Introduction
Atrial natriuretic peptide (ANP), which was first
extracted from rat atrial tissue by Baines et al, is now
known to be a member of the natriuretic peptide family (1). The natriuretic peptide family consists
of a family of 5 natriuretic peptides that include A-, B-, C-type
natriuretic peptide, urodilatin and Dendroaspis natriuretic
peptide (2). The pathophysiological
actions of various natriuretic peptides are mediated by three
different natriuretic peptide receptors; atrial natriuretic peptide
receptor-A-type (NPR-A), NPR-B- and NPR-C (3). ANP, an A-type natriuretic peptide, has
been reported to have a wide variety of biological functions, not
only in the circulatory system but also in the kidney and other
tissues (3). ANP acts to reduce the
water, sodium and adipose loads on the circulatory system, thereby
reducing blood pressure.
ANP receptors are distributed extensively in the
cochlea, including the strial vascularis (StV). Lamprecht and Meyer
zum Gottesberge (4) first reported
that ANP receptors are expressed in the inner ear of guinea pigs.
Previous studies have also identified ANP receptors in the inner
ear of guinea pigs and rats, and demonstrated their presence in the
StV, spiral ligament in the cochlea and the secretory epithelium of
the vestibular organ (4–10).
Studies suggest that ANP may be locally synthesized
and activates NPR-A in a paracrine/autocrine manner (4–8).
Pronounced immunoreactivity for ANP was observed in the spiral
ligament of the cochlear lateral wall. ANP has been demonstrated to
be expressed mainly in the cytoplasm of the StV marginal cells of
normal guinea pigs (11).
Epithelial sodium channel (ENaC) consists of three
different subunits: α, β and γ, with the α-subunit (α-ENaC) being
the most critical for channel functionality (12). Studies suggest that ENaC is
important for regulating sodium transport across epithelia. ENaC
has been shown to participate in transepithelial Na+
transport in epithelia of various organs, such as kidney, colon,
lung, sweat glands and salivary glands (13). ENaC is located in the apical
membrane of epithelia facing the endolymph, StV, spiral prominence
and spiral limbus in the inner ear. The endolymph in the cochlea is
characterized by a high K+ concentration of ~150 mM and
low Na+ concentration of ~1 mM. ENaC may be involved in
transepithelial Na+ transport through the apical
membrane and maintenance of a K-rich and Na-poor composition in the
endolymph (14).
ANP may participate in the regulation of the
water-electrolyte balance (15).
However, little is known regarding the exact mechanisms of ANP in
the regulation of the water-electrolyte balance in the cochlea.
Whether ANP has a regulatory effect on the ENaC in the StV remains
unknown. Reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) is a highly sensitive technology that allows
high throughput quantification of gene expression (16). In the present study, RT-qPCR was
used to evaluate whether ANP affects α-ENaC mRNA expression
in the mouse StV.
Materials and methods
Animals
Healthy adult Kunming mice were provided by the
Animal Care and Use committee at the Huaxi Medical School of
Sichuan University. The study was approved by the Animal Care and
Use Committee at the West China Medical School of Sichuan
University (Sichuan, China). The temporal bone was removed and StV
tissues were dissected at 4˚C, as previously described (17).
Tissues culture
StV tissues were dissected and rinsed in saline
solution prior to being placed in culture plates. All the
experimental specimens were cultured in Dulbecco's modified Eagle's
medium/nutrient mixture F-12 (1:1) containing 10% fetal bovine
serum, 50 mM glucose, 100 U/ml penicillin and 2 µ1/ml
insulin-transferrin selenium sodium mixture. StV tissues were
co-cultured with ANP (10−6 mol/1) in a standard cell
culture incubator at 37°C, 5% CO2 and 80% humidity for
different times (2, 4, 6, 12, 24 and 48 h).
RNA isolation and reverse
transcription
Total RNA from the tissues of the cultured StV in
the mouse cochlea was isolated using the TRIzol reagent (MRC,
Cincinnati, OH, USA). RNA was reverse transcribed into cDNA using
the RevertAid™ First Strand cDNA Synthesis kit (MBI Fermentas,
Inc., Burlington, ON, Canada) with the addition of random hexamer
primers. Briefly, 5 µ1 purified RNA was used as template for cDNA
synthesis in the presence of 1 µ1 Moloney murine leukemia virus
reverse transcriptase (200 units), 1 µ1 random hexamer primer, 4 µ1
5X reaction buffer, 2 µ1 hexamer 1X (Roche, Indianapolis, IN, USA),
2 µ1 dNTP mix (10 mM each) and 6 µ1 RNase-free water. After
incubation for 60 min at 42˚C, the reverse transcriptase was
inactivated at 70˚C for 10 min and the cDNAs were stored at −20˚C
until further analysis.
Primers
SCNN1A is a gene encoding the α-ENaC protein.
Primers were designed according to the NCBI GenBank sequences of
mice and were synthesized by the Sangon Biotech (Shanghai, China).
The primer sequences for RT-qPCR are as follows. Outer primers:
SCNN1A forward, CACAGCAGGTGT GCATT and reverse,
CAGCCTGCAGTTTATAGT. Inner primers for the second-round PCR
reaction: SCNN1A forward, CATTCACTCCTGCTTCCAG and reverse,
GTAGCAGTA GCCCCAGGAG; ACTB forward, GAAGATCAAGATCAT TGCTCCT
and reverse, TACTCCTGCTTGCTGATCCA.
First-round PCR reaction
The reaction mixture for the first-round PCR
contains 3 µ1 10X PCR buffer, 1.8 µ1 25 mM MgCl2, 11
µmol each primer (SCNN1A forward and reverse)
(Qiagen-Operon, Cologne, Germany), 0.36 µ1 deoxynucleoside
triphosphate mix, 0.3 µ1 Taq DNA polymerase, 5 µ1 DNA template and
water to a final volume of 30 µl. The first-round amplification was
carried out in a thermocycler under the following conditions: 45
cycles at 94˚C for 2 min, 94˚C for 20 sec and 54˚C for 30 sec, with
a final extension at 70˚C for 40 sec and at 70˚C for 5 min 4˚C.
Second-round PCR reaction
qPCR was used to determine the gene expression
profiles of α-ENaC. cDNA was amplified by RT-qPCR using an
FTC-2000 (Funglyn, Toronto, ON, Canada). Each analysis was
performed in a total volume of 30 µ1 reaction mixture containing 5
µ1 cDNA sample, 1 µ1 SYBR-Green I and 2 µ1 gene-specific forward
and reverse primers (10 µM each). Reference genes were included to
normalize the data. Amplifications were performed as follows: 45
cycles at 94˚C for 2 min, 94˚C for 20 sec and 54˚C for 20 sec, with
a final extension at 70˚C for 30 sec and at 80˚C for 20 sec.
The cycle threshold (Ct) number, defined as the
number of PCR amplification cycles required to reach fluorescent
intensity above the threshold, was determined for each gene and
each developmental time point was analyzed. Using serial dilutions
of the test sample cDNA, the standard curve was generated on the
basis of the linear relationship of existing Ct and the logarithm
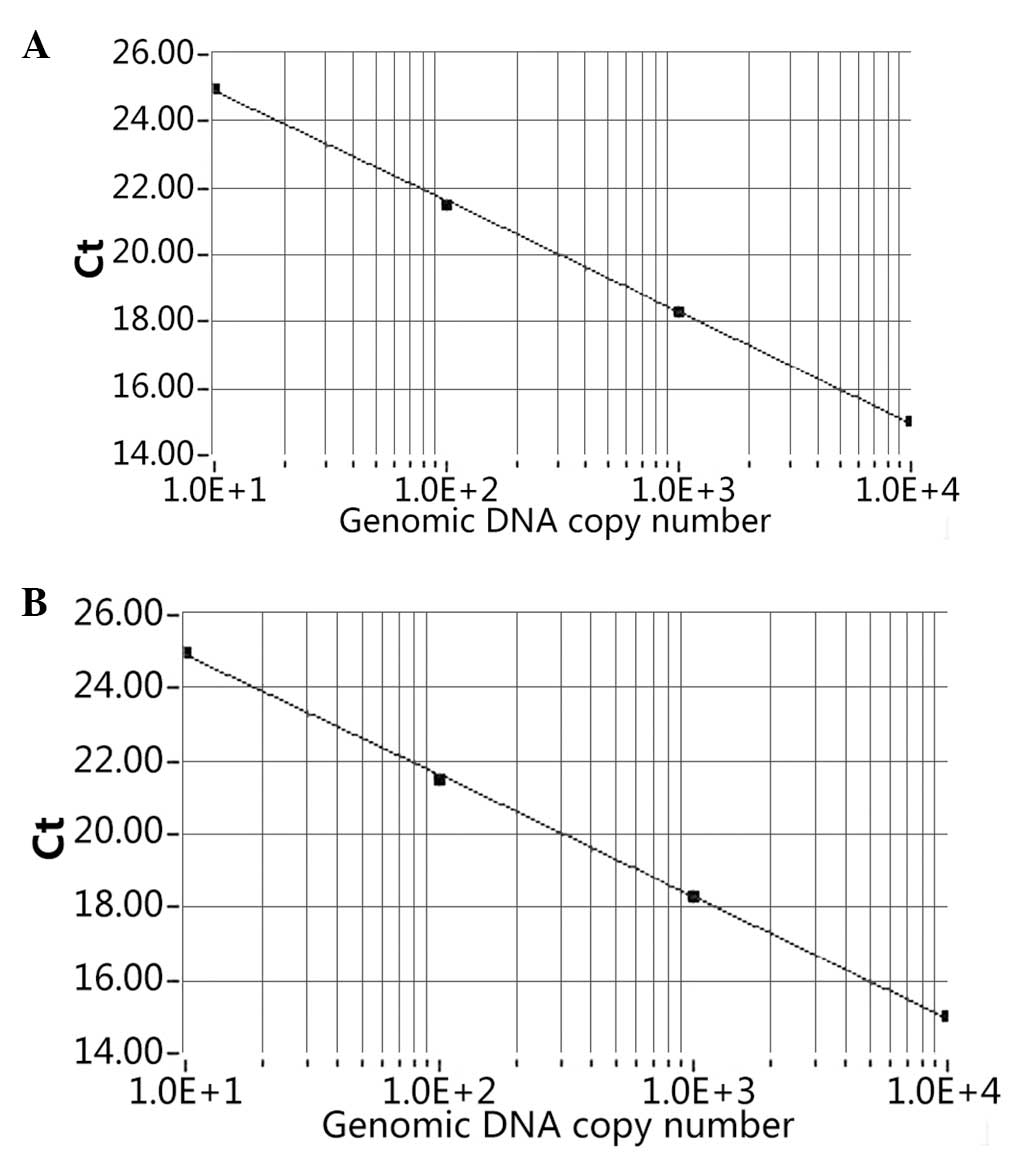
of the copy number. The SCNN1A slope of the standard curve
was shown to be −3.32 and a strong linear association was
demonstrated (R2=1.00; Fig.
1A). The ACTB standard slope of the curve was also shown
to be −3.32 and a strong linear association was demonstrated
(R2=1.00; Fig. 1B).
Analysis
Samples were normalized internally using the Ct
number of the reference gene ACTB as follows: Δ Ct (sample)
= Ct (sample)-Ct (ACTB). The mean Ct of α-ENaC in 0 h
(control) was set to a relative quantity (RQ) value of 1 using the
ΔΔ Ct, calculated as follows: ΔΔ Ct (sample) = Δ Ct (sample)-Δ Ct
(control) and RQ=2−ΔΔCt. The data is presented as mean ±
standard deviation. The one-way analysis of variance was performed
using SPSS 11.0 statistical software (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
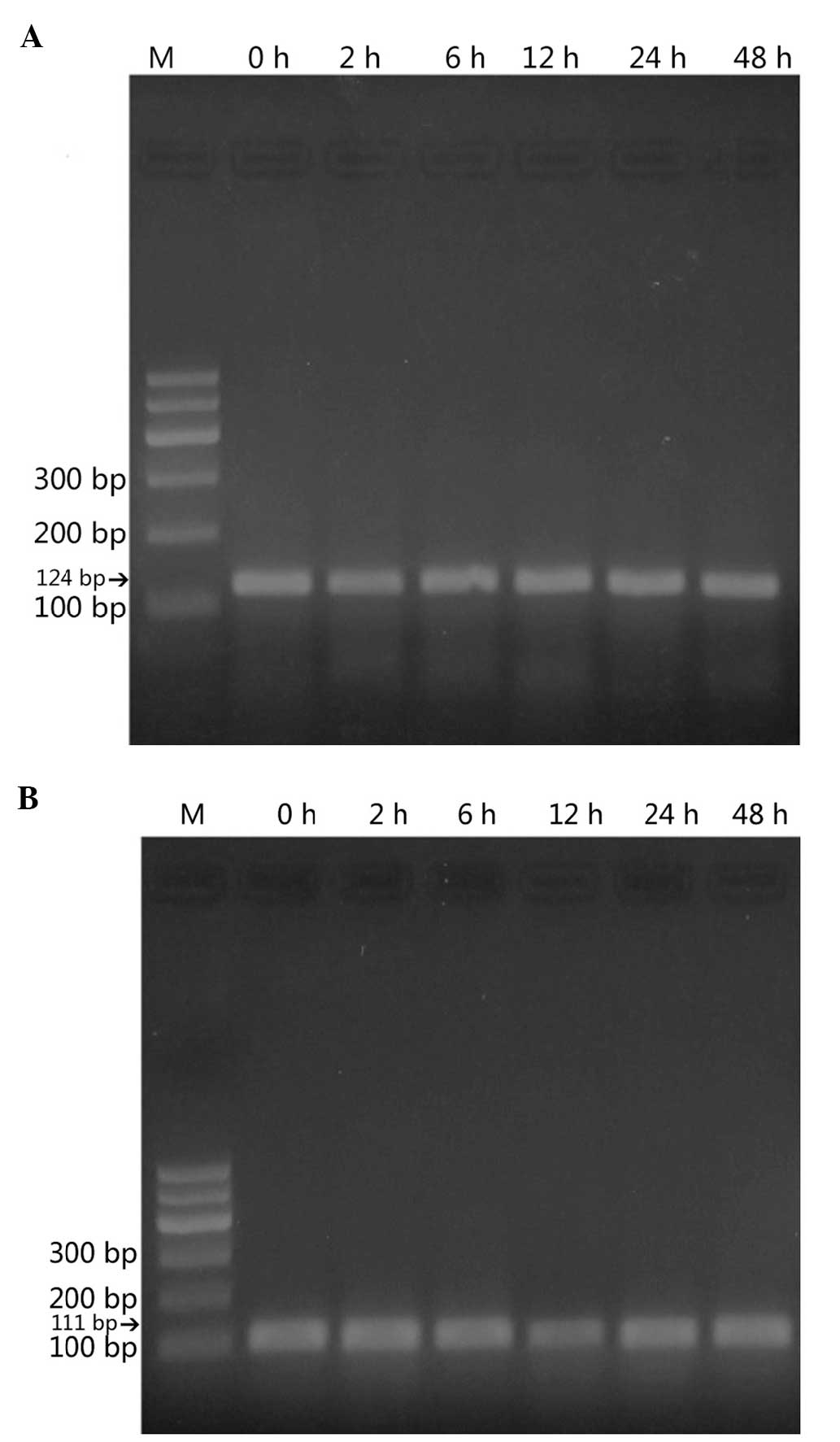
Using RT-qPCR, α-ENaC mRNA was demonstrated
to be expressed in the StV (Fig.
2). ACTB was examined as a reference gene. α-ENaC
cDNA amplified from homogenates of the amplified fragments were of
the expected sizes [124 basepairs (bp) for the α-ENaC gene
(Fig. 2A) and 111 bp for the
reference, ACTB cDNA (Fig.
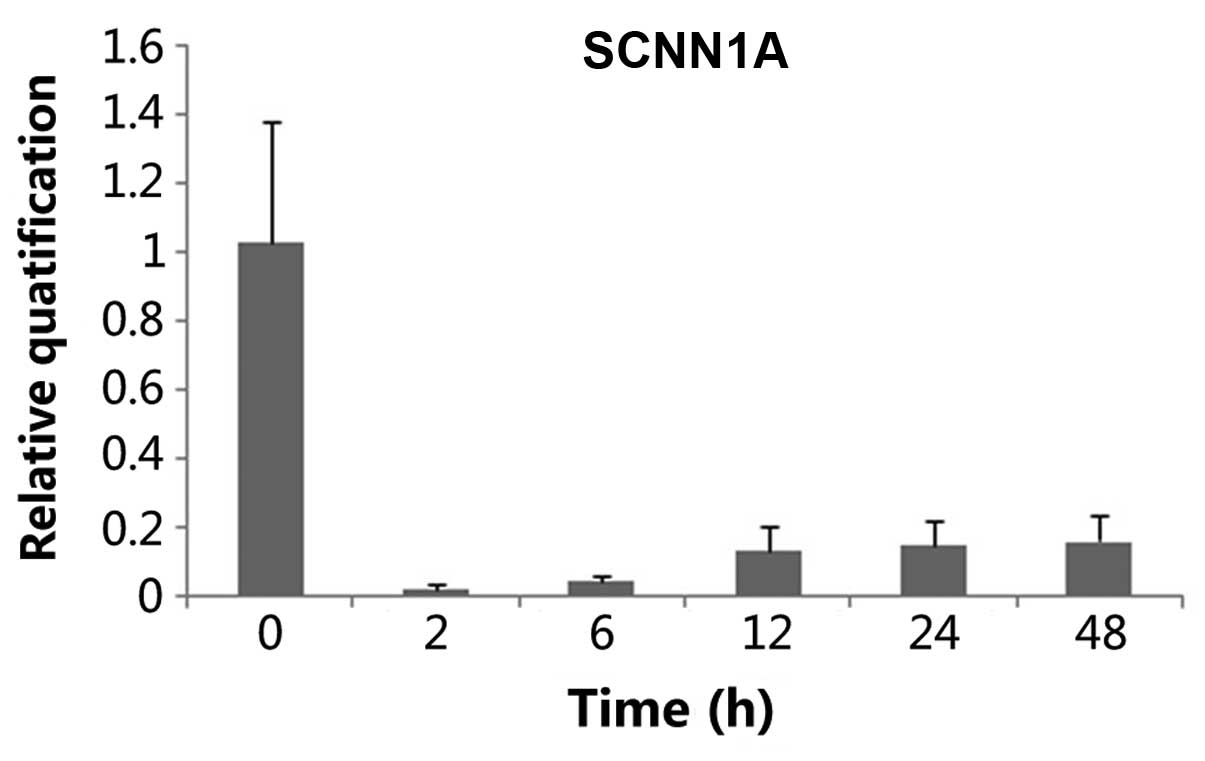
2B)]. Tissues treated with ANP (10−6 mol/1) for
different times (2, 6, 12, 24 and 48 h) showed that ANP decreased
α-ENaC mRNA expression, and at the time of 2 h a maximum
effect was reached (n=3, P<0.05) (Fig. 3). This effect was slightly reversed
after 6 h. When α-ENaC mRNA level was evaluated chronologically in
a series of cultured explants, a stability along with culture time
was noticed, after 12, 24 and 48 h respectively (Fig. 3).
Discussion
The StV is mainly responsible for generating the
high K+ and low Na+ levels in the endolymph,
which plays an important role in normal auditory function (17). The StV is bound by several types of
parasensory epithelium. Previously is has been suggested that
several different ion channels are present in these parasensory
epithelium cells, which include the IsK/KvLQT1,
Ca2+-activated nonselective cation, Cl− and
ENaC channels (12, 18). The IsK/KvLQT1 channel has been shown
to be the main pathway across the apical membrane of strial
marginal cells for secreting K+ into the lumen of the
inner ear. ENaC has been shown to participate in reabsorbing
Na+ in the apical membrane of epithelia, which is
located in the apical membrane of epithelia facing endolymph.
ENaC is critical in maintaining the sodium balance
and water-electrolyte balance. Together with basolateral
Na+/K+-ATPase, ENaC regulates sodium
reabsorption and plays a major role in the control of total body
salt and water homeostasis (12).
In numerous types of cells, it has been shown that ENaC is
regulated by a variety of extrinsic factors, such as proteolytic
cleavage, mechanical and cytoskeletal activity. ENaC has also been
shown to be under the control of different hormones, such as
aldosterone, arginine vasopressin, endothelin, insulin and ANP
(19).
We have previously shown the presence of NPR-A
transcripts in the mouse StV, as well as in the nonstrial tissue of
the cochlear lateral wall and vestibular organ by PCR (9, 10).
Gene expression of NPR-A in the StV was higher compared to
the nonstrial and vestibula (9,
10). In the present study, the
effect of ANP on the expression of the α-ENaC mRNA was
investigated in the mouse StV by the RT-qPCR technique. The study
showed that ANP significantly decreases α-ENaC mRNA
expression in the mouse StV and at the time of 2 h a maximum effect
was reached. ANP may exert its inhibitory activity on
Na+ reabsorption through suppression of ENaC gene
expression in the mouse StV.
The inhibitory effect by ANP on the α-ENaC
mRNA may reduce the clearance of Na+. Inhibition of ENaC
is expected to lead to an increase in endolymphatic Na+
concentration, subsequently resulting in an elevation in endolymph
volume. An increase of the endolymph volume may aggravate the
endolymphatic hydrops. ANP is believed to influence the ionic
homeostasis of the endolymph by regulating the ENaC in StV. This
effect may be more evident in pathological conditions, such as
severe congestive heart failure when endogenous ANP is
significantly increased.
The precise mechanisms of ANP in strial marginal
cells remain unclear. ANP was shown to mediate the majority of its
biological functions through interaction with NPR-A via cyclic
guanosine monophosphate (cGMP), a second messenger (20). ANP is a classical inhibitor of
Na+ reabsorption induced by aldosterone at renal distal
tubule and collecting duct segments, possibly via cGMP and protein
kinase G intracellular second messengers (21–23).
Previously, it has been shown that cGMP is involved in the control
of gene expression via its action on different gene-promoter
regions or by controlling the activities of transcription factors
(24, 25).
The finding of the present study that α-ENaC
is expressed in the StV supports a functional role for ENaC
in the regulation of Na+ concentration. Although the
pathophysiological significance of the inhibition of the
α-ENaC mRNA expression by ANP in the mouse StV remains
unknown in the present study, it may take part in the modulation of
homeostasis of the endolymph. Further investigation is required to
explore the detailed molecular and cellular mechanisms by which ANP
modulates the expression of the α-ENaC mRNA in the mouse
StV.
In conclusion, to the best of our knowledge, the
present study is the first to directly access the effect of ANP on
α-ENaC mRNA expression in the mouse StV. These results
indicate that ANP has a physiological importance in the regulation
of ion transport and endolymph fluid balance in the inner ear.
Acknowledgements
The authors would like to thank Ms Yanfang Chen for
her technical assistance. The present study was supported by the
National Natural Science Foundation of China (grant no. NSFC
81271079 to Dr Yuedi Tang).
Glossary
Abbreviations
Abbreviations:
|
ANP
|
atrial natriuretic peptide
|
|
ENaC
|
epithelial sodium channel
|
|
α-ENaC
|
α-subunit of the epithelial sodium
channel
|
|
StV
|
strial vascularies
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
|
ACTB
|
β-actin
|
References
|
1
|
Baines AD, De Bold AJ and Sonnenberg H:
Natriuretic effect of atrial extract on isolated perfused rat
kidney. Can J Physiol Pharmacol. 61:1462–1466. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cea LB: Natriuretic peptide family: new
aspects. Curr Med Chem Cardiovasc Hematol Agents. 3:87–98. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brenner BM, Ballermann BJ, Gunning ME and
Zeidel ML: Diverse biological actions of atrial natriuretic
peptide. Physiol Rev. 70:665–699. 1990.PubMed/NCBI
|
|
4
|
Lamprecht J and Meyer zum Gottesberge AM:
The presence and localization of receptors for atrial natriuretic
peptide in the inner ear of the guinea pig. Arch Otorhinolaryngol.
245:300–301. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koch T, Gloddek B and Gutzke S: Binding
sites of atrial natriuretic peptide (ANP) in the mammalian cochlea
and stimulation of cyclic GMP synthesis. Hear Res. 63:197–202.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seebacher T, Beitz E, Kumagami H, Wild K,
Ruppersberg JP and Schultz JE: Expression of membrane-bound and
cytosolic guanylyl cyclases in the rat inner ear. Hear Res.
127:95–102. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meyer zum Gottesberge A, Schleicher A,
Drummer C and Gerzer R: The volume protective natriuretic peptide
system in the inner ear. Comparison between vestibular and cochlear
compartments. Acta Otolaryngol Suppl. 520:170–173. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meyer zum Gottesberge A and Lamprecht J:
Localization of the atrial natriuretic peptide binding sites in the
inner ear tissue-possibly an additional regulating system. Acta
Otolaryngol Suppl. 468:53–57. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Long L, Tang Y, Xia Q, Xia Z and Liu J:
Detection of atrial natriuretic peptide receptor in the labyrinth
of the mouse inner ear. Neuro Endocrinol Lett. 29:577–580.
2008.PubMed/NCBI
|
|
10
|
Long L, Tang Y, Xia Q, Xia Z and Liu J:
The expression of atrial natriuretic peptide receptor in the mouse
inner ear labyrinth. Neuro Endocrinol Lett. 31:126–130.
2010.PubMed/NCBI
|
|
11
|
Chen HX, Wang JL, Liu QC and Qiu JH:
Distribution and location of immunoreactive atrial natriuretic
peptides in cochlear stria vascularis of guinea pig. Chin Med J
(Engl). 107:53–56. 1994.PubMed/NCBI
|
|
12
|
Loffing J and Schild L: Functional domains
of the epithelial sodium channel. J Am Soc Nephrol. 16:3175–3181.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Garty H and Palmer LG: Epithelial sodium
channels: function, structure, and regulation. Physiol Rev.
77:359–396. 1997.PubMed/NCBI
|
|
14
|
Couloigner V, Fay M, Djelidi S, et al:
Location and function of the epithelial Na channel in the cochlea.
Am J Physiol Renal Physiol. 280:F214–F222. 2001.PubMed/NCBI
|
|
15
|
Yoon YJ and Hellstrom S:
Immunohistochemical localization of alpha-atrial natriuretic
polypeptide in the rat cochlea. Acta Otolaryngol. 112:604–610.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Higuchi R, Fockler C, Dollinger G and
Watson R: Kinetic PCR analysis: real-time monitoring of DNA
amplification reactions. Biotechnology (NY). 11:1026–1030. 1993.
View Article : Google Scholar
|
|
17
|
Marcus DC and Chiba T: K+ and Na+
absorption by outer sulcus epithelial cells. Hear Res. 134:48–56.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takeuchi S, Ando M, Kozakura K, Saito H
and Irimajiri A: Ion channels in basolateral membrane of marginal
cells dissociated from gerbil stria vascularis. Hear Res.
83:89–100. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bhalla V and Hallows KR: Mechanisms of
ENaC regulation and clinical implications. J Am Soc Nephrol.
19:1845–1854. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Potter LR, Abbey-Hosch S and Dickey DM:
Natriuretic peptides, their receptors, and cyclic guanosine
monophosphate-dependent signaling functions. Endocr Rev. 27:47–72.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fried TA, Osgood RW and Stein JH: Tubular
site(s) of action of atrial natriuretic peptide in the rat. Am J
Physiol. 255:F313–F316. 1988.PubMed/NCBI
|
|
22
|
Argenzio RA and Armstrong M: ANP inhibits
NaCl absorption and elicits Cl secretion in porcine colon: evidence
for cGMP and Ca mediation. Am J Physiol. 265:R57–R65.
1993.PubMed/NCBI
|
|
23
|
Waldman SA, Rapoport RM and Murad F:
Atrial natriuretic factor selectively activates particulate
guanylate cyclase and elevates cyclic GMP in rat tissues. J Biol
Chem. 259:14332–14334. 1984.PubMed/NCBI
|
|
24
|
Kiemer AK, Bildner N, Weber NC and Vollmar
AM: Characterization of heme oxygenase 1 (heat shock protein 32)
induction by atrial natriuretic peptide in human endothelial cells.
Endocrinology. 144:802–812. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pilz RB and Casteel DE: Regulation of gene
expression by cyclic GMP. Circ Res. 93:1034–1046. 2003. View Article : Google Scholar : PubMed/NCBI
|