Introduction
Pseudomembranous enteritis (PME) is an acute
inflammatory bowel disease affecting the total population.
Clostridium difficile (C. difficile) is recognized as one of
the most important pathogens responsible for antibiotic-associated
colitis and accounts for 15–25% of nosocomial antibiotic-associated
diarrhea cases (1). Up to 70% of
infants may be asymptomatically colonized with C. difficile,
including toxigenic strains. The rates of colonization decrease
with age, falling to 6% by 2 years of age. The colonization rates
in children over the age of 2 years are similar to those in adults
(~3%) (2). Currently, the standard
treatment recommendations for mild to moderate C. difficile
infection (CDI) include metronidazole or vancomycin (1). However, the cure rates of severe CDI
treated with metronidazole and vancomycin are only 76% and 97%,
respectively. The chance of recurrent CDI within 8 weeks is 10–20%,
but once a patient experiences recurrence, the rate of further
recurrences increases to 40–65% (3). Given the effective outcome, low cost,
apparent safety and availability of fecal microbiota
transplantation (FMT), it has become an increasingly accepted
option for treatment of CDI (4).
Microbiota dysbiosis is the fundamental pathogenetic base of
PME/CDI/antibiotic-associated diarrhea, and FMT provides a rapid
solution to restore the intestinal microbiota (5). To the best of our knowledge, the
current study presents the first case of a 13-month-old infant with
severe PME who was treated with FMT in China (6, 7).
Case report
A 13-month-old male was admitted to the Shanghai
Children's Hospital (Shanghai, China) with a 2-month history of
diarrhea, loose, malodorous dark-green stools with variable mucus
and blood, and a 1.5-month history of retractable edema,
hypoalbuminemia, electrolyte disturbance and severe malnutrition.
Initially, the infant presented with acute diarrhea for 1 day,
followed by ileus and exploratory laparotomy and high ligation of
inguinal hernia on day 3 without a noticeable food allergy, family
history and vaccination. Multiple broad-spectrum antibiotics,
including ceftazidime, amoxicillin, imipenem, metronidazole and
vancomycin, were introduced daily to the patient during 2 months.
The patient had a birth weight of 2,500 g with a 20-day history of
diarrhea at the age of six months.
Physical examination revealed that the infant was
conscious, weak and responding. The vital signs were stable, the
weight was 8.4 kg and the eyelids were puffy and had dry skin and
mucous. Abdominal examinations showed abdominal distention, pitting
edema of the pretibial area and the dorsum, and normal neurological
examination. The dark-green stools were loose, malodorous and had a
frequency of 5–8 times per day with variable mucus and positive
occult blood.
The routine blood examination showed white blood
cells (WBC), 10.7–30.3 × 109/1; hemoglobin, 85–129 g/1;
platelet count, 305–616 × 109/1; and C-reactive protein,
3–19 mg/1. The blood biochemical examination demonstrated albumin,
11–38 g/1; globulin, 10–16 g/1; K+, 2.8-5.5 mmol/1;
Na+, 131–143 mmol/1; and Ca2+, 1.69-2.25
mmol/1. The routine stool examination showed WBC, 2–20/high power
(HP); red blood cells, 0–15/HP; and positive fecal occult blood
test. Multiple stool cultivation was negative. The fecal bacteria
population test showed 50% enterobacteria and 50% enterococcus on
admission. Fecal C. difficile toxin A and B was negative
during days 11–13 of hospitalization. Other laboratory tests showed
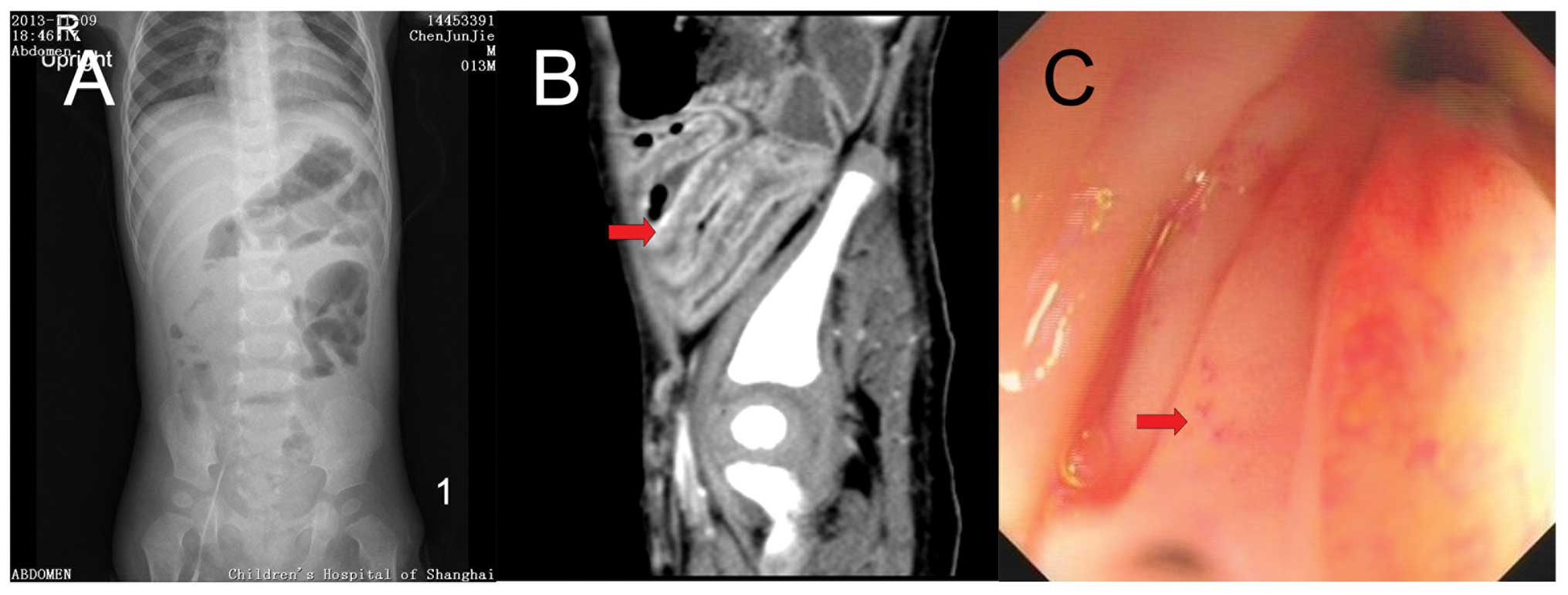
no significant abnormalities. Abdominal images, including
ultrasound, X-ray and enhanced computed tomography revealed a
swallowed-segmented intestinal wall, cellulitis, ascites and
pleural effusion. Colonoscopy on day 16 of hospitalization showed
left colitis (Fig. 1).
The condition of the infant was improved with
nutritional support, correction of electrolytes, acid-base
disturbance, supply of albumin, and intravenous immunoglobulin.
Edema gradually faded on day 7 and completely disappeared on day 14
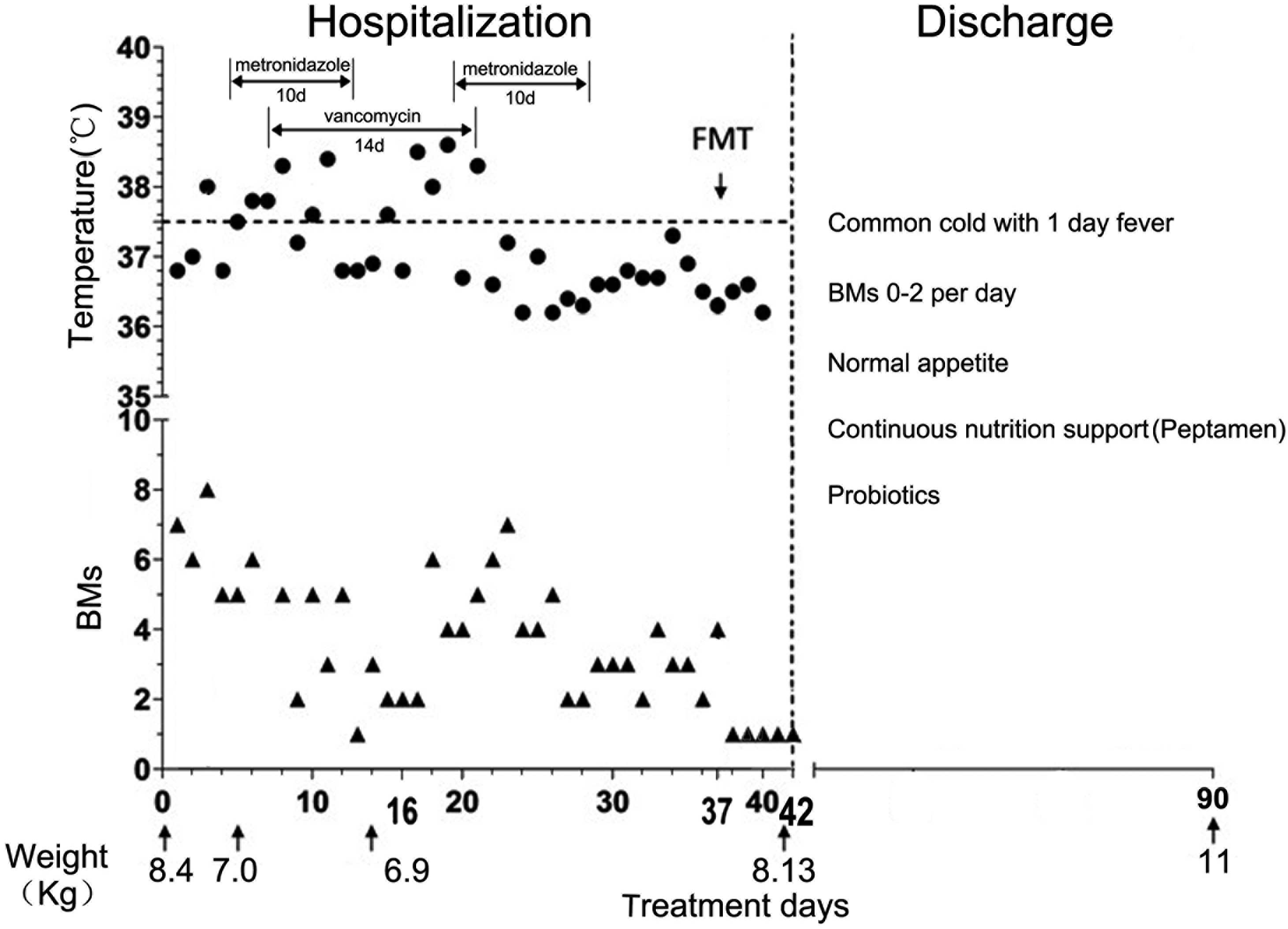
following hospitalization. The patient was treated with two rounds
of oral metronidazole for 10 days, with vancomycin for 14 days, and
finally a single FMT (the feces donor was the mother of the
patient) was performed via a nasal jejunal feeding tube on day 96
following the initiation of the disease, which was day 37 from
admission. The patient completely recovered and was released on day
5 post-FMT. The temperature, stool frequency, weight, major
antibiotics and medical procedure patterns are shown in Fig. 2. Normal appetite, normal bowel
movement frequency and satisfactory weight were exhibited during
the 4 months post-FMT.
Discussion
Based on the clinical features and the response of
whole treatment, severe PME was the final diagnosis of the infant
in the present study. The diagnosis of PME was based on the
following reasons: i) Diarrhea was persistent, and there were
intestinal mucosae in the stools more than once; ii) the patient
presented risk factors, including multiple broad-spectrum
antibiotics application, long-term fasting, exploratory laparotomy
and high ligation of inguinal hernia; and iii) although the use of
metronidazole and vancomycin in the Children's Hospital (Jiangxi,
China) was ineffective, empirical treatment with adequate dosage
and duration of metronidazole combined with vancomycin showed
partial effectiveness in Shanghai Children's Hospital (Shanghai,
China). The effectiveness of FMT was positive.
According to the guidelines by the Society for
Healthcare Epidemiology of America (SHEA) and the Infectious
Diseases Society of America (IDSA) (1), the definition for the usual
presentation of CDI includes the following findings: i) The
presence of diarrhea, defined as passage of ≥3 unformed stools in
≤24 consecutive hours; and ii) a stool test result positive for the
presence of toxigenic C. difficile or its toxins, or
colonoscopic or histopathological findings demonstrating
pseudomembranous colitis. The guideline also indicated the
difficulties for CDI diagnosis. SHEA-IDSA defined severe CDI as
leukocytosis with a WBC of ≥15,000/l or a serum creatinine level
≥1.5 times the premorbid level (1).
The European Society of Clinical Microbiology and Infectious
Diseases defined severe CDI as serum albumin <3 g/dl plus one of
the following; WBC >15×109/l and abdominal tenderness
(3). Although, C. difficile
toxin tests during the hospitalization were all negative, possibly
due to the antibiotic treatment prior to the toxin tests.
Colonscopy was not performed until the infant reached a stable
general condition (day 16 of hospitalization). Relatively late
timing may be the reason for missing the typical image findings and
possible positive C. difficile detection.
In the present case, diarrhea was persistent, serum
albumin was <1g/d1, and WBC was >15×109/1 for many
examinations, with the highest being 30×109/1. Abdominal
images revealed swallowed-segmented intestinal sigmoid colon and
rectum. The temperature of the patient was >38.5°C. All the
parameters of the present case assisted in the diagnosis of severe
CDI. In China, there are only a few case reports regarding severe
CDI (8–11). None of the studies are supported by
the toxin, fecal bacteria population test and culture. Due to the
population size of China, antibiotic abuse and the few cases
reported, severe CDI is extremely under-recognized, particularly in
children. In the next few years, more progress is required for
pediatric CDI in China, including clinical diagnosis, treatment and
research.
References
|
1
|
Cohen SH, Gerding DN, Johnson S, Kelly CP,
Loo VG, McDonald LC, et al: Clinical practice guidelines for
Clostridium difficile infection in adults: 2010 update by the
society for healthcare epidemiology of America (SHEA) and the
infectious diseases society of America (IDSA). Infect Control Hosp
Epidemiol. 31:431–455. 2010.PubMed/NCBI
|
|
2
|
Sammons JS, Toltzis P and Zaoutis TE:
Clostridium difficile Infection in children. JAMA Pediatr.
167:567–573. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Surawicz CM, Brandt LJ, Binion DG,
Ananthakrishnan AN, Curry SR, Gilligan PH, et al: Guidelines for
diagnosis, treatment, and prevention of Clostridium difficile
infections. Am J Gastroenterol. 108:478–498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brandt LJ, Aroniadis OC, Mellow M,
Kanatzar A, Kelly C, Park T, et al: Long-term follow-up of
colonoscopic fecal microbiota transplant for recurrent Clostridium
difficile infection. Am J Gastroenterol. 107:1079–1087. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Borody TJ, Warren EF, Leis SM, Surace R,
Ashman O and Siarakas S: Bacteriotherapy using fecal flora: toying
with human motions. J Clin Gastroenterol. 38:475–483. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Russell G, Kaplan J, Ferraro M and
Michelow IC: Fecal bacteriotherapy for relapsing Clostridium
difficile infection in a child: a proposed treatment protocol.
Pediatrics. 126:e239–242. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van Nood EI, Vrieze A, Nieuwdorp M,
Fuentes S, et al: Duodenal infusion of donor feces for recurrent
Clostridium difficile. N Engl J Med. 368:407–415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He JQ, Li JD and Mo QH: Severe
pseudomembranous enterocolitis-one case report and review of
literature. Hainan Med J. 19:137–138. 2008.(In Chinese).
|
|
9
|
Li WH, Liu XL and Tian YL: Clinical
analysis of 62 psendomembranons colitis patient. Chongqing Med J.
41:2379–2381. 2001.(In Chinese).
|
|
10
|
Wang L, Gao J and Cheng WP: Clinical
analysis of 9 psendomembranons colitis patient. Pract Clin Med.
13:22–30. 2012.(In Chinese).
|
|
11
|
Zhang L, Zhang YD, Duan LF, Liang Y and
Gong QM: Severe pseudomembranous colitic induced by antibiotics in
1 patient. Chin J Clin Pharmacol. 26:684–686. 2010.(In
Chinese).
|