Introduction
Regeneration is a complex event that occurs in
several vertebrates and invertebrates (1). For regeneration to occur, one of the
earliest signaling events following a lesion is the production of
cells that are capable of rebuilding lost structures. The way that
these events occur and the types of cells involved differ between
animal groups (2).
The choice of planarians as a regeneration study
model is based on their plasticity, regeneration ability, rapid
response and adult stem cell reservoir (3). Cell and molecular characterization of
planarian stem cells has been achieved with various tools, such as
inhibition of the function of genes involved in regeneration via
RNA interference, western blotting, in situ hybridization
and cell isolation by flow cytometry (4–8). These
tools are extremely important alternatives for understanding the
mechanisms associated with the maintenance and role of stem cells
in vivo (9).
Stem cells, also known as neoblasts, are located in
the parenchyma and account for ~20–30% of the whole cells of an
individual and may present morphological subtypes (10–13).
Neoblasts are characterized by i) the absence of morphological
cytodifferentiation signs; ii) chromatoid body or large cytoplasmic
ribonucleoprotein granules; iii) large nuclei with dispersed
chromatin, or large nucleoli; iv) reduced cytoplasm containing
numerous ribosomes; v) little endoplasmic reticulum; vi)
perinuclear germ granules; and vii) X-ray sensitivity (8, 9,
11, 14).
The evolutionary conservation and expression of
neoblasts in mammalian genes is associated with the maintenance and
expression of pluripotency (15).
Pluripotency transcription factors in mammalian stem cells are
regulated by key genes, such as OCT4, SOX2 and NANOG.
OCT4 is a member of the octamer-binding subgroup of the
Pit-Oct-Unc transcription factor family (16, 17).
Due to a lack of information on the establishment of
planarian primary neoblast cultures, particularly of Girardia
tigrina, the present study proposed a methodology for cell
culture establishment and characterization of neoblasts.
Materials and methods
Animals
Specimens (n=24) of planarian Girardia
tigrina were collected from the urban stretch of the Paraiba do
Sul River in the municipality Jacarei (SP, Brazil). Specimen
selection account ed for criteria, such as absence of lesions and
perfect morphology. The selected animals were maintained in vials
containing non-chlorinated water and were fasted for seven days
until disintegration.
Disintegration and cultivation
The specimens were disintegrated as follows: 24
planarians were bathed in antibiotic and ultrapure water for 30 min
each. The specimens were incubated in trypsin (Sigma-Aldrich, St.
Louis, MO, USA) and antibiotic-antimycotic (Gibco®, Life
Technologies, New York, NY, USA) (1:1; TA) in the dark for 48 h.
The partially disintegrated material was homogenized with a pipette
and centrifuged (Excelsa Baby I; Fanem, Sao Paulo, SP, Brazil) at
2,360 x g for 5 min. The cell sediment was resuspended in 1 ml of
Iscove's modified Dulbecco's medium (IMDM) supplemented with 10%
fetal calf serum (Gibco®) and 1% (v/v) antibiotic and
antimycotic solution.
Cell viability
Cell viability was assessed with trypan blue
(Sigma-Aldrich). Approximately 100 cells were counted in a Leica DM
IL microscope (Leica Microsystems GmbH, Wetzlar, Germany),
including stained and non-stained cells, to determine the
proportion of membrane damage at different temperatures.
Cell fractionation
Following incubation in TA, the specimens were
centrifuged and the segment was resuspended in 6 ml of IMDM
supplemented with 10% fetal calf serum and 1% antibiotic and
antimycotic solution. For neoblast purification, 6 ml of cells was
suspended in 6 ml of a solution of Histopaque® 1077
(Sigma-Aldrich). The cell suspension was centrifuged (Fanem) at
3,248.93 x g for 45 min. Following centrifugation, three
interphases were visualized and cells were harvested from each one.
This isolation procedure was performed in triplicate with five
replications and cell culture was monitored for 10 weeks.
Analysis tools used to evaluate the
culture for total and interphase cells
Disintegration and purification were complete after
48 h incubation in TA. The total cell culture samples and
interphases were analyzed by optical and fluorescence microscopy.
Optical microscopy showed the following cellular aspects: a)
Nuclear cytoplasmic ratio; b) morphology; and c) cell size. For
fluorescence microscopy, total cells and neoblast-enriched cell
interphases were resuspended in 20 µ1 sterile phosphate-buffered
saline (PBS) and incubated with mouse anti-OCT4 (1:200) (AB-3209;
Merck Millipore, Darmstadt, Germany) primary antibody for 60 min at
room temperature. Subsequently, the cells were washed with PBS and
incubated with fluorescein isothiocyanate-rat anti-mouse (04-6111;
Invitrogen, Life Technologies, New York, NY, USA) secondary
antibody at room temperature for 45–60 min. Nuclei were detected by
4′,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich) staining.
Images were captured using a Leica DM IL microscope fitted with a
Leica DFC310 FX camera (Leica Microsystems GmbH). After 10 days of
cultivation, 50 µ1 of cell culture was collected in Eppendorf tubes
and centrifuged for 5 min at 2,360 x g. Subsequently, the cells
were resuspended and stained as previously described.
For scanning electron microscopy, 100 µ1 of the
control and irradiated groups were collected and centrifuged at
4,588.8 x g for 10 min. Round coverslips were coated with a 0.1%
solution of poly-L-lysine in water (molecular weight, 400,000;
Sigma-Aldrich). The pellet was resuspended in PBS and the cells
were placed on coverslips coated with poly-L-lysine and incubated
for 20 min at room temperature. At the end of this period, the
coverslips were washed to remove non-adhered cells, fixed with
0.25% glutaraldehyde and 2% paraformaldehyde (Sigma-Aldrich) in 0.1
M phosphate buffer and incubated for 10 min. After fixation, the
samples were washed with 0.1 M phosphate buffer and dehydrated with
acetone and hexamethyldisilazane for 10 min. The coverslips were
gold-palladium coated in a high vacuum evaporator on a
rotating-tilting stage and observed in a Zeiss EVO MA 10 scanning
electron microscope (Carl Zeiss Microscopy GmbH, Königsallee,
Germany).
Results
Disintegration of the specimens
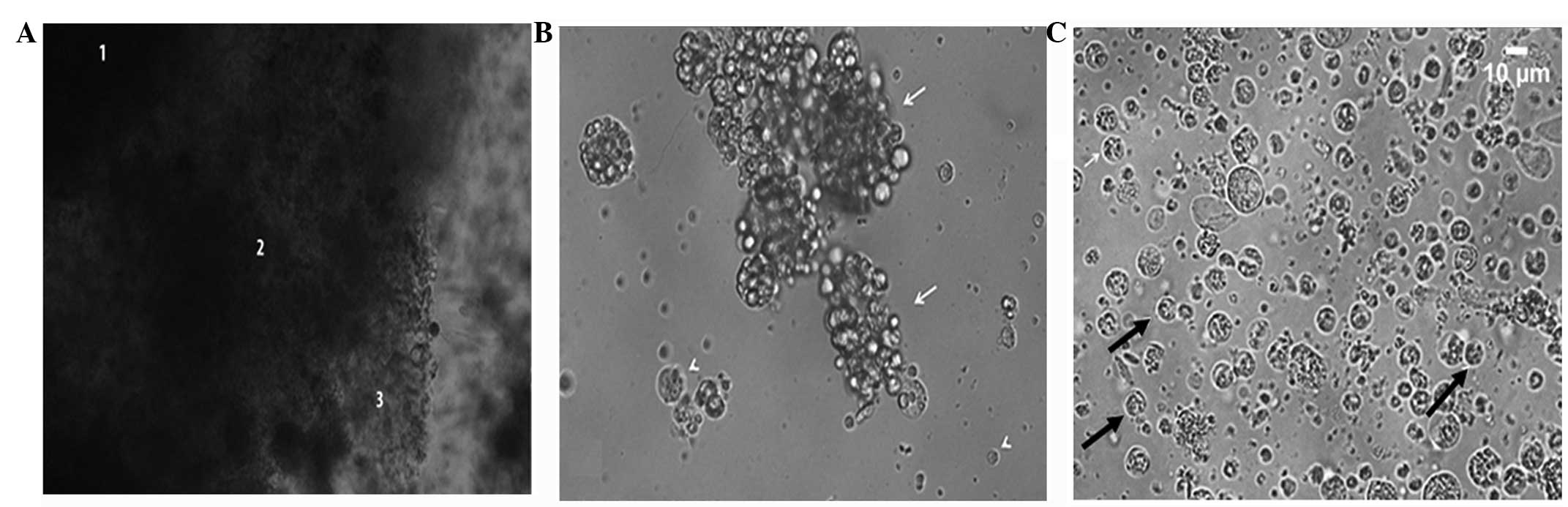
After an incubation period of 48 h, the TA solution
efficiently disintegrated the specimens and three regions with
different levels of fragmentation were identified (Fig. 1). Following this period, the
specimens were centrifuged to further total disintegration. Optical
microscopy analysis revealed cell aggregates; some of which were
isolated for characterization. The presence of mucus during
cultivation following specimen disintegration had a double effect,
as it contributed positively to cell adhesion to the bottom of the
culture plate and hindered the characterization of cell types due
to the formation of cell aggregates (Fig. 1B). Total cell decoupling occurred in
four days.
Assessment of membrane integrity
Trypan blue was used due to the few mitochondria in
the neoblasts. Membrane integrity was assessed through cell count.
At the temperatures of 4, 18 and 37˚C, the cell membrane integrity
was preserved with values of 63.81, 51.92 and 4.35%,
respectively.
Interphases following
purification
Following complete purification, three interphases
were obtained: Interphase 1 (I1), I2 and I3. Neoblasts predominated
in I1 (Fig. 1C), with the presence
of other cell types in smaller amounts. Fig. 1C shows that the neoblast nucleus was
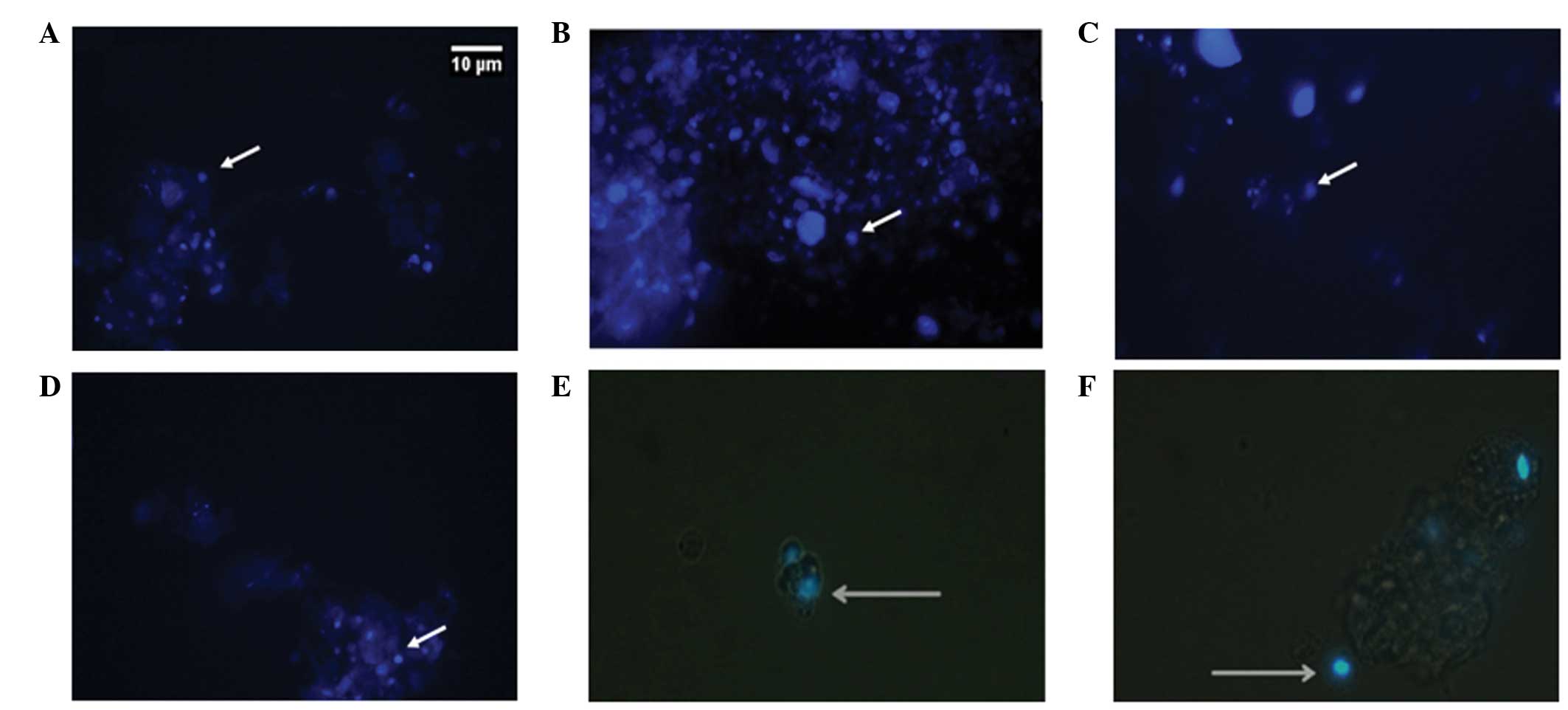
large and the cytoplasm was reduced, which was confirmed by
fluorescent staining with DAPI (Fig.
2) and OCT-4 (Fig. 3A and
B).
Discussion
The isolation of a pure neoblast cell fraction and
its culture for a long period of time allowed the inclusion of
Girardia tigrina among the model systems for the study of
the stem cell signalization and genetic circuitry. However, there
are limited studies on the establishment of cultures for the
analysis of neoblast biology. Planarian Girardia tigrina, a
species commonly found in Brazil, has been used as a model in our
stem cells studies.
The neoblast cultures were established from a
planarian pool after fasting for 7–15 days, depending on the
species used (4–6, 18).
Experiments with Dugesia tahitiensis and Dugesia
polychroa used 24-h baths (7,
8). In the present study, two
30-min antibiotic and sterile ultrapure water (1:1) baths were
performed, in contrast to the aforementioned studies. Regarding the
disintegration solutions and the cultivation medium, another
relevant factor is the osmolarity relative to the studied specimen.
Trypsin and pronase are digestive enzymes used to disperse cells
and when specimens were incubated with trypsin (0.25%) at 4˚C,
testicular cell membrane damage and cell viability were reduced,
demonstrating the effectiveness of this cell dissociation technique
(9). The methodology adopted in the
present study for the disintegration of Girardia tigrina
involved incubation in a TA solution for 48 h. Therefore, this is
opposed to studies that used maceration, mechanical disintegration
and enzymatic dissociation methods (8) and fragment disintegration in a
Holtfreter's 5/8 trypsin solution with subsequent gentle
homogenization (5).
Trypsin may damage the cell membrane and thus affect
the cell membrane integrity. In the present study, three incubation
temperatures (4, 18 and 37˚C) were tested for disintegration in a
TA solution and cell viability was assessed with trypan blue
staining. At 4 and 18˚C, smaller damage rates occurred,
respectively (9). Cell viability
was only greatly compromised by 4.35% at 37˚C, confirming the
thermal tolerance results obtained for Girardia tigrina.
After 96 h at 33˚C, <50% of the specimens died (11).
Neoblast characterization through light and
fluorescence microscopy showed rounded morphology and large nuclei
when compared to the small cytoplasm, corroborating with several
previous studies (12, 19). Besides morphological
characterization, the neoblasts were labeled with antibody
anti-OCT4 (Fig. 3), which is
specific for mammalian stem cells and features homology to
pluripotency factors and target genes between neoblast
trascriptomes and proteomes (13).
These results are consistent with those of others studies (13, 17),
demonstrating that OCT4 is a good neoblast label, independent of
the use of a planarian species as a model, as this gene
evolutionarily conserved pluripotency.
In general, neoblasts comprise from ~20 to 30% of
the cells that constitute the proliferative compartment known as
blastema (7). The methods employed
for disintegration and gradient centrifugation were efficient.
Following purification, the proportion of neoblasts recovered in I1
was 15%, in accordance with the studies mentioned above.
In conclusion, disintegration with a TA solution was
efficient and 18˚C is a suitable incubation temperature for
Girardia tigrina specimens. IMDM supplemented with fetal
bovine serum (10%) was adequate for the establishment and
maintenance of a planarian primary cell culture. Ultimately,
antibody anti-OCT4 allowed the identification of neoblasts
following purification in the Histopaque® 1077 gradient
and may be useful in regeneration and proliferation studies of stem
cells applied to cell biology and tissue engineering.
Acknowledgements
The authors would like to thank the Foundation for
Research Support of the State of Sao Paulo for grant no.
2009/15206-8 and National Council for Scientific and Technological
Development for grant no. 480993/2010-6.
References
|
1
|
Isaeva VV, Akhmadieva AV, Aleksandriova
IaN and Shukaliuk AI: Morphofunctional organization of reserve stem
cells providing for asexual and sexual reproduction of
invertebrates. Ontogenez. 40:83–96. 2009.(In Russian). PubMed/NCBI
|
|
2
|
Wenemoser D and Reddien PW: Planarian
regeneration involves distinct stem cell responses to wounds and
tissue absence. Dev Biol. 344:979–991. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Salo E and Agata K: Planarian
regeneration: a classic topic claiming new attention. Int J Dev
Biol. 56:3–4. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sato K, Shibata N, Orii H, et al:
Identification and origin of the germline stem cells as revealed by
the expression of nanos-related gene in planarians. Dev Growth
Differ. 48:615–628. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Higuchi S, Hayashi T, Hori I, et al:
Characterization and categorization of fluorescence activated cell
sorted planarian stem cells by ultrastructural analysis. Dev Growth
Differ. 49:571–581. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tasaki J, Shibata N, Nishimura O, et al:
ERK signaling controls blastema cell differentiation during
planarian regeneration. Development. 138:2417–2427. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Behensky C, Schurmann W and Peter R:
Quantitative analysis of turbellarian cell suspensions by
fluorescent staining with acridine orange and video microscopy.
Belg J Zool. 131 (Suppl 1):131–136. 2001.
|
|
8
|
Schurman W, Betz S and Peter R: Separation
and subtyping of planarian neoblasts by density-gradient
centrifugation and staining. Hydrobiologia. 383:117–124. 1998.
View Article : Google Scholar
|
|
9
|
Terraciano PB, Paz AHR, Horn MM, et al:
Isolation of rat testis cells from enzymatic digestion using
trypsin at 4˚C. HCPA. 24:163–164. 2004.(In Spanish).
|
|
10
|
Salo E and Baguna J: Regeneration and
pattern formation in planarians. II. Local origin and role of cell
movements in blastema formation. Development. 107:69–76. 1989.
|
|
11
|
de Campos Velho NMR: Planarians limnic
(Tricladida: Dugesiidae) as models for regeneration experiments in
microgravity and hypergravity environments. (unpublished PhD
thesis)Universidade do Vale do Rio dos Sinos2011
|
|
12
|
Pellettieri J and Sanchez Alvarado A: Cell
turnover and adult tissue homeostasis: from humans to planarians.
Annu Rev Genet. 41:83–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rink JC: Stem cell systems and
regeneration in planaria. Dev Genes Evol. 223:67–84. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hayashi T, Asami M, Higuchi S, Shibata N
and Agata K: Isolation of planarian X-ray-sensitive stem cells by
fluorescence-activated cell sorting. Dev Growth Differ. 48:371–380.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Onal P, Grun D, Adamidi C, et al: Gene
expression of pluripotency determinants is conserved between
mammalian and planarian stem cells. EMBO J. 31:2755–2769. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tantin D: Octtranscription factors in
development stem cells insights and mechanisms. Development.
140:2857–2866. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao FQ: Octamer-binding transcription
factors: genomics and functions. Front Biosci (Landmark Ed).
18:1051–1071. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bender CE, Fitzgerald P, Tait SW, et al:
Mitochondrial pathway of apoptosis is ancestral in metazoans. Proc
Natl Acad Sci USA. 109:4904–4909. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shibata N, Umesono Y, Orii H, Sakurai T,
et al: Expression of vasa(vas)-related genes in germline cells and
totipotent somatic stem cells of planarians. Dev Biol. 206:73–87.
1999. View Article : Google Scholar : PubMed/NCBI
|