypertension showed that the lowest prevalence was in Yi (3.28%),
and was higher for the Korean (22.95%), Tibetan (21.04%) and
Mongolian (20.22%) populations, and for Xinjiang Kazakh and Han it
was 18.97 and 13.88%, respectively (10). Using the Xinjiang Tianshan mountains
as the boundary, China is divided into North and South regions. The
Northern winter is long and cold so therefore exercise outdoors is
minimal, and the local residents are carnivorous, whereas in the
Southern region the winter is shorter, and vegetable and fruit
intake is relatively higher, which also generates certain body
weight and blood lipid characteristics. Kawamura et al
(11) studied the region of Balikun
(Xinjiang Northern) and found that the prevalence of hypertension
in the Han population was 42%, but in the Hotan prefecture
(Xinjiang Southern) the Han prevalence of hypertension was 27%. The
aim of the present study was to investigate the correlation between
Hcy and the gene polymorphisms of its metabolic enzymes,
CBS844ins68 and MTHFR C677T, and EH in patients from the
North of Xinjiang.
Materials and methods
Ethics statement
Written informed consent was obtained from all the
participating patients prior to enrollment in the study. The study
was approved by the Institutional Ethics Committee at the First
Affiliated Hospital of Shihezi University School of Medicine
(Shihezi, China) and conducted in accordance with the Ethical
Guidelines of the Declaration of Helsinki.
Study subjects
Between May 2012 and March 2013, 200 patients with
EH (47.01±8.06 years) and 200 control subjects (45.23±9.88 years)
were recruited sequentially from the Chinese Han population in
Xinjiang Shihezi. All the patients fulfilled the hypertension
diagnosis standard of the World Health Organization/International
Society of Hypertension in 2003 and secondary hypertension
(12), cardiomyopathy, diabetic and
other patients were excluded. The control subjects inclusion
criteria were systolic blood pressure <140 mmHg and diastolic
blood pressure <90 mmHg, no use of antihypertensive medication,
no family history of hypertension, and a medical history of liver,
kidney and thyroid problems, diabetes and others were excluded.
Specimen collection
After 12–14 h fasting, 4 ml venous blood was
collected into an EDTA-tube, mixed and stored at −80°C for genomic
DNA extraction. Another tube of heparin was used and plasma was
centrifuged for 1 h at 3,000 × g within 10 min of collection. The
plasma was separated for biochemical testing.
Detection of Hcy and biochemical
analyses
Using an AU 2700 biochemical analyzer (Olympus,
Tokyo, Japan), the Hcy concentration was determined by enzymatic
cycling assay (Nine Strong Biological Co., Ltd., Beijing, China),
and the study participated in the National ‘863’ Project quality
control of Hcy. Other biochemical analyses were performed by Roche
7600 (Roche Diagnostics, Madison, WI, USA), according to the
manufacturer’s instructions.
Genotype analysis
Genomic DNA was extracted from the blood samples of
the study subjects using a blood genome DNA extraction kit
(GeneCore BioTeke Co., Ltd., Beijing, China). The primers were
designed as described previously (4).
Polymerase chain reaction (PCR)
amplification of the CBS844ins68 and MTHFR C677T polymorphisms
PCR was performed in a total volume of 2 μl
containing 3.0 μl DNA, 0.5 μl of each primer, 12.5 μl PCR mix
(including dNTPs and MgCl2; Shanghai Sangon Biological
Engineering Technology and Services Co., Ltd., Shanghai, China) and
distilled water 8.5 μl. Amplification was carried out at Biometra
(Göttingen, Germany) using a gradient automatic PCR amplification
instrument (negative controls without DNA template). The reaction
of CBS844ins68 was performed as follows: Initial
denaturation was at 95°C for 3 min; denaturation at 95°C for 30 sec
and annealing at 57°C for 30 sec, with extension for 60 sec at
72°C, repeated for 35 cycles, followed by a final extension at 72°C
for 5 min. The CBS844ins68 PCR product was analyzed in 2%
agarose gel electrophoresis directly, and the gel bands and typing
were observed under an imager.
The reaction of MTHFR C677T was carried out
as follows: initial denaturation was at 95°C for 5 min;
denaturation at 95°C for 60 sec and annealing at 57°C for 60 sec,
with extension for 60 sec at 72°C, repeated for 36 cycles, followed
by a final extension at 72°C for 5 min. The MTHFR C677T
product (198 bp) was digested using HinfI (Fermentas,
Waltham, MA, USA), and the digested products were run on a 9%
agarose gel and stained with silver nitrate 35 min, observed and
genotyped in the UV gel imager.
Statistical analysis
Statistical analysis was performed with the SPSS
software, version 17.0 (SPSS, Inc., Chicago, IL, USA). Each result
was calculated as mean ± standard deviation. Hardy-Weinberg
equilibrium was used to confirm whether the sample had a group
representation, the differences in genotype and allelic frequency
between the ethnic groups were evaluated with Fisher’s exact test
or χ2 tests as appropriate. The odds ratio (OR) was
calculated together with its 95% confidence interval (CI).
P<0.05 was considered to indicate a statistically significant
difference. Linkage disequilibrium (LD) and haplotype analysis was
performed using SHEsis software (13), and the LD coefficient (D′)<0.2
was considered to have no LD, D′>0.5 had a LD, D′>0.8 had a
strong LD and D′=1 is in complete LD. r2 was the
relevant factor.
Results
Characteristics of the subjects
The analysis of the clinical data in the EH and
normal tensive (NT) groups (400 cases were selected) is shown in
Table I. There were no significant
differences (P>0.05) between the two groups for age, urea,
creatinine, uric acid, glucose, high-density lipoprotein
cholesterol (HDL-C), apolipoprotein (apo) A, apo B and
lipoprotein(a) (Lpa). In systolic blood pressure, diastolic blood
pressure, body mass index, triglyceride (TG), total cholesterol
(TC) and low-density lipoprotein cholesterol (LDL-C), EH was
significantly higher compared to the NT group (P<0.05; Table II). In the two groups, the level of
Hcy in males was higher compared to females, but was not
statistically significant (Table
III).
 | Table IGeneral clinical data analysis of EH
and NT groups. |
Table I
General clinical data analysis of EH
and NT groups.
| Characteristics | EH group (n=200) | NT group (n=200) | T | P-value |
|---|
| Age, years | 47.01±8.06 | 45.23±9.88 | 1.958 | 0.051 |
| SBP, mmHg | 152.38±9.60 | 115.51±12.18 | 33.605 | 0.001a |
| DBP, mmHg | 101.82±10.15 | 76.34±8.50 | 27.213 | 0.001a |
| BMI,
kg/m2 | 27.07±2.50 | 24.23±1.68 | 13.283 | 0.003a |
| BUN, mmol/l | 5.03±1.51 | 4.86±1.18 | 1.277 | 0.202 |
| CR, mmol/l | 73.13±16.22 | 72.32±13.75 | 0.538 | 0.591 |
| UA, mmol/l | 280.70±90.55 | 272.72±69.37 | 0.989 | 0.323 |
| FPG, mmol/l | 5.31±1.09 | 5.21±0.84 | 0.966 | 0.335 |
| TG, mmol/l | 1.45±0.85 | 0.97±0.46 | 6.923 | 0.001a |
| TC, mmol/l | 4.71±1.12 | 4.09±0.53 | 6.981 | 0.002a |
| HDL-C, mmol/l | 1.24±0.40 | 1.19±0.21 | 1.477 | 0.141 |
| LDL-C, mmol/l | 2.83±0.84 | 2.45±0.52 | 5.263 | 0.001a |
| Apo A, mmol/l | 1.42±0.34 | 1.37±0.27 | 1.580 | 0.115 |
| Apo B, mmol/l | 0.96±0.28 | 0.94±0.22 | 0.838 | 0.403 |
| Lpa, mmol/l | 217.07±179.23 | 199.71±75.59 | 1.262 | 0.208 |
 | Table IIHcy level in the NT and EH
groups. |
Table II
Hcy level in the NT and EH
groups.
| Groups | Hcy, μmol/l | T | P-value |
|---|
| NT (n=200) | 10.33±2.04 | 0.28 | 0.001a |
| EH (n=200) | 16.05±7.59 | | |
 | Table IIIHcy level in the different
genders. |
Table III
Hcy level in the different
genders.
| Groups | Female, μmol/l | Male, μmol/l | T | P-value |
|---|
| EH (112/88) | 15.29±7.27 | 17.01±7.93 | −1.598 | 0.112 |
| NT (107/93) | 10.13±2.12 | 10.56±1.93 | −1.497 | 0.136 |
Polymorphism detection
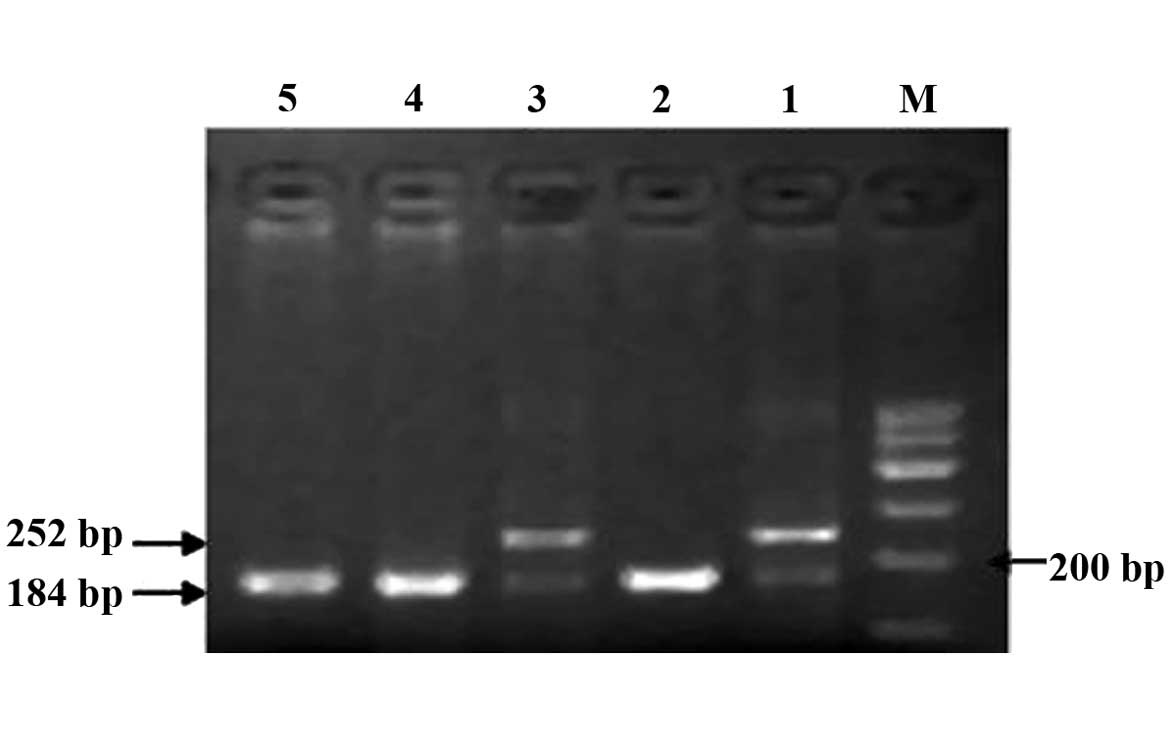
The CBS844ins68 gene polymorphism is shown in
Fig. 1. The detection of the PCR
products of CBS844ins68 obtained two genotypes: Homozygous
wild-type deletion/deletion (DD) (184 bp) and heterozygous genotype
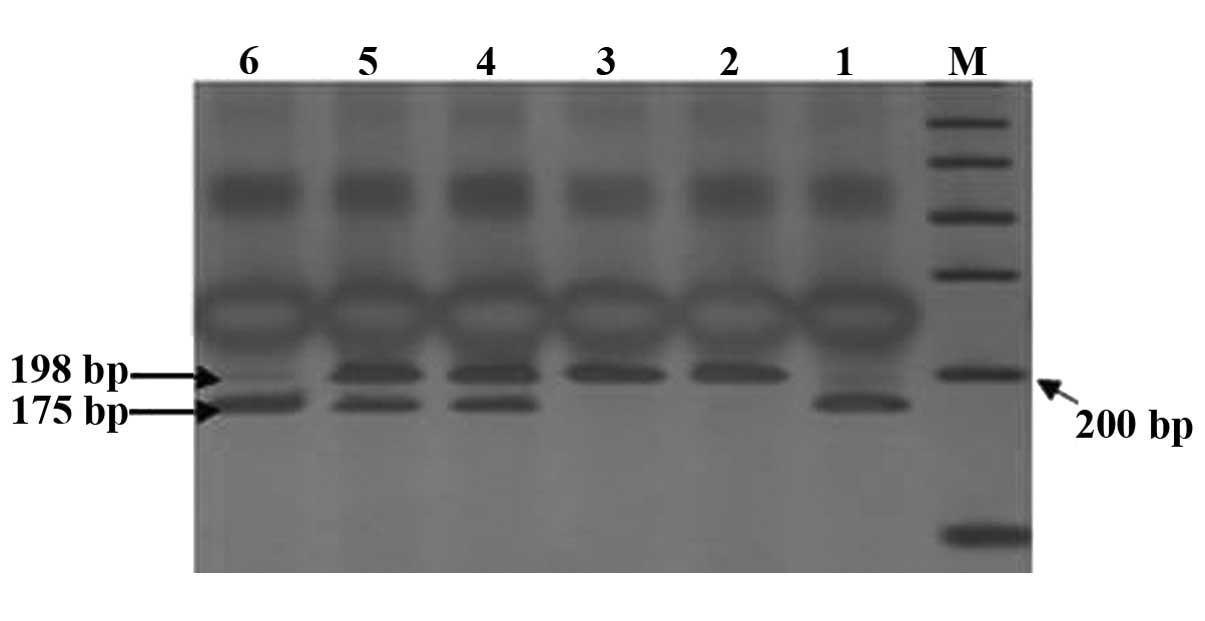
deletion/insertion (DI) (184 and 252 bp). Through digestion, the
cleavage products of MTHFR C677T detected three genotypes:
Wild-type CC (198 bp), heterozygous genotype CT (198, 175 and 23
bp, but only 198 and 175 bp were visible on the imager; since the
cleavage product of the gene was extremely small, 23 bp was not
visible when visualized), homozygous mutant genotype TT (175 and 23
bp, but only 175 bp was visible) (Fig.
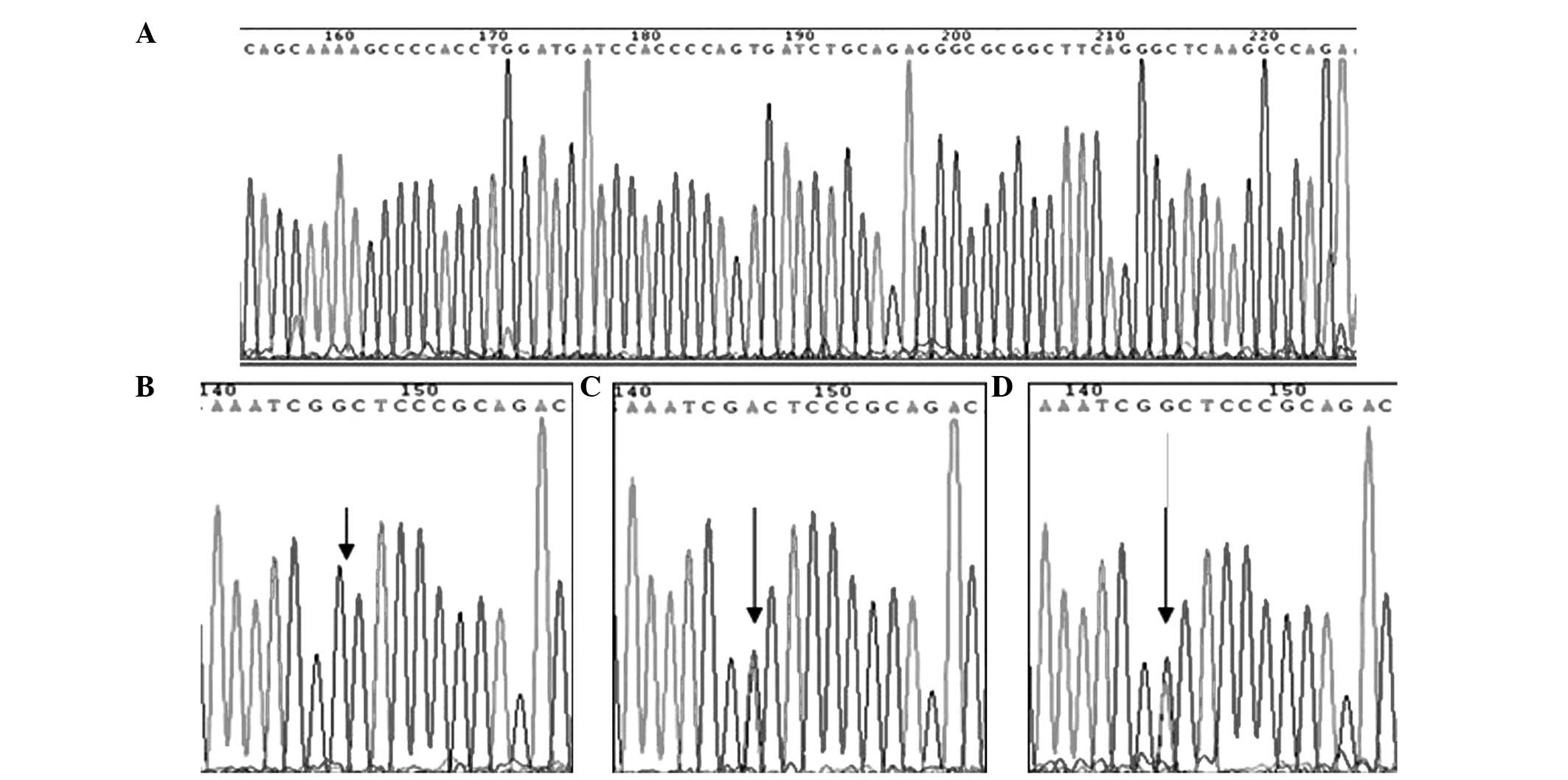
2). The sequencing results are shown in Fig. 3.
As shown in Table
IV, there was no significant difference (P>0.05) between the
CBS844ins68 genotype and allele frequency distribution. In
the two groups, the MTHFR C677T gene had three genotypes;
CC, CT and TT. The three genotype frequencies in the EH group were
19.5, 49.5 and 31% and in the NT group were 32.5, 44.5 and 25%,
respectively. There were differences in the overall genotype and
allele frequency distribution in the two groups. The risk of
suffering from EH for the CT+TT genotype is 1.812 times higher than
that of the CC genotype, and this was statistically significant
(P<0.05), as shown in Table V.
This indicates that the MTHFR C677T polymorphism is
associated with the development of EH and that the T allele may be
present in the genetic susceptibility genes.
 | Table IVComparison of the CBS844ins68
genotypes and alleles in the EH and NT groups. |
Table IV
Comparison of the CBS844ins68
genotypes and alleles in the EH and NT groups.
| Genotypes, n
(%) | Alleles, n (%) |
|---|
|
|
|
|---|
| Groups | DD | DI | II | D | I |
|---|
| EH (n=200) | 191 (95.5) | 9 (4.5) | 0 (0) | 391 (97.75) | 9 (2.25) |
| NT (n=200) | 188 (94.0) | 12 (6.0) | 0 (0) | 388 (97.00) | 12 (3.00) |
| χ2 | | | 0.452 | | 0.440 |
| P-value | | | >0.05 | | 0.05 |
 | Table VComparison of the MTHFR C677T
genotypes and alleles in the EH and NT groups. |
Table V
Comparison of the MTHFR C677T
genotypes and alleles in the EH and NT groups.
| Genotype, n
(%) | Alleles, n (%) |
|---|
|
|
|
|---|
| Groups | CC | CT | TT | C | T |
|---|
| EH (n=200) | 39 (19.5) | 99 (49.5) | 62 (31.0) | 177 (44.25) | 223 (55.75) |
| NT (n=200) | 61 (30.5) | 89 (44.5) | 50 (25.0) | 211 (52.75) | 189 (47.25) |
| χ2 | | | 6.658 | | 5.785 |
| P-value | | | <0.05 | | <0.05 |
Associations between the two genotype and
Hcy level
The level of Hcy had no significant difference in
CBS844ins68 DD and DI type (P>0.05) and there was a
significant difference in the MTHFR C677T CC, CT and
TT-types (P<0.05), as shown in Table VI.
 | Table VIComparison of the two genotypes and
Hcy levels. |
Table VI
Comparison of the two genotypes and
Hcy levels.
| Genotype | No. | Hcy, μmol/l | P-value |
|---|
|
CBS844ins68 | DD | 379 | 13.20±6.32 | >0.05 |
| DI | 21 | 12.98±4.85 | |
| MTHFR
C677T | CC | 100 | 11.54±4.63 | |
| CT | 188 | 13.86±7.04 | <0.05 |
| TT | 112 | 13.55±5.87 | <0.05 |
Analysis of MTHFR C677T, CBS844ins68
combined genotype
The two genes were composed of six types of joint
genotypes: CC/DD, CC/DI, CT/DD, CT/DI, TT/DD and TT/DI. As
CBS844ins68 type II was not detected, the CC/II, CT/II and
TT/II combined genotype were not found. Through the analysis, the
frequency distribution of the CC/DD gene type in EH was lower than
that of the NT group, and the difference was statistically
significant (P<0.05, Table
VII). OR, 0.569 showed that the CC/DD gene type was a
protective factor of hypertension, and the frequency distribution
of the remaining joint genotype between the two groups showed no
significant difference (P>0.05).
 | Table VIIDistribution of MTHFR C677T
and CBS844ins68 combined genotype frequency. |
Table VII
Distribution of MTHFR C677T
and CBS844ins68 combined genotype frequency.
| MTHFR
C677T |
CBS844ins68 | EH (n=200) (%) | NT (n=200) (%) | χ2 | P-value | OR (95% CI) |
|---|
| CC | DD | 37 (18.5) | 57 (28.5) | 5.563 | 0.018a | 0.569
(0.356–0.912) |
| CC | DI | 2 (1.0) | 4 (2.0) | 0.677 | 0.411 | 0.495
(0.090–2.733) |
| CT | DD | 95 (47.5) | 85 (42.5) | 1.010 | 0.315 | 1.224
(0.825–1.816) |
| CT | DI | 4 (2.0) | 4 (2.0) | 0.000 | 1.000 | 1.000
(0.247–4.055) |
| TT | DD | 59 (29.5) | 46 (23.0) | 2.182 | 0.140 | 1.401
(0.895–2.193) |
| TT | DI | 3 (1.5) | 4 (2.0) | 0.145 | 0.703 | 0.746
(0.165–3.378) |
For the T-test statistical analysis, the joint
genotype CC/DD, CT/DD, TT/DD and TT/DI of Hcy levels were
significantly different in the two groups (P<0.05; Table VIII) and Hcy levels in CC/DI and
CT/DI genotypes showed no statistically significant difference
(P>0.05).
 | Table VIIILevel of plasma Hcy in MTHFR
C677T and CBS844ins68 combined genotype. |
Table VIII
Level of plasma Hcy in MTHFR
C677T and CBS844ins68 combined genotype.
| MTHFR
C677T |
CBS844ins68 | EH-Hcy
(μmol/l) | NT-Hcy
(μmol/l) | T | P-value |
|---|
| CC | DD | 13.28±6.67 | 10.44±2.37 | 2.94 | 0.004a |
| CT | DD | 17.14±8.33 | 10.16±1.87 | 7.55 | 0.003a |
| TT | DD | 16.04±6.84 | 10.43±1.84 | 5.40 | 0.002a |
| CC | DI | 10.20±1.98 | 11.83±2.74 | −0.73 | 0.506 |
| CT | DI | 17.80±7.10 | 10.60±2.24 | 1.93 | 0.101 |
| TT | DI | 17.73±2.45 | 9.53±2.07 | 4.82 | 0.005a |
MTHFR C677T, CBS844ins68 polymorphism in
LD, analysis of LD
Use of the SHEsis software analysis showed D′=0.216
and r2=0.02. D′<0.2 was considered to have no LD and
D′>0.5 with LD, where r2 represents the correlation
coefficient. The results indicate that MTHFR C677T and
CBS844ins68 have a weak linkage.
MTHFR C677T and CBS844ins68 gene
polymorphism in different countries and regions
In this study, the CBS844ins68 genotype and
allele frequencies of the North Xinjiang Han population were
compared to various countries. There were a significant difference
in the CBS844ins68 genotype and allele frequencies of the
North Xinjiang Han population and Italy, Japan, Australia, Beijing
of China (P<0.05, Table IX),
but no significant difference was apparent for Germany and the
Czech Republic (P>0.05). As shown in Table X, a comparison of the MTHFR
C677T polymorphism in populations of various countries and
regions was performed. There were a significant difference in the
MTHFR C677T polymorphism and Australia, the United Kingdom,
India, Turkey (P<0.05), but there was no statistically
significant difference in South Korea (P>0.05). When compared to
domestic Tianjin, Hebei, Yunnan, Shandong and Hainan, there was a
statistical significance (P<0.05), and the difference was more
clear in Northern compared to Southern China.
 | Table IXCBS844ins68 gene polymorphism
in different countries and regions. |
Table IX
CBS844ins68 gene polymorphism
in different countries and regions.
| | Genotype, n
(%) | Allele, n (%) | | |
|---|
| |
|
| | |
|---|
| Countries | No. | DD | DI | II | D | I | χ2 | P-value |
|---|
| Germany | 229 | 207 (90.4) | 22 (9.6) | 0 (0.0) | 436 (95.2) | 22 (4.8) | 1.825 | 0.177 |
| Pakistan | 872 | 754 (86.5) | 115 (13.2) | 3 (3.4) | 1623 (93.1) | 121 (6.9) | 8.672 | 0.003a |
| Italy | 102 | 88 (86.3) | 14 (13.7) | 0 (0.0) | 190 (93.1) | 14 (6.9) | 4.893 | 0.027b |
| Czech | 400 | 356 (89.0) | 44 (11.0) | 0 (0.0) | 756 (94.5) | 44 (5.5) | 3.746 | 0.053 |
| Japan | 393 | 393 (100.0) | 0 (0.0) | 0 (0.0) | 786 (100.0) | 0 (0.0) | 23.800 | 0.001a |
| Australia | 186 | 150 (80.6) | 34 (18.3) | 2 (1.1) | 334 (89.8) | 38 (10.2) | 16.570 | 0.002a |
| China |
| Beijing | 375 | 366 (97.6) | 9 (2.4) | 0 (0.0) | 741 (98.8) | 9 (1.2) | 4.715 | 0.030b |
| Han in
Xinjiang | 200 | 188 (94.0) | 12 (6.0) | 0 (0.0) | 388 (97.0) | 12 (3.0) | | |
 | Table XMTHFR C677T polymorphism in
different countries and regions. |
Table X
MTHFR C677T polymorphism in
different countries and regions.
| | Genotype, n
(%) | Allele, n (%) | | |
|---|
| |
|
| | |
|---|
| Countries | No. | CC | CT | TT | G | T | χ2 | P-value |
|---|
| Australia | 393 | 175 (44.5) | 183 (46.6) | 35 (8.9) | 533 (67.8) | 253 (32.2) | 25.720 | 0.001a |
| America | 898 | 402 (44.8) | 407 (45.3) | 89 (9.9) | 1211 (67.4) | 585 (32.6) | 30.880 | 0.003a |
| Korea | 1700 | 540 (31.8) | 863 (50.8) | 297 (17.5) | 1943 (57.1) | 1457 (42.9) | 2.818 | 0.093 |
| India | 103 | 85 (82.5) | 18 (17.5) | 0 (0.0) | 188 (91.3) | 18 (8.7) | 89.670 | 0.003a |
| Turkey | 45 | 5 (11.1) | 16 (35.6) | 24 (53.3) | 26 (28.9) | 64 (71.1) | 16.750 | 0.002a |
| China |
| Tinjing | 932 | 199 (21.4) | 450 (48.3) | 283 (30.4) | 848 (45.5) | 1016 (54.5) | 6.970 | 0.008a |
| Hebei | 475 | 172 (36.2) | 223 (46.9) | 80 (16.8) | 567 (59.7) | 383 (40.3) | 5.543 | 0.019 |
| Yunnan | 124 | 53 (42.7) | 52 (41.9) | 19 (15.3) | 158 (63.7) | 90 (36.3) | 7.500 | 0.006a |
| Shangdong | 1052 | 154 (14.6) | 469 (44.6) | 429 (40.8) | 777 (36.9) | 1327 (63.1) | 35.200 | 0.004a |
| Hainan | 3016 | 1763 (58.5) | 1061 (35.2) | 192 (6.4) | 4587 (76.0) | 1445 (24.0) | 107.400 | 0.003a |
| Han in
Xinjiang | 200 | 61 (30.5) | 89 (44.5) | 50 (25.0) | 211 (52.8) | 189 (47.3) | | |
Discussion
Using the Hardy-Weinberg equilibrium, the MTHFR
C677T and CBS844ins68 genotypes were shown to achieve a
genetic balance in the Han population with regard to the
distribution frequency, which had a group representation. Analysis
of the clinical data in the EH and NT groups (400 cases were
selected) is shown in Table I.
There were no significant differences (P>0.05) between the two
groups for age, urea, creatinine, uric acid, glucose, HDL-C, apo A,
apo B and Lpa, but for systolic blood pressure, diastolic blood
pressure, body mass index, TG, TC and LDL-C, EH was significantly
higher compared to the NT group (P<0.05). The plasma Hcy levels
in EH were significantly higher compared to those in the NT group
(P<0.05) (Table II) and between
males and females there were no significant differences (P>0.05)
(Table III).
The results of the χ2 test and
correlation analysis showed that gene polymorphisms of the Hcy
metabolism enzymes MTHFR C677T and CBS844ins68 were
present in the North Xinjiang Han population. This has relevance in
MTHFR C677T polymorphism and susceptibility of EH, and
significant differences were found in the allele and genotype
frequencies between the two groups (P<0.05), as shown in
Table V. The relative risk of
suffering from EH in the CT+TT genotype was 1.812-fold higher than
that of the CC genotype (OR, 1.812; 95% CI, 1.142–2.874), and the
results of the C allele relative risk analysis were OR, 1.407 and
95% CI, 1.065–1.858. These results indicate that the changes
observed for the MTHFR C677T gene polymorphism are one of
the genetic factors of EH, thus the T allele may be a risk factor.
No correlation was found between the CBS844ins68 gene
polymorphism and alleles in the Xinjiang Han patients (Table IV). Thus, I allele is probably not
a predisposing factor. Analysis of the single-nucleotide
polymorphism using factors including sample selection, sample size
and ethnicity showed that a larger cohort of samples and the
application of different ethnicities in case-control studies are
required to improve the understanding of EH pathogenesis.
In the present study, the Hcy levels in two points
genotypes were analyzed. The results showed that the level of Hcy
had no significant difference in CBS844ins68 DD and DI types
(P>0.05), and there was a significant difference in the MTHFR
C677T CC, CT and TT-types (P<0.05), as shown in Table VI.
The two points genotypes were composed of six kinds
of joint genotypes: CC/DD, CC/DI, CT/DD, CT/DI, TT/DD and TT/DI;
and as CBS844ins68 type II was not detected, the CC/II,
CT/II and TT/II combined genotype were not found. Through the
analysis, the frequency distribution of the CC/DD gene type in EH
was lower than that of the NT group, the difference was
statistically significant (P<0.05) and OR, 0.569 showed that the
CC/DD gene type was a protective factor of hypertension (Table VII). The frequency distribution of
the remaining joint genotype between the two groups showed no
significant difference (P>0.05). Joint genotype CC/DD, CT/DD,
TT/DD and TT/DI of the Hcy levels were significantly different in
the two groups (P<0.05) and Hcy levels in the CC/DI and CT/DI
genotypes showed no statistically significant difference
(P>0.05) (Table VIII). This
indicates that the combined genotypes, CC/DD, CT/DD, TT/DD and
TT/DI, may affect Hcy levels and further affect the occurrence and
development of EH.
In addition, the present study compared the
CBS844ins68 polymorphisms and allele frequencies in the
populations of various countries and regions. Table IX shows that the CBS844ins68
genotype and allele frequencies of the North Xinjiang Han
population were significantly different compared to Italy (14), Japan (15), Australia (7), Beijing of China (16) (P<0.05); and no significant
difference (P>0.05) was apparent for Germany (17) and the Czech Republic (18). A comparison of the MTHFR
C677T polymorphism in populations of various countries and
regions was performed (Table X). In
comparison to Australia (7), the
United Kingdom (19), India
(20) and Turkey (21) the difference was statistically
significant (P<0.05), but there was no statistically significant
difference in South Korea (22)
(P>0.05). When compared to domestic Tianjin, Hebei, Yunnan,
Shandong and Hainan (5) there was a
statistical significance (P<0.05), and the difference was more
clear in Northern compared to Southern China. Therefore, the gene
polymorphisms of the Hcy metabolism enzymes, MTHFR and
CBS, and allele frequencies exist in different ethnicities
and nationalities. This difference may affect certain MTHFR
C677T and CBS844ins68 gene polymorphism-associated
diseases.
In conclusion, the preliminary results of the
present study show the single-nucleotide polymorphisms of the Hcy
metabolism enzymes, MTHFR C677T and CBS844ins68,
exist in the Han population. The CBS844ins68 gene
polymorphism may not be associated with the development of EH,
however the MTHFR C677T gene polymorphism may influence the
level of Hcy and is closely associated with the occurrence of EH.
The T allele may be a genetic susceptible gene of EH. However, the
exact mechanism of EH in the Xinjiang Han and other populations
remains to be determined in future studies.
Acknowledgements
The present study was supported by the National High
Technology Research and Development Program (‘863’ Program; grant
no. 2011AA02A111).
References
|
1
|
Petramala L, Acca M, Francucci CM and
D’Erasmo E: Hyperhomocysteinemia: a biochemical link between bone
and cardiovascular system diseases? J Endocrinol Invest. 32(Suppl
4): 10–14. 2009.PubMed/NCBI
|
|
2
|
Lu D and Lu XC: Research progress of
homocysteine and its metabolic enzyme gene polymorphisms and
cardiovascular disease. Lab Med Clin. 9:844–845. 2012.(In
Chinese).
|
|
3
|
Zhang L, Yin RX, Liu WY, Miao L, et al:
Association of methylenetetrahydrofolate reductase C677T
polymorphism and serum lipid levels in the Guangxi Bai Ku Yao and
Han populations. Lipids Health Dis. 9:1232010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fowdar JY, Lason MV, Szvetko AL, Lea RA,
et al: Investigation of homocysteine-pathway-related variants in
essential hypertension. Int J Hypertens 2012.
1909:232012.PubMed/NCBI
|
|
5
|
Yang B, Liu Y, Li Y, Fan S, et al:
Geographical distribution of MTHFR C677T, A1298C and MTRR A66G gene
polymorphisms in China: findings from 15357 adults of Han
nationality. PLoS One. 8:e579172013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alghasham A, Settin AA, Ali A, Dowaidar M,
et al: Association of MTHFR C677T and A1298C gene polymorphisms
with hypertension. Int J Health Sci (Qassim). 6:3–11. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lucock M, Yates Z, Martin C, Choi JH, et
al: Hydrogen sulphide-related thiol metabolism and nutrigenetics in
relation to hypertension in an elderly population. Genes Nutr.
8:221–229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yakub M, Moti N, Parveen S, Chaudhry B, et
al: Polymorphisms in MTHFR, MS and CBS genes and homocysteine
levels in a Pakistani population. PLoS One. 7:e332222012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li AF, Zheng H, Xu YM, Zhao XJ, et al: The
association between gene polymorphisms of homocysteine
metabolism-related enzymes and ischemic cerebrovascular diseases in
Chinese Henan Han population. Life Sci J. 9:403–408. 2012.
|
|
10
|
The National Blood Pressure Survey
Cooperative Group. Prevalence and variation trends of hypertension
in China. J Hypertens. 23(S1)1995.(In Chinese).
|
|
11
|
Kawamura H, Jumabay M, Mitsubayashi H,
Izumi Y, et al: 24-hour blood pressure in Uygur, Kazakh and Han
elderly subjects in China. Hypertens Res. 23:177–185. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Whitworth JA; World Health Organization,
International Society of Hypertension Writing Group. 2003 World
Health Organization (WHO)/International Society of Hypertension
(ISH) statement on management of hypertension. J Hypertens.
21:1983–1992. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang K, Fu DJ, Julien D, Braun A, et al:
Chip-based genotyping by mass spectrometry. Proc Natl Acad Sci USA.
96:10016–10020. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Olivieri O, Friso S, Trabetti E, Girelli
D, et al: Homocysteine and atheromatous renal artery stenosis. Clin
Exp Med. 1:211–218. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Le Marchand L, Donlon T, Hankin JH,
Kolonel LN, Wilkens LR and Seifried A: B-vitamin intake, metabolic
genes, and colorectal cancer risk (United States). Cancer Causes
Control. 13:239–248. 2002.PubMed/NCBI
|
|
16
|
Bi XH, Zhao HL, Zhang ZX, Liu Q, et al:
Association analysis of CbetaS 844ins68 and MTHFD1 G1958A
polymorphisms with Alzheimer’s disease in Chinese. J Neural Transm.
117:499–503. 2010.PubMed/NCBI
|
|
17
|
Ott N, Geddert H and Sarbia M:
Polymorphisms in methionine synthase (A2756G) and cystathionine
beta-synthase (844ins68) and susceptibility to carcinomas of the
upper gastrointestinal tract. J Cancer Res Clin Oncol. 134:405–410.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Orendác M, Musková B, Richterová E,
Zvárová J, et al: Is the common 844ins68 polymorphism in the
cystathionine beta-synthase gene associated with atherosclerosis? J
Inherit Metab Dis. 22:674–675. 1999.PubMed/NCBI
|
|
19
|
Mitrou PN, Watson MA, Loktionov AS, et al:
MTHFR (C677T and A1298C) polymorphisms and risk of sporadic distal
colorectal adenoma in the UK Flexible Sigmoidoscopy Screening Trial
(United Kingdom). Cancer Causes Control. 17:793–801. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Poduri A, Mukherjee D, Sud K, Kohli HS, et
al: MTHFR A1298C polymorphism is associated with cardiovascular
risk in end stage renal disease in North Indians. Mol Cell Biochem.
308:43–50. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Onrat ST, Akci O, Söylemez Z, Onrat E and
Avşar A: Prevalence of myocardial infarction polymorphisms in
Afyonkarahisar, Western Turkey. Mol Biol Rep. 39:9257–9264. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cui LH, Shin MH, Kweon SS, Kim HN, Song
HR, et al: Methylenetetrahydrofolate reductase C677T polymorphism
in patients with gastric and colorectal cancer in a Korean
population. BMC Cancer. 10:2362010. View Article : Google Scholar : PubMed/NCBI
|