Introduction
Su Xiao Jiu Xin dripping pill (SXJXDP) is a
traditional Chinese medicine (TCM), which is widely used to treat
cardiovascular and cerebrovascular diseases. SXJXDP is composed of
Chuanxiong (Rhizoma Chuanxiong) and Bing pian
(Borneolum) (1).
Phytochemical studies have shown that SXJXDP contains numerous
types of compounds, such as borneol and isoborneol from
Borneolum, lactones (ligustilide and
n-butylphthalide) and organic acids (ferulic acid) from
Rhizoma Chuanxiong (2,3).
Pharmacological studies have also revealed that major active
components, including borneol, isoborneol, ligustilide,
n-butylphthalide and ferulic acid, are implicated in the
treatment of cardiovascular and cerebrovascular diseases (4–7).
Therefore, an efficient method should be developed to detect these
compounds absorbed in the buccal mucosa.
SXJXDP is sublingually administered and can be
absorbed by the rich blood flow in the buccal mucosa. As a
non-keratinising epithelial tissue, the buccal mucosa provides a
number of advantages in drug permeability, prevents first-pass
metabolism in the liver exhibiting good compliance depending on a
particular drug and contains efficient enzymatic flora (8,9).
Therefore, the characteristic of drug absorption in the human
buccal mucosa should be determined to provide relevant guidelines.
However, to the best of our knowledge, the release pattern and
absorption characteristics of the main bioactive components of
SXJXDP in the human buccal mucosa have not yet been reported.
In the present study, borneol, isoborneol,
n-butylphthalide and ligustilide were detected by gas
chromatography-mass spectrometry (GC-MS). Ferulic acid was analysed
by high-performance liquid chromatography (HPLC). The circulating
system involved in the drug absorption in the buccal mucosa was
observed to investigate the absorption characteristics of the five
main bioactive components, which are associated with cardiovascular
and cerebrovascular diseases. Furthermore, an absorption curve was
illustrated and permeability coefficient was calculated. The study
aimed to establish efficient methods and explain the absorption
characteristics of SXJXDP.
Materials and methods
Materials
The standard compounds of borneol, isoborneol,
ligustilide, n-butylphthalide and ferulic acid were
purchased from the National Institutes for Food and Drug Control
(Beijing, China). HPLC-grade acetonitrile, n-hexane and
dichloromethane were purchased from Oceanpak Alexative Chemical
(Gothenburg, Sweden). Pure water was purchased from Wahaha Group
Co. (Hangzhou, China). SXJXDP was purchased from the No. 6
Pharmaceutical Factory of Tianjin Zhongxin Pharmaceutical Co.
(Tianjin, China). Six healthy volunteers underwent training and
signed an informed consent prior to the experiment. Our study was
approved by the ethics comitee of Tanjin University of Traditional
Chinese Medicine. These volunteers gargled pure water prior to the
experiment. The presence or absence of adverse reactions was
closely observed during the process and whether the mucosa remains
normal or becomes abnormal following the completion of the
experiment was assessed.
In total, 15 pills were dissolved in 50 ml purified
water and added to the circulating fluid storage cup of the
circulating devices. The buccal mucosa devices used in the
experiment were prepared in accordance with previously described
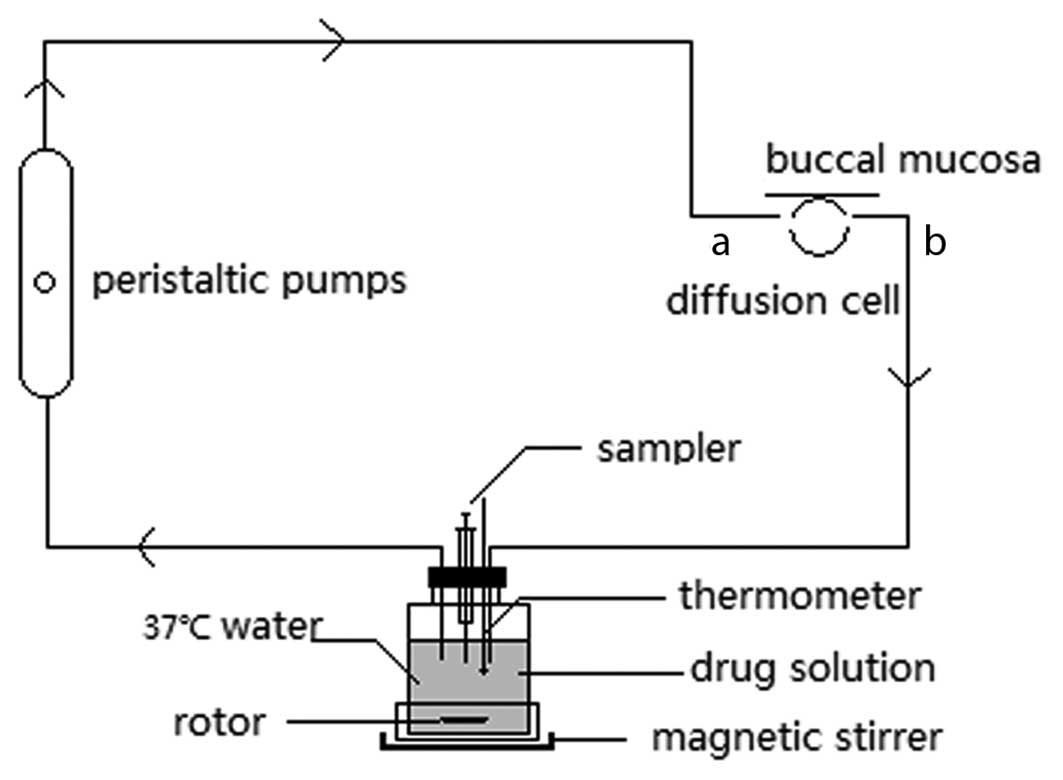
methods (10,11). Fig.
1 illustrates the diffusion cell used in the study. This
diffusion cell is composed of polytetrafluoroethylene and comprises
two sides; a-side functions as an inlet of circulating fluid and
b-side serves as an outlet of circulating fluid (10,11).
The circulating system was equipped with a magnetic stirrer and
performed under the following conditions: A suitable modulation
speed and solution temperature of 37°C. At 0, 1, 3, 5, 10, 20, 30,
45, 60 and 90 min, 300 µl of the solution was obtained. Subsequent
to performing the sampling, the same volume of blank solution was
immediately injected as a supplementary sample.
Chromatography
Borneol, isoborneol, ligustilide and
n-butylphthalide were analysed using a GC-MS-QP-2010 SE
(Shimadzu, Japan) with a column Rtx-5MS type at an inlet
temperature of 280°C, an ion source temperature of 200°C, a flow
rate of 1 ml/min helium carrier gas and an injection volume of 1 µl
in a splitless mode.
Borneol and isoborneol samples were prepared under
the following chromatographic conditions. In brief, 50 µl sample
was obtained and 300 µl n-hexane were added. Subsequently,
dichloromethane (9:1 v/v) liquid-to-liquid extraction was performed
by vortexing for 1 min, and the vortexed extract was centrifuged at
9,168 x g for 5 min. An aliquot of the supernatant was subsequently
obtained. The temperature program started at 70°C for 2 min and was
increased at a rate of 5°C/min until 120°C was reached. Temperature
was ramped at 50°C/min until a final temperature of 280°C was
achieved. Temperature was maintained constantly at 280°C for 2
min.
Ligustilide and n-butylphthalide samples were
prepared under the following chromatographic conditions. In brief,
100 µl sample was obtained and 100 µl n-hexane was added.
Subsequently, dichloromethane (9:1 v/v) liquid-to-liquid extraction
was performed by vortexing for 1 min. The vortexed extract was
centrifuged at 9,168 x g for 5 min. An aliquot of the supernatant
was subsequently obtained. The temperature program started at 70°C
for 2 min and was increased at a rate of 5°C/min until 120°C was
reached. Temperature was ramped at 50°C/min until a final
temperature of 300°C was reached, and was subsequently maintained
constantly at 300°C for 2 min.
Ferulic acid sample was prepared under the following
chromatographic conditions. In brief, 100 µl sample was filtered
using a 0.45 µm membrane and injected into HPLC-LC-20AT (Shimadzu,
Japan). HPLC was performed in a Diamonsil C18 column (4.6×250 mm, 5
µm) with a mobile phase of methanol: 1% acetic acid aqueous
solution (42:58 v/v) at a flow rate of 1 ml/min. The column
temperature was maintained at 25°C and the injection volume was 20
µl. The wavelength of the detector was set at 321 nm.
The cumulative absorption rate was calculated to
evaluate drug absorption and was obtained using the following
equation: (F0 -
Ft)/F0 × 100%, where
Ft is the liquid concentration at time t
and F0 is the initial concentration of the
solution.
The permeability coefficient was calculated to
evaluate drug permeability. Permeability coefficient was obtained
using the following equations: J = dQ/dt =
P × (Cd - Cr) ≈ P
× Cd, P = J/Cd × 60,
where Q is the drug cumulative infiltration capacity in
µg/cm2; t is the time in min; P is the
permeability coefficient in cm2/s; and
Cd and Cr are the drug
concentrations of the supply cell and the accepting cell in µg/ml,
respectively. Additionally, Cd >>
Cr.
Under these conditions, three sensitive and reliable
analytical methods were developed to simultaneously quantify
borneol, isoborneol, ligustilide and n-butylphthalide by
GC-MS and analyse ferulic acid by HPLC. The representative
chromatograms of the compounds indicated that the developed method
was free of any evident interference.
Results and Discussion
GC-MS and HPLC methods were validated in terms of
linearity, precision, accuracy, stability and recovery. Calibration
curves were constructed at five different concentration levels from
the following concentration ranges: 2–200 µg/ml for borneol; 2–400
µg/ml for isoborneol; 1–100 µl/ml for ligustilide; 0.1–15 µl/ml for
n-butylphthalide; and 1.081–16.215 µg/ml for ferulic acid.
Each calibration curve was obtained from five different
concentrations of the five bioactive analytes by linear regression
analysis. The obtained correlation coefficient values (r>0.999)
indicated good correlations between the concentrations and their
peak responses within the test ranges. Stability test results
showed that the processed samples could be re-injected after 0, 2,
4, 8 and 12 h; furthermore, the relative standard deviation (RSD)%
of the stabilities of these five bioactive analytes ranged,
1.11–8.71%, which was within acceptable ranges. The precision of
these methods was determined by repeated injections (n=6) of the
standard solutions; the accuracy of these methods was determined by
injections (n=6) of equally treated samples. The RSD% values of the
precision and the accuracy of five bioactive analytes ranged,
1.12–5.01% and 1.85–8.71%, respectively. The recoveries of the
GC-MS analytes were compared to a standard solution following
extraction using known concentrations at three different levels.
The recoveries of the HPLC analytes were determined by spiking the
samples with known amounts of the standard solution at three
different concentration levels. The recoveries of the five analytes
were as follows: 88.96–91.63% for borneol; 86.27–87.84% for
isoborneol; 84.67–89.25% for ligustilide; 84.72–88.99% for
n-butylphthalide; and 100.07–103.77% for ferulic acid.
Overall, the recoveries of each of the analytes at three different
concentrations were consistent and >80%. These results
demonstrated that all the values were present in permissible ranges
and the methods were accurate (Table
I).
 | Table IResult of the method validation. |
Table I
Result of the method validation.
| Calibration
curve | | | | Recovery |
|---|
|
| Precision, | Accuracy, | Stability, |
|
|---|
| Compound | Regression
equation | r | RSD% | RSD% | RSD% | Low | Med | High |
|---|
| Borneol |
y=1.311×105x−3.329×104 | 0.9998 | 3.58 | 2.44 | 2.63 | 88.96 | 91.63 | 90.61 |
| Isoborneol |
y=4.148×104x+3.203×105 | 0.9994 | 4.54 | 2.95 | 2.46 | 86.80 | 87.84 | 86.27 |
| Ligustilide |
y=2.594×104x−2.317×104 | 0.9998 | 5.01 | 4.86 | 8.71 | 88.77 | 89.25 | 84.67 |
|
n-butylphthalide |
y=1.631×105x−8.477×103 | 0.9996 | 2.73 | 8.71 | 3.86 | 87.50 | 88.99 | 84.72 |
| Ferulic acid |
y=1.112×105x−3.830×103 | 0.9998 | 1.12 | 1.85 | 1.11 | 103.77 | 100.32 | 100.07 |
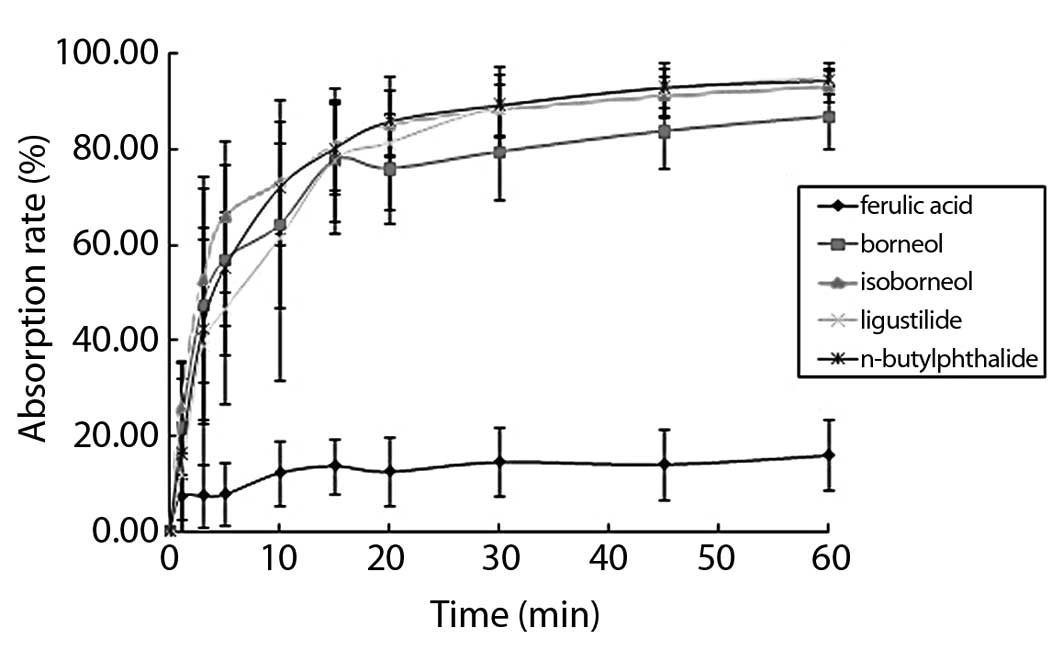
The buccal mucosa absorption curve showed that the
five types of components reached a plateau in 10–20 min. During
this period, the maximum cumulative absorption rate was almost
obtained. Subsequently, the cumulative absorption rate was
significantly decreased. The cumulative absorption rates of
borneol, isoborneol, ligustilide and n-butylphthalide showed
that the maximum accumulation in 60 min were 86.70, 93.16, 94.25
and 93.16%, respectively.
The accumulation of the four components was almost
complete. In the buccal mucosa absorption curve of ferulic acid in
60 min, the maximum absorption rate was 15.91% with incomplete
absorption and each component exhibited different performances
(Fig. 2).
Ferulic acid yielded the lowest permeability
coefficient; by comparison, the four other components exhibited
relatively similar permeability coefficients. However, the
penetration ability of ferulic acid in the buccal mucosa was ~10
times weaker than that of the four other components (Table II).
 | Table IIPermeability coefficient of buccal
mucosa. |
Table II
Permeability coefficient of buccal
mucosa.
| Compound | P, cm/s |
|---|
| Borneol |
1.12×10−2 |
| Isoborneol |
1.17×10−2 |
| Ligustilide |
1.45×10−2 |
|
n-butylphthalide |
1.34×10−2 |
| Ferulic acid |
1.93×10−3 |
Previous studies showed that SXJXDPs are stable
during absorption (1). This
phenomenon can account for the decrease in drug concentration in
vitro, indicating that this decrease is not caused by drug
degradation; instead, this decrease occurs as a result of drug
absorption into the bloodstream. Therefore, residual drug method
was applied in the present study to evaluate absorption.
Components undergo rapid sublingual absorption as
the buccal mucosa is extremely thin and rich in blood vessels.
These components can enter blood circulation within an extremely
short time to alleviate symptoms and provide first-aid relief. Drug
absorption occurs in two passages, via intracellular and
intercellular routes, which primarily exhibit passive diffusion
influenced by several physicochemical properties, such as lipid
solubility, dissociation degree, molecular weight and permeability
effect.
In general, drugs with low molecular weights are
easily absorbed by mucosa capillaries. The five main bioactive
components exhibit molecular weights <200 Da; as such, these
components can pass through the buccal mucosa membrane and into the
bloodstream. As a drug-promoting agent in TCM, borneol exhibits
strong absorption and permeation abilities, and can also promote
the absorption of other substances in the buccal mucosa to a
certain extent (12).
Lipophilic drugs can easily penetrate intercellular
channels filled with lipid. This penetration is possible as
sublingually administered drugs permeability coefficients closely
associated with the oil-water partition, K. K is
extremely high when fat-soluble compounds are insoluble in saliva.
By contrast, K is extremely low when compounds are strongly
hydrophilic and membrane permeability is weak. The Ideal K
ranges, 40–2,000 (13,14). Borneol, isoborneol,
n-butylphthalide and ligustilide are fat-soluble compounds;
therefore, these compounds are absorbed more efficiently and
rapidly in the buccal mucosa compared to ferulic acid. In contrast
to the four fat-soluble compounds, ferulic acid exhibits strong
hydrophilicity, poor permeation ability, poor absorption and weak
permeability in the buccal mucosa. In addition, the pKa values of
ferulic acid are 4.56 and 8.65; the main form of ferulic acid in
saliva (pH=6.6–7.1) (15) is a
mono-anion (16). Drugs are
transported across the cell membrane in an absorbable molecular
state. However, the present form of ferulic acid is one of the key
reasons of its low absorption. Therefore, drug absorption in the
buccal mucosa is an extremely complicated process.
In conclusion, the GC-MS and HPLC methods were
established in the present study to detect the bioactive components
of SXJXDP. These methods may be suitably applied to elucidate the
characteristics and permeabilities of drugs in the buccal mucosa of
healthy volunteers, as validated by selectivity, linearity,
precision and recovery test results. Novel efficient methods should
be developed to implement comprehensive quality control strategies
of drugs absorbed in the buccal mucosa.
Acknowledgements
The present study was financially supported by the
Large Variety of Technological Innovation of Su Xiao Jiu Xin
Dripping Pill (grant nos. 2011ZX09201-201 and
2011ZX09201-201-2).
References
|
1
|
Sun SR, Huang X and Zhang L: Advances in
studies on pharmacokinetics, pharmacodynamics and clinical research
of Su Xiao Jiu Xin Wan. Chin Tradit Herb Drugs. 33:89–91. 2002.
|
|
2
|
Wei Y, Huang W and Gu Y: Online isolation
and purification of four phthalide compounds from Chuanxiong
rhizoma using high-speed counter-current chromatography coupled
with semi-preparative liquid chromatography. J Chromatogr A.
1284:53–58. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alaerts G, Merino-Arevalo M, Dumarey M, et
al: Exploratory analysis of chromatographic fingerprints to
distinguish rhizoma Chuanxiong and rhizoma Ligustici.
J Chromatogr A. 1217:7706–7716. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiang L, Jiang P, Zhan C, et al: The serum
metabolomic study of intervention effects of the traditional
Chinese medicine Shexiang Baoxin Pill and a multi-component
medicine polypill in the treatment of myocardial infarction in
rats. Mol BioSyst. 8:2434–2442. 2012. View Article : Google Scholar
|
|
5
|
Diao X, Deng P, Xie C, et al: Metabolism
and pharmacokinetics of 3-n-butylphthalide (NBP) in humans: the
role of cytochrome P450s and alcohol dehydrogenase in
biotransformation. Drug Metab Dispos. 41:430–444. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feng Z, Lu Y, Wu X, et al: Ligustilide
alleviates brain damage and improves cognitive function in rats of
chronic cerebral hypoperfusion. J Ethnopharmacol. 144:313–321.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alam MA, Sernia C and Brown L: Ferulic
acid improves cardiovascular and kidney structure and function in
hypertensive rats. J Cardiovasc Pharmacol. 61:240–249. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Patel VF, Liu F and Brown MB: Modeling the
oral cavity: in vitro and in vivo evaluations of
buccal drug delivery systems. J Control Release. 161:746–756. 2012.
View Article : Google Scholar
|
|
9
|
Campisi G, Paderni C, Saccone R, Di Fede
O, Wolff A and Giannola LI: Human buccal mucosa as an innovative
site of drug delivery. Curr Pharm Des. 16:641–652. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adrian CL, Olin HB, Dalhoff K and Jacobsen
J: In vivo human buccal permeability of nicotine. Int J
Pharm. 311:196–202. 2006. View Article : Google Scholar
|
|
11
|
Zhang X, Wang L, Yang X, et al: Study on
the correlation between buccal absorption in healthy volunteers and
release rate in vitro of Glycyrrhizin in Qingyan Drop Pills. Chin
Med J Res Prac. 24:60–61. 2010.
|
|
12
|
Senel S and Hincal AA: Drug permeation
enhancement via buccal route: possibilities and limitations. J
Control Release. 72:133–144. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yuji K and Toshikiro K: Drug absorption by
oral mucosa-mechanism of absorption and promoter. Int. J Clin Exp
Med. 145-147:468–470. 1988.
|
|
14
|
Zhou Y, Li W, Chen L, Ma S, Ping L and
Yang Z: Enhancement of intestinal absorption of akebia saponin D by
borneol and probenecid in situ and in vitro. Environ
Toxicol Pharmacol. 29:229–234. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leon-Carmona JR, Alvarez-Idaboy JR and
Galano A: On the peroxyl scavenging activity of hydroxycinnamic
acid derivatives: mechanisms, kinetics, and importance of the
acid-base equilibrium. Phys Chem Chem Phys. 14:12534–12543. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bikov A, Galffy G, Tamasi L, Lazar Z,
Losonczy G and Horvath I: Exhaled breath condensate pH is
influenced by respiratory droplet dilution. J Breath Res.
6:0460022012. View Article : Google Scholar : PubMed/NCBI
|