Introduction
The association between epilepsy and fear has
attracted increasing attention alongside epilepsy research. Fear
has been described as a crucial symptom of epilepsy, even in the
oldest Chinese medical book. A large number of clinical cases of
patients with temporal lobe epilepsy seizures often have
inexplicable fear attack as the aura (1). Numerous patients reported the emotion
of fear prior to seizures (2).
Mesial temporal lobe epilepsy is commonly associated with ictal
fear (3), and temporal lobectomy
patients showed a general impairment in fear conditioning (4,5).
However, the association between seizure-modulated fear and the
underlying basics of neuronal mechanisms remains unclear.
Recent studies (6)
identified stathmin as one of the key controlling molecules in
learning and innate fear. Stathmin-knockout mice exhibit a
decreased memory in amygdala-dependent fear conditioning and fail
to recognize danger in innately aversive environments (7). The stathmin protein binds to tubulin
and inhibits microtubule assembly and promotes microtubule
catastrophes (8,9). Overexpression of stathmin can lead to
disassembly of microtubules (10).
Therefore, stathmin is predicted to play a crucial
role in associating the two processes; epilepsy seizures and fear
conditioning. In the present study, the expression of stathmin in
the epileptic rats was analyzed in detail with fear to confirm this
hypothesis.
Materials and methods
Animals and epilepsy model
Adult male Sprague-Dawley rats (180–220 g) were used
in the study. All the experimental procedures were approved by the
Animal Research Committee of the Ningxia Medical University
(Ningxia, China) and performed in accordance with the Chinese
Animal Welfare Act for the use and care of laboratory animals. Rats
were divided into four groups at random: Normal control (control),
fear conditioning (fear), epilepsy (epilepsy) and epilepsy treated
with fear groups (epilepsy + fear). The rats with epilepsy were
triggered with pilocarpine and raised for 30 days after the
epilepsy seizure. Each group comprised of six rats.
The epileptic model was established with
LiCl/pilocarpine as previously described (10). The rats were injected
intraperitoneally (IP) with LiCl (127 mg/kg) on day 1 and
administered pilocarpine injection (30 mg/kg) 18–24 h later.
Epileptic seizures of racine Ⅳ-V grade were chosen for the
experiments. Epileptic rats were administered chloral hydrate (300
mg/kg) injection IP after 1 h of seizures to suppress the seizures.
The normal control rats were administered the saline injection,
respectively.
Fear conditioning and freezing
time
The rats were placed in the conditioning chamber
(23×23×35 cm) for 5 min before the loudspeaker stimulus. A sonic
wave (1 min, 1,000 Hz, 75 Db) was delivered through the loudspeaker
as the conditioned stimulus. Electrical foot shock (3 sec, 1 mA)
stimulation occurred from the metal grid floor as the unconditioned
stimulus. The context was a 1 min tone followed by the 3 sec foot
shock, which was repeated 30 times (Fig. 1A). Following training in the
chamber, the rats were returned to their home cages. The training
was performed in 4 consecutive days. The rats of the fear and the
epilepsy treated with fear groups underwent this training
procedure. The control and the epilepsy groups had the same
conditioned stimulus of the tone, but without the unconditioned
electric stimulation at the foot shock.
Freezing time was used as an index of fear
conditioning. Freezing was defined as immobility, excluding
respiratory movements with a freezing posture. Rats remained still,
sluggish, curled or crouched whilst breathing, and had a slight
rocking motion. The total time of the freezing posture was called
the freezing time and was measured starting from day 5 of training.
The freezing time was measured immediately after the tone
stimulation (without foot shock) and within 30 min. The behavioral
activities were recorded by video.
Serum corticosterone (COR) and
C-reactive protein (CRP) radioimmunoassay
The rats were anaesthetized and subsequentely
decapitated to collect the whole blood with centrifuge tubes. Blood
was incubated at 37̊C for 30 min. Serum was collected after 10 min
centrifugation at 1,688 x g. Serum COR and CRP concentrations were
measured by the radioimmunoassay kits, following the manufacturer's
instructions. COR and hs-CRP radioimmunoassay kits were purchased
from the Beijing Huaying Biotechnology Research Institute (Beijing,
China). The radioimmunoassay was analyzed on the BN II Nephelometer
(Dade Behring Marburg GmbH, Marburg, Germany) analyzer.
Brain tissue preparation,
immunohistochemistry and western blotting
Brains were dissected and fixed routinely. A
stainless steel rat brain matrix (175–300 g, coronal; RWD Life
Science Co., Ltd., Shenzhen, China) was used to define the brain
tissue blocks, including the hippocampus and insular cortex,
respectively. A total of 15-µm coronal serial sections were
prepared routinely. The boundary of the hippocampus and insular
cortex was defined in accordance with the atlas of Paxinos and
Watson (11). Ten sections,
including the hippocampus or insular cortex, from each animal were
chosen.
Immunohistochemistry was performed routinely. The
stathmin protein 1 antibody (1:80; Proteintech, Inc., Chicago, IL,
USA) and microtubule-associated protein (MAP2) antibodies (1:100;
Bioworld, Visalia CA, USA) were used. The avidin-biotin kit
(Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China)
was used for the second antibody incubation and
3,3′-diaminobenzidine staining. All the sections were
counterstained with hematoxylin. The
immunohistochemistry-positivity of the images was assessed semi
quantitatively by automatically measuring the optical
densities.
Western blotting was performed routinely. Antibodies
were used at the following concentrations: Stathmin 1 (1:300; cat
no. ab47328); phosphorylated stathmin 1 (1:500; cat no. ab47398;
Abcam, Cambridge, MA, USA); microtubule-associated protein (1:800;
cat no. 17490-1-AP; Bioworld); α-tubulin (1:5,000; cat no.
66031-1-Ig Proteintech); GAPDH (1:5,000; cat no. TA-08; Bioss,
Beijing, China); and horseradish peroxidase-conjugated goat
anti-rabbit immunoglobulin G (cat no. ZB-2301; ZSGB-BIO, Beijing,
China). Quantization of relative band densities was performed by
scanning densitometry.
Statistical analysis
All the data were processed using SPSS 17.0 software
(SPSS, Inc., Chicago, IL, USA). Mean ± standard deviation that the
measurement data using one-way ANOVA; same object point in time
measurement data using a repeated measures analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Fear is strengthened following
epilepsy
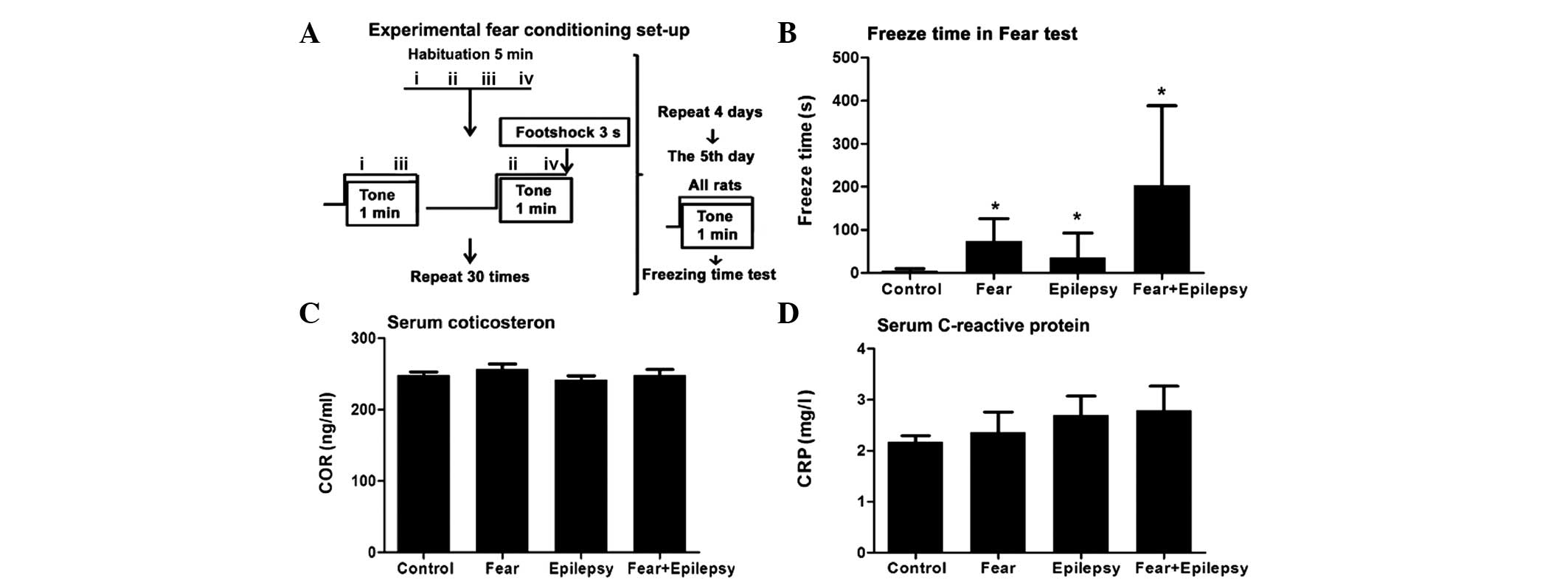
Rats (n=6) in the normal control exhibited an
extremely short freezing time, from 2 to 14 sec, with an average of
5 sec. Whereas the epileptic rats (n=6, and epilepsy after 30 days)
had a longer freezing time, from 10 to 152 sec, with an average of
36 sec, which was ~5–6-fold longer compared to the normal control
(Fig. 1B).
Rats with fear training by tone and foot shock
(fear) only (n=6) exhibited an increased freezing time immediately
following the shock, from 15 to 152 sec, with an average of 74 sec.
This was ~10–12-fold longer compared to the control and ~2-fold
longer compared to the epilepsy group. Therefore, the contextual
fear has occurred in these rats (F=4.521, P<0.05).
The rats of the epilepsy + fear (n=6) group had the
longest freezing time among the four groups. The average freezing
time was 203 sec, which was approximately three times higher
compared to the fear group. As these rats have experienced
epileptic seizures during the past 30 days, the increased freezing
time revealed a significantly strengthened effect of epileptic
seizures on the learned fear of the tone-shock contextual. These
results indicate that these rats not only have the memory regarding
the context in which they received the electrical stimulation, but
have also developed a strong aversive response to the tone and the
environment associated with a painful experience.
Systemic stress responses increase
accompanied epilepsy and fear
Psychological processes, such as fear, are always
accompanied with systemic stress responses. A systemic stress
response includes objective signs of stress, such as high serum
levels of adrenocorticotropic and cortisol, elevation of
immunoreactions and an increase in arterial blood pressure
(12). In the present study, the
serum COR and inflammatory biomarker, CRP, were chosen as the
biological indices of psychological stress caused by the fear.
The COR and CRP levels of the fear and the epilepsy
+ fear rats elevated when compared to the control. However, there
was no statistical significance when compared to the control
(Fig. 1C and D).
Epilepsy and fear increase the
expression of stathmin
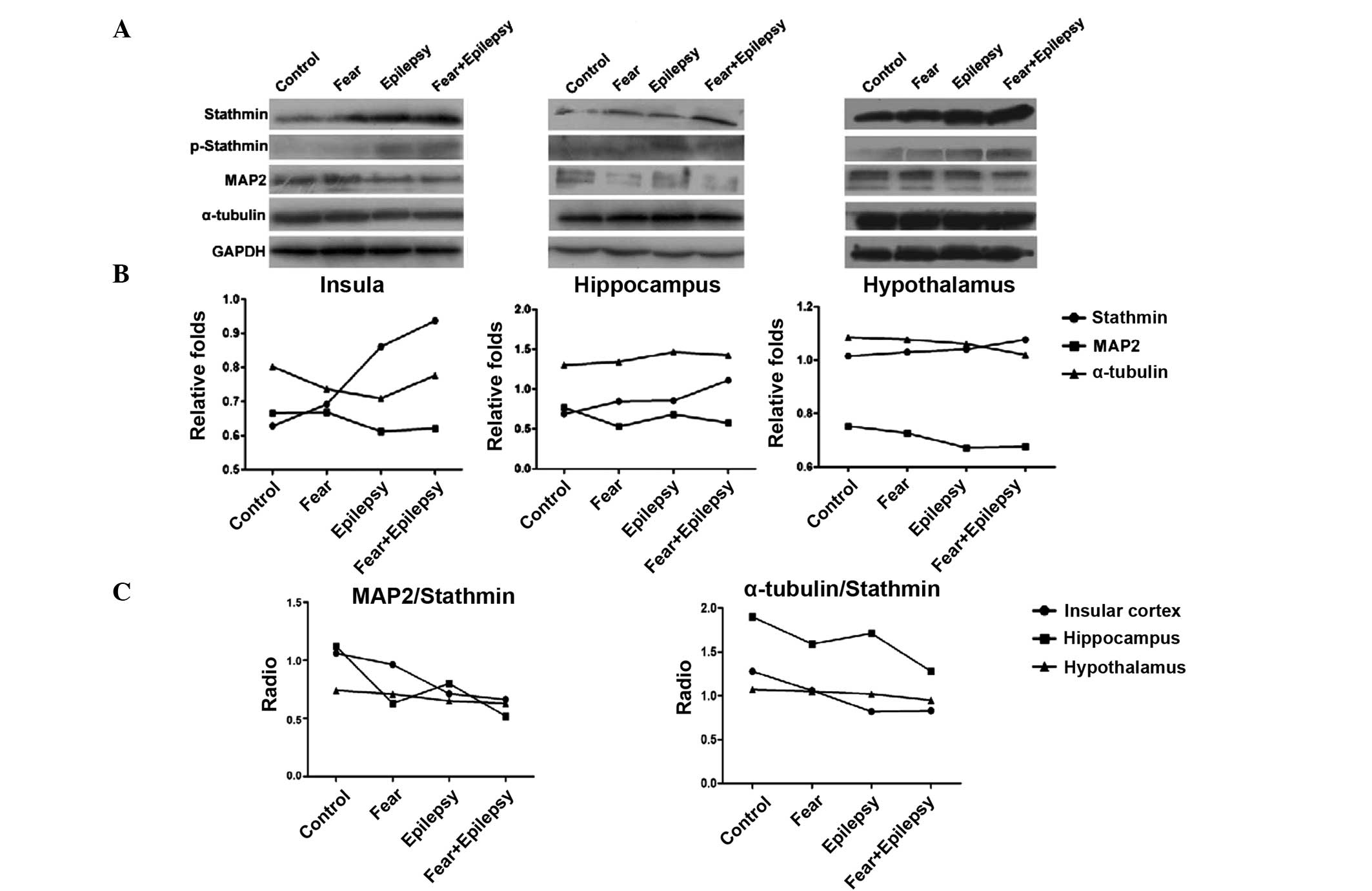
According to the results of the western blotting, as
shown in Fig. 2, the expression of
stathmin in the three areas of the brain increased in a similar
sequence. The highest expression of stathmin was observed in the
epilepsy + fear rats. Fear conditioning (the fear group) increased
the expression of stathmin slightly, whereas the epilepsy + fear
treatment increased it dramatically. The stathmin expression level
of the epilepsy group was also significantly increased, more than
that of the fear group, particularly in the insular cortex and
hypothalamus, but less in the hippocampus (insula F=75.863,
P<0.05; hippocampus F=36.698, P<0.05; and hypothalamus
F=150.761, P<0.05).
Stathmin is a phospho-protein that binds to tubulin
and regulates microtubule dynamics. The binding of stathmin to
tubulin and its ability to disrupt the microtubules is modulated
through its phosphorylation on multiple serine residues. These
microtubule destabilizing activities are suppressed by
phosphorylation of stathmin (9).
Therefore, the expression levels of phosphorylated stathmin were
examined by western blotting. The phosphorylated stathmin increased
slightly in the epilepsy + fear conditioning rats, in all three
areas of the brain. The increasing effects on phosphorylated
stathmin were stronger in the epilepsy group compared to the fear
group, suggesting that phosphorylated stathmin was more sensitive
in the epileptic brain to the contextual stimulus of tone.
Western blotting analysis revealed that the
expression of MAP2 decreased inversely with stathmin in the
epilepsy and the fear groups in all three areas of the brain. The
most significant decrease was observed in the epilepsy + fear group
(Fig. 2). The expression of
α-tubulin was not significantly changed in all the groups and in
all three areas of the brain.
The microtubule stability was indicated by the ratio
of MAP2 and α-tubulin/stathmin. In all three areas of the brain
tested, epilepsy + fear had the lowest ratio, indicating that the
microtubule stability was lowest. The most evident change of this
ratio occurred in the insular cortex, instead of the hippocampus
(Fig. 2).
Stathmin expression peaks at 30 days
after epileptic seizures
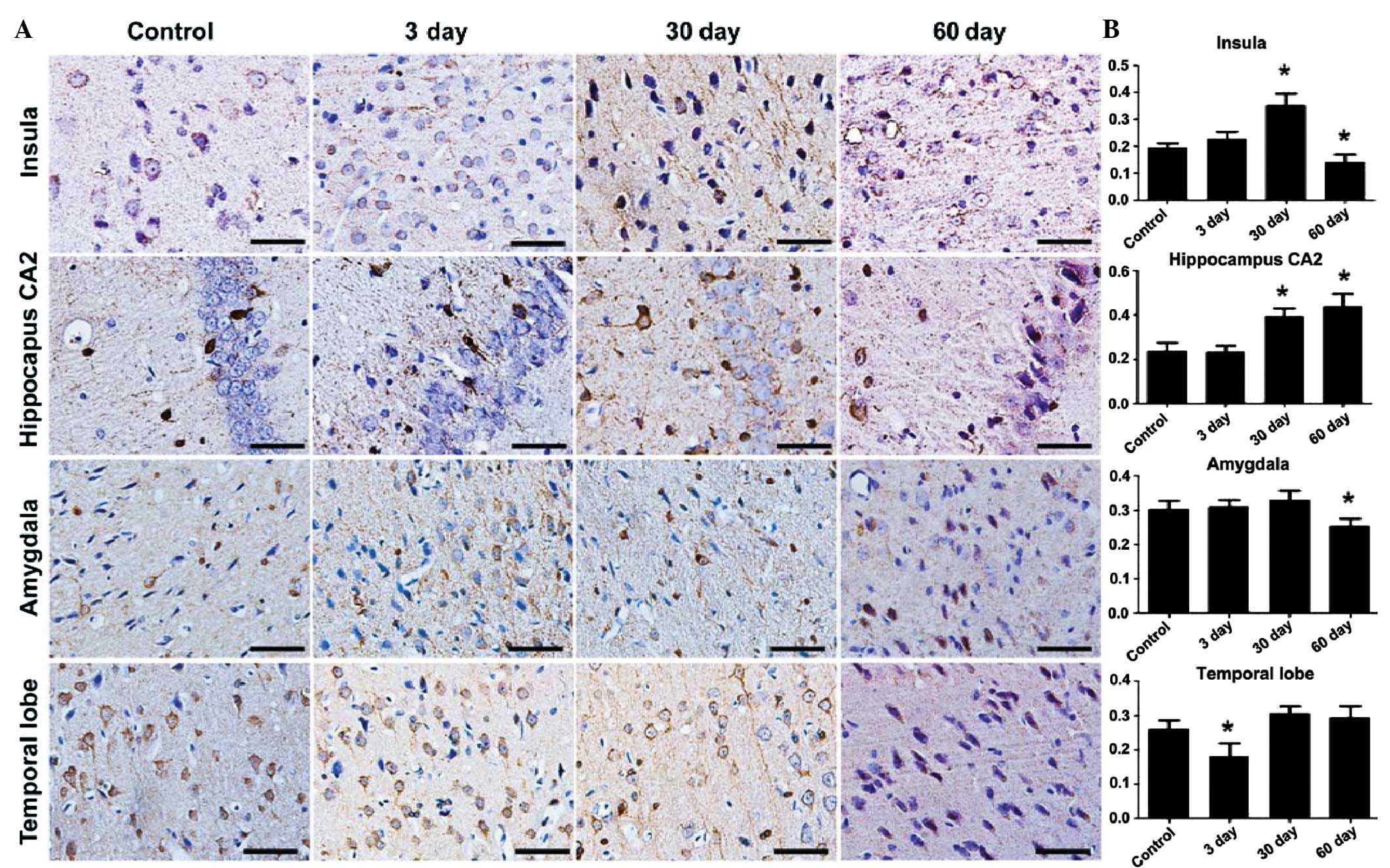
To further analyze the variation of stathmin
expression during the development of epilepsy, the
immunohisotochemical patterns in the temporal cortex and the
hippocampus were examined at 3, 30 and 60 days after the epileptic
seizures. The stathmin expression started to increase at 3 days and
reached its peak after 30 days of epilepsy in the local areas of
the insular cortex. The reinforced stathmin expression in the
dendrites was even more noticeable in the local areas of the
insular, which was located at the IV layer of the cortex. Evident
variation patterns were also observed in the hippocampus CA2
region, but not in the amygdale and temporal lobe cortex (Fig. 3). Stathmin expression declined to
the normal control level in 60 days, in all the areas tested
(insula F=52.018, P<0.05; hippocampus F=42.074, P<0.05;
amygdala F=12.699, P<0.05; and temporal lobe F=21.469,
P<0.05).
Discussion
Epileptic seizures can lead to psychological
deficits, including learning and memory deficits, fear, language
impairment and depression. In certain temporal lobe epileptic
patients, intense ictal fear was the main feature of epileptic
seizures (14). The present study
confirmed that epilepsy can strongly aggravate or strengthen the
fear conditioning and makes the epileptic rats more sensitive to
the fear stimulation, as demonstrated by a longer freezing
time.
Stathmin is essential for regulation of the innate
and learned fear. Those rats with a longer freezing time exhibited
a higher expression level of stathmin. Epilepsy alone, without the
fear conditioning, has certain increasing effects on stathmin
expression, which reached its peak at 30 days after seizure,
particularly in the insular instead of the hippocampus.
Microtubules and cytoskeleton play crucial roles
during the neuronal processes of epilepsy and fear (15). Stathmin strongly increases the minus
end catastrophe frequency and induces rapid treadmilling of bovine
brain microtubules (16). The MAP2
expression decreased inversely with the increase of stathmin. This
result indicates that the microtubule stability plays a crucial
role in the epilepsy associated fear conditioning.
The insular lobe has also been shown to generate
interictal and ictal discharges in the majority of temporal lobe
epileptic patients analyzed with depth electrodes positioned in
this area (17). In the present
study, the most evident changes of stathmin expression occurred in
the insular cortex and the hippocampus, instead of the amygdale.
This result suggests that the insular cortex may play a more
important role in the correlation of fear and epilepsy.
In conclusion, epilepsy can strongly aggravate the
sensation of fear, as exhibited by a longer freezing time. This is
evidenced by an increase in stathmin expression and an inverse
decrease of microtubule stability, particularly in the insular
cortex.
Acknowledgements
The present study was supported by grants from the
Chinese National 973 Projects (nos. 2011CB512115 and 2012CB722408)
to Tao Sun, the National Natural Science Foundation of China (nos.
30960150 and 31260246) to Qikuan Hu, the Natural Science Foundation
of Ningxia (no. NZ12172) to Linna Zhang and the Funds of Ningxia
Medical University (no. XT200909) to Qikuan Hu.
References
|
1
|
Chiesa V, Gardella E, Tassi L, Canger R,
Lo Russo G, Piazzini A, Turner K and Canevini MP: Age-related
gender differences in reporting ictal fear: analysis of case
histories and review of the literature. Epilepsia. 48:2361–2364.
2007.PubMed/NCBI
|
|
2
|
Ryan S and Räisänen U: ‘The brain is such
a delicate thing’: an exploration of fear and seizures among young
people with epilepsy. Chronic Illn. 8:214–224. 2012. View Article : Google Scholar
|
|
3
|
Reynders HJ, Broks P, Dickson JM, Lee CE
and Turpin G: Investigation of social and emotion information
processing in temporal lobe epilepsy with ictal fear. Epilepsy
Behav. 7:419–429. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weike AI, Hamm AO, Schupp HT, Runge U,
Schroeder HW and Kessler C: Fear conditioning following unilateral
temporal lobectomy: dissociation of conditioned startle
potentiation and autonomic learning. J Neurosci. 25:11117–111124.
2005. View Article : Google Scholar
|
|
5
|
Hlobil U, Rathore C, Alexander A, Sarma S
and Radhakrishnan K: Impaired facial emotion recognition in
patients with mesial temporal lobe epilepsy associated with
hippocampal sclerosis (MTLE-HS): Side and age at onset matters.
Epilepsy Res. 80:150–157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang L.-Ν..LI H.-Υ..Tao H..HU Q.-Κ.:
Expression of stathmin gene in brain of epilepsy rats. ShanDong
Med. 41:24–28. 2012.(In Chinese).
|
|
7
|
Shumyatsky GP, Malleret G, Shin RM,
Takizawa S, Tully K, Tsvetkov E, Zakharenko SS, Joseph J, Vronskaya
S, Yin D, Schubart UK, Kandel ER and Bolshakov VY: Stathmin, a gene
enriched in the amygdala, controls both learned and innate fear.
Cell. 123:697–709. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Niethammer P, Bastiaens P and Karsenti E:
Stathmin-tubulin interaction gradients in motile and mitotic cells.
Science. 303:1862–1866. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Curmi PA, Gavet O, Charbaut E, Ozon S,
Lachkar-Colmerauer S, Manceau V, Siavoshian S, Maucuer A and Sobel
A: Stathmin and its phosphoprotein family: general properties,
biochemical and functional interaction with tubulin. Cell Struct
Funct. 24:345–357. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cassimeris L: The oncoprotein 18/stathmin
family of microtubule destabilizers. Curr Opin Cell Biol. 14:18–24.
2002. View Article : Google Scholar
|
|
11
|
Glien M, Brandt C, Potschka H, Voigt H,
Ebert U and Löscher W: Repeated low-dose treatment of rats with
pilocarpine: low mortality but high proportion of rats developing
epilepsy. Epilepsy Res. 46:111–119. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paxinos G and Watson C: The Rat brain in
Stereotaxic Coordinates. 3rd. Academic Press; Sydney: pp. 51–73.
2007
|
|
13
|
Dockray S and Steptoe A: Positive affect
and psychobiological processes. Neurosci Biobehav Rev. 35:69–75.
2010. View Article : Google Scholar
|
|
14
|
Biraben A, Taussig D, Thomas P, Even C,
Vignal JP, Scarabin JM and Chauvel P: Fear as the main feature of
epileptic seizures. J Neurol Neurosurg Psychiatry. 70:186–191.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gardiner J and Marc J: Disruption of
normal cytoskeletal dynamics may play a key role in the
pathogenesis of epilepsy. Neuroscientist. 16:28–39. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Manna T, Thrower D, Miller HP, Curmi P and
Wilson L: Stathmin strongly increases the minus end catastrophe
frequency and induces rapid treadmilling of bovine brain
microtubules at steady state in vitro. J Biol Chem. 281:2071–2078.
2006. View Article : Google Scholar
|
|
17
|
Isnard J, Guénot M, Sindou M and Mauguière
F: Clinical manifestations of insular lobe seizures: a
stereo-electroencephalographic study. Epilepsia. 45:1079–1090.
2004. View Article : Google Scholar : PubMed/NCBI
|