Introduction
Extended-spectrum β-lactamases (ESBLs) are the
plasmid-mediated bacterial enzymes that have been found in enteric
Gram-negative bacteria and are important pathogens in nosocomial
infections (1). The importance of
ESBLs in the spread of multiple resistances among Gram-negative
bacteria was thoroughly recognized (2). The majority of ESBLs are derived from
broad-spectrum β-lactamases, TEM-1 and SHV-1. CTX-M and OXA-type
enzymes are novel families of ESBLs (3). There are nine distinct structural
families, TEM, SHV, CTX-M, PER, VEB, GES, TLA, BES and OXA, which
are classified based on comparisons of deduced amino acid sequences
(4). TEM and SHV enzymes were
included in the first ESBL variant and they remain in the most
prevalent types of ESBL (5–7). The first SHV type of an ESBL isolate
was reported in Germany in 1983 (8). SHV is one of the most common genotypes
of ESBL (9, 10).
Recently, ESBLs have increased in prevalence due to
the use of extended-spectrum cephalosporins (ESC). The resistance
to ESC among Enterobacteriaceae has occurred worldwide (11). β-lactams were the most frequently
prescribed antibiotics accounting for ~50% of global antibiotic
administration. Therefore, ESBLs presented a big challenge to
treatment (12). ESBL-producing
isolates caused outbreaks of infections that lead to serious
antibiotic management concerns with significant economic causatives
(13). The main predictor of
mortality caused by ESBL-producing isolates was initially
inadequate antimicrobial treatment (14). Escherichia coli (E. coli) and
Klebsiella pneumoniae (K. pneumoniae) were the most popular
ESBL-producing isolates (15). In
addition, ESBLs have been found in other Enterobacteriaceae and
Pseudomonas aeruginosa (3).
The resistance to the 3rd-generation cephalosporins
was mainly due to the production of ESBLs enzymes, which are
hydrolyzing oxyimino-cephalosporins inhibited by clavulanic acid
(16). ESBL enzymes, such as SHV,
TEM and CTX-M, were all mediated by the specific plasmids.
Conjugation experiments and plasmid identification were conducted
to examine the transferability of resistance to ESCs (11). Traditionally, the scope of the
problem caused by ESBLs was assessed by considering the
microbiological width (the type of microorganisms involved),
antibiotic depth (the number of antibiotics hydrolyzed by ESBL) and
geographical breadth (the global burden of resistance). Otherwise,
the clinical impact of antibiotic resistance, such as the effect on
mortality, was extremely difficult to define (1).
To delineate the trends of ESBL-producing isolates,
the frequency of SHV in Chengdu (China) were detected by assessing
the clinical isolates obtained from hospitals in Chengdu between
2010 and 2011. Confirmation testing of ESBLs-producing isolates was
performed by the double-disk synergy test. The minimum inhibitory
concentration (MIC) of the ESBLs-producing isolates to antibiotics
was obtained by a microdilution method. Polymerase chain reaction
(PCR) and DNA sequencing measurement were also performed to
determine the genotypes and mutations of the SHV β-lactamase gene
(blaSHV). Additionally, we hypothesized that the
ESBL gene may be transferred among Enterobacteriaceae.
Materials and methods
Bacterial isolates
A total of 223 isolates, including E. coli
(n=153) and K. pneumoniae (n=70), were collected from
hospitals in Chengdu between 2010 and 2011 (all the patients
provided written consent). The ethics committee of Sichuan
University approved the study. E. coli ATCC25922, K.
pneumoniae ATCC700603 and a SHV E. coli isolate were
used as quality control strains.
Screening and confirmation for
ESBL
The ESBL phenotype screening test was performed
according to the disk diffusion method recommended by the National
Committee for Clinical Laboratory Standards (NCCLS) on
Mueller-Hinton agar (Sigma-Aldrich, St. Louis, MO, USA) using
commercial antibiotic disks. The antibiotics used were ceftazidime,
aztreonam, cefotaxime and ceftriaxone (all 30 µg/disk; all Binhe
Microorganism Reagent Co., Ltd., Hangzhou, Zhejiang, China). The
size of the inhibition holes was measured after the Mueller-Hinton
plates (Aoboxing Bio-Tech Co., Ltd., Beijing, China) were incubated
at 35˚C for 16 h. When the size of the inhibition hole was 22 mm
for ceftazidime, ≤ 27 mm for aztreonam, ≤ 27 mm for cefotaxime or ≤
25 mm for ceftriaxone, the occurrance of any of these circumstances
showed the possibility of ESBLs. The double-disk synergy testing
method was performed to confirm ESBL. The antibiotics that were
used for confirmation testing in ESBLs were cefotaxime (30
µg/disk), cefotaxime-clavulanate (30 µg/disk) (Binhe), ceftazidime
(30/10 µg/disk) and ceftazidime-clavulanate (30/10 µg/disk) (Binhe
Microorganism Reagent Co., Ltd.). When the difference of diameter
in any pair of disks was ≥ 5 mm, the isolate was confirmed to
produce ESBLs.
Extracting the plasmid DNA and
amplification of ESBL genes
The plasmid DNA of ESBL-producing isolates was
extracted with the Plasmid Mini Kit I (Omega Bio-Tek, Norcross, GA,
USA) and stored at −20˚C. The presence of β-lactamase genes was
investigated by PCR (Bio-Rad, Hercules, CA, USA). The primers
aiming to amplify the SHV genes were designed by the Primer Premier
5.0 (PREMIER Biosoft, Palo Alto CA, USA) according to the original
SHV gene sequence from GenBank; accession no. AF124984. The primers
were as follows: blaSHV1, 5′CTTATTTGTCGCTTC
TTT3′; and blaSHV2 5′TTATGGCGTTACCTTTG3′. In the
25 µ1 PCR system was 1 µ1 (10 pmol/µl) primer
blaSHV1, 1 µl (10 pmol/µl) primer
blaSHV2, 1 µl (10 ng/µl) plasmid, 12.5 µl 2X Taq
Master mix (Tiangen Biotech, Co., Ltd., Beijing, China) and 9.5 µl
sterile water. PCR amplification was performed under the following
conditions: Initial denaturation at 94˚C for 3 min, denaturation at
94˚C for 45 sec, annealed at 58˚C for 45 sec and extended at 72˚C
for 1 min. After 35 cycles, the amplification was finally extended
at 72˚C for 7 min. The PCR products were detected by 2% agarose gel
electrophoresis (Sigma-Aldrich).
Transconjugation experiments
The amplification products of K. pneumoniae
F8, including novel mutations, and K. pneumoniae F25,
including the blaSHV-18 gene to the carrier
pUCm-T (Sangon Biotech, Shanghai, China) with
ampicillin-resistance, were conjugated. The carrier, including the
target segments, were transformed to competent cell: E. coli
DH5 α (Sangon Biotech). The bacterial strains transformed were
selected on Mueller-Hinton agar with ampicillin-resistance with a
concentration of 50 µg/ml ampicillin (Binhe Microorganism Reagent
Co., Ltd.). The combinations are known as DH5 α-F8 and DH5 α-F25 in
the following section. The plasmid DNA of DH5 α-F8 and DH5 α-F25
were extracted after incubation at 35˚C for 16–18 h. The plasmids
were amplified and primer blaSHV1 and
blaSHV2 were analyzed with 2% agarose gel
electrophoresis (Sigma-Aldrich).
Drug-sensitivity testing of
ESBL-producing isolates, DH5 α-F8 and DH5 α-F25
The MIC was obtained by the microdilution method
according to the standards for antimicrobial susceptibility testing
(NCCLS document M100-S9, 2008). The antibiotic concentrations were
512, 256, 128, 64, 32, 16, 8, 4, 2, 1, 0.5, 0.25, 0.125, 0.06, 0.03
and 0.015 mg/l, respectively. The antibiotics used were penicillin,
amoxicillin, oxacillin, cefoxitin, ceftazidime, ceftriaxone,
cefotaxime, cefoperazone, cefepime, ampicillin-sulbactam,
piperacillin-tazobactam, cefoperazone-sulbactam, imipenem,
aztreonam, netilmicin, levofloxacin and gentamicin (all Binhe
Microorganism Reagent Co., Ltd.).
Results
Detection of ESBLs
A total of 151 ESBLs were detected in 112 (74.67%)
E. coli and 39 (55.7%) of K. pneumoniae isolates. The
total relevance ratio was 67.7%. PCR products were ~1,021 base
pairs and there were 28 isolates, including
blaSHV gene in 16 E. coli and 12 K.
pneumoniae isolates. The proportion of gene
blaSHV was 18.5%.
Blast search of SHV
The results of Blast searches revealed that there
were nine isolates of SHV-28, seven of SHV-11, four of SHV-1, three
of SHV-12, one of SHV-31 and one of SHV-18. There were another
three isolates that included novel mutations and their nucleotide
sequence had no difference between each other and cannot be found
in the NCBI reference genome. The novel sequence was submitted to
GenBank with an accession no. JX192924. The gene sequence used,
was: 1 ctttgggctc ctcatgatgt attgtg gtta tgcgtcatat tcgcctgtgt
attatctccc 61 tgttagccac cctgccgctg gcggtacaag ccagcccgca
gccgcttgag caaattaaac 121 aaagcgaaag ccagctgtcg ggccgcgtag
gcatgataga aatggatctg gccagcggcc 181 gcacgctgac cgcctggcgc
gccgatgaac gctttcccat gatgagcacc tttaaagtag 241 tgctctgcgg
cgcagtgctg gcgcgggtgg atgccggtga cgaacagctg gagcgaaaga 301
tccactatcg ccagcaggat ctggtggact actcgccggt cagcgaaaaa caccttgccg
361 acggcatgac ggtcggcgaa ctctgcgccg ccgccattac catgagcgat
aacagcgccg 421 ccaatctgct gctggccacc gtcggcggcc ccgcaggatt
gactgccttt ttgcgccaga 481 tcggcgacaa cgttacccgc cttgaccgct
gggaaacgga actgaatgag gcgcttcccg 541 gcgacgcccg cgacaccact
accccggcca gcatggccgc gaccctgcgc aagctgctga 601 ccagccagcg
tctgagcgcc cgttcgcaac ggcagctgct gcagtggatg gtggacgatc 661
gggtcgccgg accgttgatc cgctccgtgc tgccggcggg ctggtttatc gccgataaga
721 ccggagctgg cgaacggggt gcgcgcggga ttgtcgccct gcttggcccg
aataacaaag 781 cagagcgcat tgtggtgatt tatctgcggg atacgccggc
gagcatggcc gagcgaaatc 841 agcaaatcgc cgggatcggc gcggcgctga
tcgagcactg gcaacgctaa cccggcggtg 901 gcgcgcgcgt tatccggctc
gtagcactcg cagctgccgg gcgatatgac tggcggcggc 961 atcggagaga
tgccgtcggt aatgatggtg gtgaaccggg tcaaagtatc acccccataa 1021 c; the
coding region was from 33 to 893.
These five known genotypes of
blaSHV included 10 mutational sites. However,
these changes do not lead to amino acid changes at position of 112,
260, 268 and 274, which were so-called nonsense mutations. Other
mutations are represented in Table
I. Three isolates, including novel mutations, contain five
mutational sites: Base 121 at amino acid position 35 changes from
‘T’ to ‘A’ and the amino acid Leu is substituted by Gln; recorded
as L35Q in GenBank. Base 36 in the coding region changes from ‘T’
to ‘C’, with a Tyr-to-His substitution; base 89 from ‘C’ to ‘A’,
with a His-to-Gln substitution; base 353 from ‘T’ to ‘C’ and base
494 from ‘C’ to ‘T’, but the amino acid of these two positions does
not change.
 | Table I.Mutations of the known SHV genotypes
of bla. |
Table I.
Mutations of the known SHV genotypes
of bla.
| Mutation sites | Quondam bases | Bases after
mutation | Quondam amino
acid | Amino acid after
mutation | Mutational rates
(%) |
|---|
| 7(Y7F) | A | T | Tyr | Phe | 32.1 |
| 8(I8F) | A | T | IIe | Phe | 3.6 |
| 35(L35Q) | T | A | Leu | Gln | 39.2 |
| 43 (R43S) | C | A | Arg | Ser | 3.6 |
| 238 (G238S or
G238A) | G | A or C | Gly | Ser or Ala | 10.7, 3.6 |
| 240 (E240K) | G | A | Glu | Lys | 17.9 |
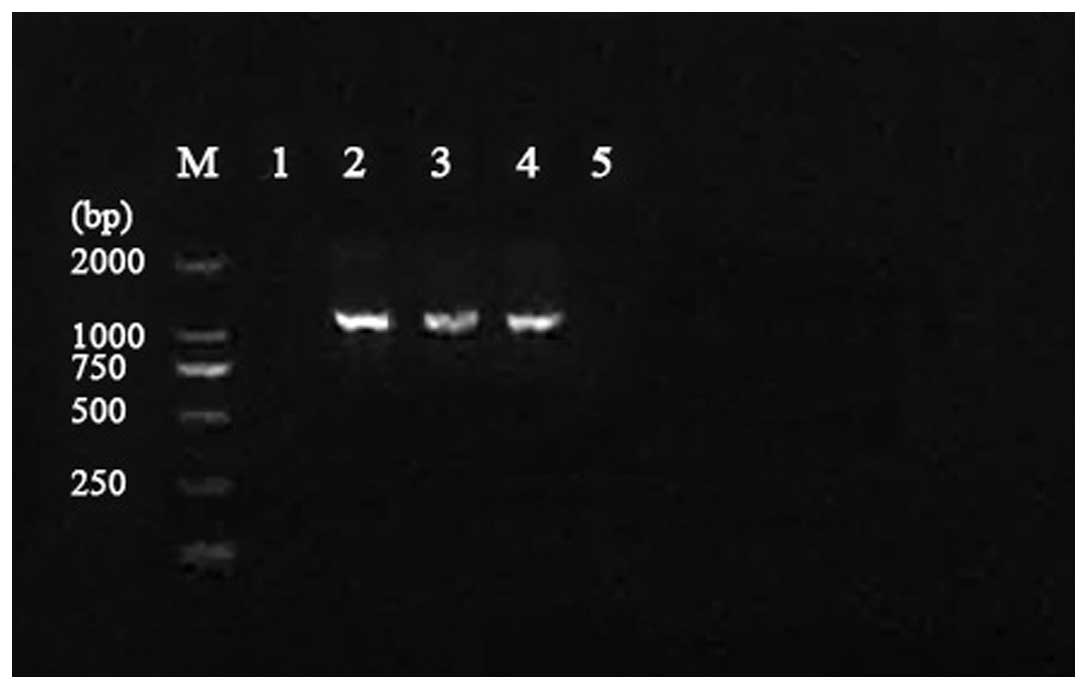
Extraction of DH5 α plasmids
The plasmid DNA of DH5 α-F8 and DH5 α-F28 were
extracted, as shown in Fig. 1. The
results showed that the gene sequence was identical to that of
JX192924. This accounted for the gene segments with novel mutations
successfully transconjugating to E. coli DH5 α. There are
three mutational amino acids that differ from the original
(accession no. EU441172); H3F, Q20H and Q31L. Additionally, this
mutation has not emerged since the first SHV-28 strain was
detected.
Antimicrobial susceptibility
testing
The results of the antimicrobial susceptibility
testing are shown in Table II. The
MIC of DH5 α-F8 and DH5 α-F25 to 17 antibiotics are shown in
Table III. A total of 28
ESBL-producing isolates were all sensitive to imipenem, and all
resistant to penicillin, amoxicillin and oxacillin. The
sensitive-rates to cefoperazone/sulbactam, piperacillin/tazobactam
and cefepime were all >75%. The resistant-rates to the 2nd and
3rd-generation cephalosporins and levofloxacin were high. DH5 α-F8
and DH5 α-F25 were resistant to penicillin, amoxicillin and
oxacillin, and sensitive to imipenem, the 3rd and 4th-generation
cephalosporins and the β-lactamase inhibitor compound, except for
piperacillin/tazobactam. Compared to DH5 α-F25, DH5 α-F8 was
sensitive to levofloxacin, netilmicin and gentamicin.
 | Table II.Results of 28extended - spectrum
β-lactamases (ESBL) - producing isolates to 17antibiotics. |
Table II.
Results of 28extended - spectrum
β-lactamases (ESBL) - producing isolates to 17antibiotics.
| 28 ESBL-producing
strains |
|---|
|
|
|---|
| Antibiotics | Resistance (%) | Intermediary (%) | Sensitive (%) |
|---|
| Oxacillin | 100.0 | 0.0 | 0.0 |
| Penicillin | 100.0 | 0.0 | 0.0 |
| Amoxicillin | 100.0 | 0.0 | 0.0 |
| Cefoxitin | 95.9 | 4.1 | 0.0 |
| Cefotaxime | 92.7 | 0.0 | 8.3 |
| Ceftazidime | 45.8 | 16.7 | 37.5 |
| Cefoperazone | 87.5 | 4.1 | 8.3 |
| Ceftriaxone | 92.7 | 0.0 | 8.3 |
| Cefepime | 11.0 | 12.5 | 76.5 |
|
Amoxicillin/clavulanate | 83.4 | 8.3 | 8.3 |
|
Piperacillin/tazobactam | 8.3 | 16.6 | 75.0 |
|
Cefoperazone/sulbactam | 4.2 | 12.5 | 83.3 |
| Aztreonam | 59.9 | 0.0 | 40.1 |
| Imipenem | 0.0 | 0.0 | 100.0 |
| Levofloxacin | 66.7 | 8.3 | 33.0 |
| Netilmicin | 37.5 | 20.8 | 41.7 |
| Gentamicin | 40.3 | 8.3 | 33.0 |
 | Table III.Minimum inhibitory concentration
(MIC) of the transconjugation isolates, DH5 α- F8 and DH5 α- F25,
to 17antibiotics. |
Table III.
Minimum inhibitory concentration
(MIC) of the transconjugation isolates, DH5 α- F8 and DH5 α- F25,
to 17antibiotics.
| MIC, mg/1 |
|---|
|
|
|---|
| Antibiotics | DH5 α- F8 L35Q 36th
base 89th base | DH5 α- F25 G238A
E240K |
|---|
| Oxacillin | 512 | 512 |
| Penicillin | 512 | 512 |
| Amoxicillin | 256 | 256 |
| Cefoxitin | 2 | 4 |
| Cefotaxime | 0.03 | 4 |
| Ceftazidime | 0.25 | 8 |
| Cefoperazone | 4 | 32 |
| Ceftriaxone | 0.125 | 0.25 |
| Cefepime | 0.06 | 0.06 |
|
Amoxicillin/clavulanate | 32 | 16 |
|
Piperacillin/tazobactam | 64 | 64 |
|
Cefoperazone/sulbactam | 4 | 4 |
| Aztreonam | 16 | 8 |
| Imipenem | 0.015 | 0.015 |
| Levofloxacin | 64 | 0.03 |
| Netilmicin | 32 | 0.5 |
| Gentamicin | 128 | 0.06 |
Discussion
Currently, antibiotic resistance is a severe
problem. As opposed to methicillin-resistant Staphylococcus
aureus and vancomycin-resistant Enterococci,
ESBLs-producing strains are another class of primary pathogenic
bacteria (17, 18). ESBLs mediated by plasmids are common
in Enterobacteriaceae, particularly in E. coli and K.
pneumoniae, which have been reported to cause serious
infections in hospitals, as well as in the community in different
parts of the world (19, 20). A previous study indicated that the
present disk diffusion criteria underestimate the prevalence of
ESBL-producing strains (21). All
the phenomena suggest that the trends of ESBLs-producing isolates
strains may be underestimated.
SHV-2 is the first reported genotype of bla,
which has only one mutation site at codon 238. The change from
glycine to serine induces the hydrolysis of the 3rd-generation
cephalosporin (15). Subsequent to
the identification of SHV-2, numerous types of SHV genotypes have
been found successively. One study identified SHV-8 with an
Asp-to-Asn substitution at amino acid position 179 in a bacteremia
patient that had received multiple courses of antimicrobial agents,
including ceftazidime. The collection of blood isolates from the
same patient suggested the in vivo evolution of resistance
under selective pressure of treatment with cephalosporins (1).
With regards to the high presence ratio in Chengdu
of the ESBL SHV genotypes, SHV-1, SHV-11 and SHV-28 were the most
popular types. A total of 28 ESBL-producing isolates showed a high
resistance-rate to oxacillin, amoxicillin, penicillin, cefoxitin,
levofloxacin and gentamicin. Similarly, Jain et al (22) also reported that ESBL-producing
organisms were resistant to ampicillin, cotrimoxazole, tetracycline
and gentamicin. According to our study, the results indicated that
broad-spectrum penicillins, such as amoxicillin and penicillin,
were ineffective to the infection of ESBL-producing isolates. In
addition, β-lactamase inhibitor compounds, such as clavulanic acid
and sulbactam, are able to enhance the sensitivity of
ESBL-producing isolates to β-lactamase (23). Tazobactam enhanced the antibacterial
function of piperacillin as a new type of β-lactamase inhibitor. In
the present study, the drug-sensitivity testing showed that
piperacillin/tazobactam was superior to amoxicillin/clavulanate.
For the prior use of clavulanate, is is possible that the bacterium
was less sensitive compared to tazobactam and sulbactam. The
4th-generation cephalosporin and β-lactamase inhibitor compound are
effective to ESBL, while the carbapenems, such as imipenem, are
most available to ESBL. DH5 α-F8 and DH5 α-F25 were resistant to
penicillin, oxacillin, amoxicillin, amoxicillin/clavulanate,
aztreonam and piperacillin/tazobactam, and sensitive to 3rd and
4th-generation cephalosporins and cefoperazone/sulbactam. The MIC
of DH5 α-F25 to the 3rd-generation cephalosporin was higher than
that of DH5 α-F8. DH5 α-F25 includes two mutations at codons 238
and 240. These codons did not mutate in DH5 α-F8. A previous study
reported that mutations at codons 238 and 240 are relevant to the
resistance to cefotaxime and ceftazidime (24). The MIC of the original isolates of
F8 and F25 showed a high resistant-rate compared to DH5 α-F8 and
DH5 α-F25, which suggested that the original isolates performed
other resistant mechanisms leading to the resistance to the
3rd-generation cephalosporin. Further study is required to confirm
their resistant mechanism. Multi-resistance isolates are now common
in the clinic, so the choosing of antibiotics should be based on
the drug-sensitivity testing. The 3rd-generation cephalosporin
should be replaced by cephamycin, a β-lactamase inhibitor compound
and carbapenem.
In conclusion, the present study demonstrated that
ESBL was mediated by the plasmids. The ESBL-producing members, such
as E. coli and K. pneumoniae, showed a high
resistance-rate to numerous types of antibiotics. Novel mutations
have existed in Chengdu from at least 2010. Further study is
required to determine its biochemical character.
References
|
1
|
Rahal JJ: Extended-spectrum
beta-lactamases: how big is the problem? Clin Microbiol Infect. 6
(Suppl 2):2–6. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cohen ML: Epidemiology of drug resistance:
implications for a post-antimicrobial era. Science. 257:1050–1055.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bradford PA: Extended-spectrum
beta-lactamases in the 21st century: characterization, epidemiology
and detection of this important resistance threat. Clin Microbiol
Rev. 14:933–951. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gniadkowski M: Evolution and epidemiology
of extended-spectrum beta-lactamases (ESBLs) and ESBL-producing
microorganisms. Clin Microbiol Infect. 7:597–608. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kliebe C, Nies BA, Meyer JF,
Tolxdorff-Neutzling RM and Wiedemann B: Evolution of plasmid-coded
resistance to broad-spectrum cephalosporins. Antimicrob Agents
Chemother. 28:302–307. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Knothe H, Shah P, Krcmery V, Antal M and
Mitsuhashi S: Transferable resistance to cefotaxime, cefoxitin,
cefamandole and cefuroxime in clinical isolates of Klebsiella
pneumoniae and Serratia marcescens. Infection. 11:315–317. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sirot D, Sirot J, Labia R, et al:
Transferable resistance to third-generation cephalosporins in
clinical isolates of Klebsiella pneumoniae: identification of
CTX-1, a novel beta-lactamase. J Antimicrob Chemother. 20:323–334.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Coudron PE, Moland ES and Sanders CC:
Occurrence and detection of extended-spectrum beta-lactamases in
members of the family Enterobacteriaceae at a veterans medical
center: seek and you may find. J Clin Microbiol. 35:2593–2597.
1997.PubMed/NCBI
|
|
9
|
Tzouvelekis LS and Bonomo RA: SHV-type
beta-lactamases. Curr Pharm Des. 5:847–864. 1999.PubMed/NCBI
|
|
10
|
Paterson DL, Hujer KM, Hujer AM, et al:
Extended-spectrum beta-lactamases in Klebsiella pneumoniae
bloodstream isolates from seven countries: dominance and widespread
prevalence of SHV- and CTX-M-type beta-lactamases. Antimicrob
Agents Chemother. 47:3554–3560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shaheen BW, Nayak R, Foley SL, et al:
Molecular characterization of resistance to extended-spectrum
cephalosporins in clinical Escherichia coli isolates from companion
animals in the United States. Antimicrob Agents Chemother.
55:5666–5675. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livermore DM: Beta-lactamase-mediated
resistance and opportunities for its control. J Antimicrob
Chemother. 41 (Suppl D):25–41. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jonathan N: Screening for
extended-spectrum beta-lactamase-producing pathogenic
enterobacteria in district general hospitals. J Clin Microbiol.
43:1488–1490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oteo J, Perez-Vazquez M and Campos J:
Extended-spectrum [beta]-lactamase producing Escherichia coli:
changing epidemiology and clinical impact. Curr Opin Infect Dis.
23:320–326. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Du Bois SK, Marriott MS and Amyes SG: TEM-
and SHV-derived extended-spectrum beta-lactamases: relationship
between selection, structure and function. J Antimicrob Chemother.
35:7–22. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Canton R and Coque TM: The CTX-M
beta-lactamase pandemic. Curr Opin Microbiol. 9:466–475. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jacoby GA: Epidemiology of
extended-spectrum beta-lactamases. Clin Infect Dis. 27:81–83. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dashti AA, Jadaon MM, Gomaa HH, Noronha B
and Udo EE: Transmission of a Klebsiella pneumoniae clone
harbouring genes for CTX-M-15-like and SHV-112 enzymes in a
neonatal intensive care unit of a Kuwaiti hospital. J Med
Microbiol. 59:687–692. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pitout JD, Nordmann P, Laupland KB and
Poirel L: Emergence of Enterobacteriaceae producing
extended-spectrum beta-lactamases (ESBLs) in the community. J
Antimicrob Chemother. 56:52–59. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang SS, Lee MH and Leu HS: Bacteremia
due to extended-spectrum beta-lactamase-producing
Enterobacteriaceae other than Escherichia coli and Klebsiella. J
Microbiol Immunol Infect. 39:496–502. 2006.PubMed/NCBI
|
|
21
|
Huijbers PM, Graat EA, Haenen AP, et al:
Extended-spectrum and AmpC β-lactamase-producing Escherichia coli
in broilers and people living and/or working on broiler farms:
prevalence, risk factors and molecular characteristics. J
Antimicrob Chemother. 69:2669–2675. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jain A, Roy I, Gupta MK, Kumar M and
Agarwal SK: Prevalence of extended-spectrum
beta-lactamase-producing Gram-negative bacteria in septicaemic
neonates in a tertiary care hospital. J Med Microbiol. 52:421–425.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jacoby GA and Carreras I: Activities of
beta-lactam antibiotics against Escherichia coli strains producing
extended-spectrum beta-lactamases. Antimicrob Agents Chemother.
34:858–862. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harada S, Ishii Y and Yamaguchi K:
Extended-spectrum beta-lactamases: implications for the clinical
laboratory and therapy. Korean J Lab Med. 28:401–412. 2008.
View Article : Google Scholar : PubMed/NCBI
|