Introduction
Central neurocytomas (CNC), World Health
Organization (WHO) grade II, are relatively rare central nervous
system (CNS) tumors that are often located in the ventricular
system, particularly the septum, the third ventricle and the
lateral ventricles. However, this entity has also been reported
outside the ventricular system, and thus, was termed as
extraventricular neurocytoma (EVN), which exhibits a wide
variability in respect of morphological characteristics,
cellularity and proliferation rate and are more frequently
associated with aggressive histological features (1). The present study reports an unusual case
of sellar neurocytoma and reviews the associated studies.
Case report
A 56-year-old male patient with a past history of
hypertension and left ocular trauma presented with a headache
following a head injury. A neoplasm of the sellar region was
occasionally identified from the computed tomography (CT) that was
performed in a local hospital. Physical examination on admission
revealed a well-developed and nourished male. There were no signs
of hypophysial dysfunction on admission. Ophthalmological tests
disclosed bitemporal hemianopsia and decreased visual acuity in the
left eye (0.2). Asymmetry in bilateral pupillary size was found;
3.5 and 2.5 mm in the left and right, respectively. Direct
pupillary light reflex of the left eye was slower than the right,
while the indirect pupillary light reflex of both was the same. The
remainder of the neurological examination was normal.
Routine laboratory tests revealed a mildly decreased
cortisol (8 a.m.) level (6.8 µg/dl; normal range, 8.7–22.4 µg/dl).
The remaining pituitary hormone was normal.
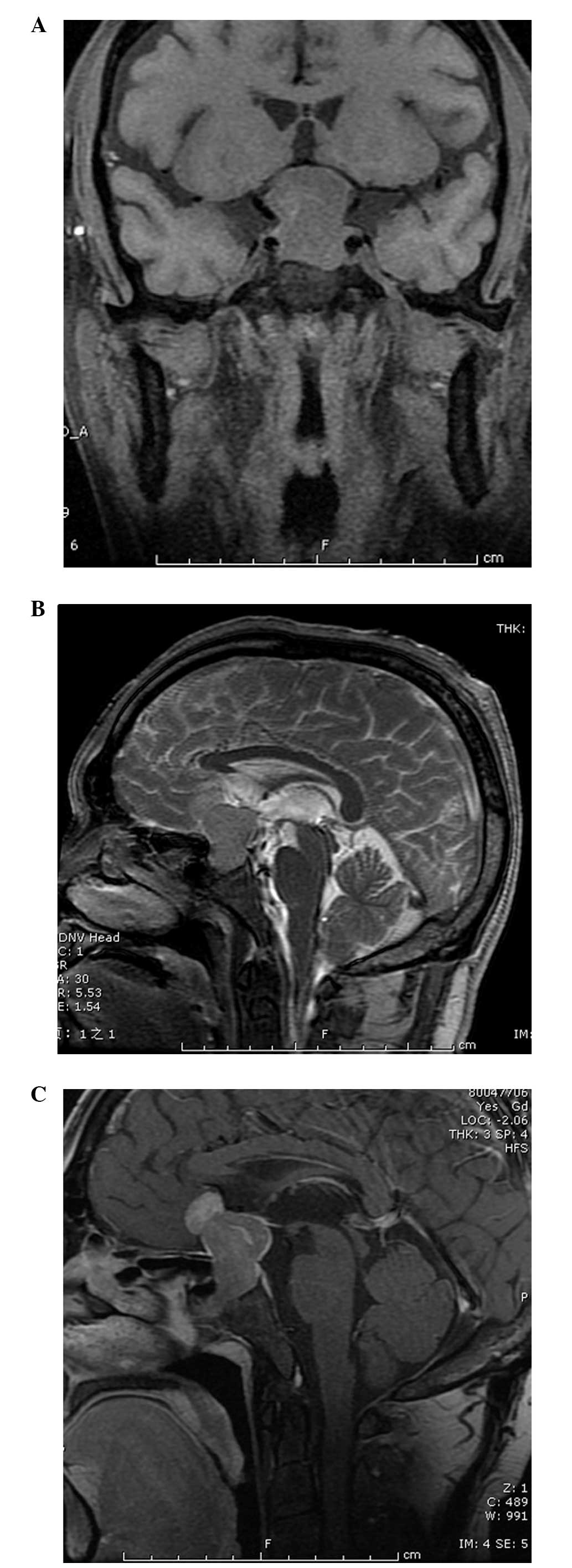
A CT scan revealed a sellar region tumor without
calcification. Coronal T1-weighted magnetic resonance imaging (MRI)
(Fig. 1A) of the patient showed a
well-defined dumbbell-shaped pituitary mass (3×2 cm) that was
hypointense to the brain parenchyma with compression of the optic
chiasm and the third ventricle. On sagittal T2-weighted MRI
(Fig. 1B) studies, the signal was
isointense to the surrounding grey matter with infiltration of the
sphenoid sinus and suprasellar space. Following the intravenous
administration of gadolinium (Fig.
1C), the neoplasm was enhanced heterogeneously. Magnetic
resonance angiography depicted that the tumor closely adhered to
the bilateral internal carotid arteries, without encasement.
Therefore, pre-surgical radiological diagnosis was possibly a
pituitary macroadenoma.
Following successful endotracheal intubation using
general anesthesia, the patient was in the supine position with
head rightward by ~45 degrees. An extended left side pterional
cranioctomy was performed, the carotid cistern and optic chiasm
cistern were opened, the left internal carotid artery and optic
nerve were separated and finally the tumor was exposed under the
surgical microscope. The tumor was of a soft texture, was red in
color and there was no abundant bleeding. Satisfactory clearance of
the neoplasm was achieved by piecemeal resection without
postoperative complications.
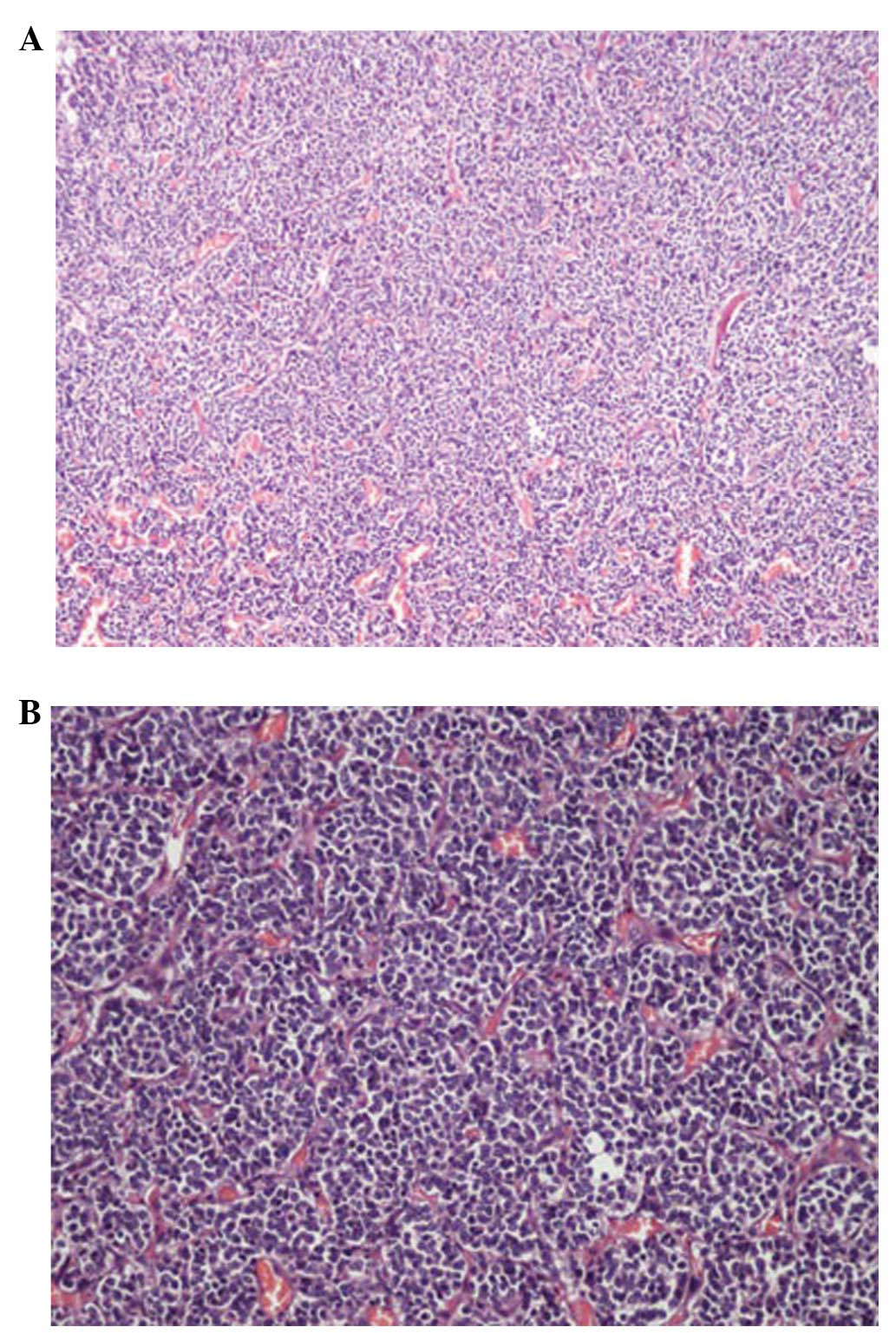
Histological examination of the tumor revealed a
neurocytoma composed of uniform sheets, small-to-medium-sized tumor
cells with round regular nuclei and salt-and-pepper chromatin,
along with scant cytoplasm (Fig. 2A and
B). These cells are closely arrayed, with a background of
filamentous matrix having a neuropil-like quality. Thin-walled
capillary-sized vessels, arranged in a linear arborizing pattern,
were observed. Immunohistochemically, synaptophysin (SYN),
chromogranin-A (CGA) and neuron-specific enolase (NSE) were
positive, while luteinizing hormone (LH), follicle-stimulating
hormone (FSH), growth hormone (GH), prolactin (PRL),
adrenocorticotropic hormone (ACTH), thyroid stimulating hormone
(TSH), glial fibrillary acidic protein (GFAP), S-100, nestin and
epithelial membrane antigen (EMA) were negative and Ki-67
proliferation index was at a level of 0–1%. Finally, the WHO
grading of the entity was II.
Discussion
CNC, first named by Hassoun in 1982, accounts for
0.25–0.5% of all CNS tumors and usually affects young adults of
approximately thirty years of age, with a range between eight days
and 67 years (2,3). This type of entity is usually located
within the ventricular system, particularly the foramina of Monro.
However, central neurocytoma-like neoplasms that arise within brain
parenchyma have been described in different studies. EVN, first
reported in 1989, is generally observed to affect children and
young adults, with cases ranging from 5 to 76 years (median, 34
years), without gender predominance (4). According to previous studies, the
cerebral hemisphere, thalamus, cerebellum, pons, amygdala, pineal
gland, retina and spinal cord have been documented as the location
of EVN. The present study is the fourth case of EVN in the sellar
region (5,6).
Analyzing the four case reports for the clinical
manifestation, decreased visual acuity and hemianopsia are common.
Endocrine tests are usually normal.
Although the radiographic differentiation of EVNs
and other tumors has not been well described, EVNs have their own
imaging characteristic. Generally, EVNs are discrete, sometimes
large, complex and variably enhancing masses (5). The content of EVNs can be solid, cystic
or both, with or without calcification. On T1-weighted MRI images,
the signal of solid mass was isointense or slightly hypointense,
while on T2 hyperintense images, there is a moderate to strong
enhancement following gadolinium administration. However, Aralasmak
et al (7) reported one case
without enhancing. Therefore, they believed that enhancement is not
always necessary for EVNs. Peritumoral edema can be observed in
certain cases and hemorrhage of tumors was sporadically reported
(8). Proton MR spectroscopic studies
of EVNs demonstrate the following typical changes: Elevated
choline, decreased N-acetylaspartate and decreased creatine
(5,9).
When a parenchymal tumor with cystic necrosis, calcification and
extensive enhancement in a young patient was encountered, EVN
should be in the range of differential diagnosis.
The diagnosis of EVNs was based on the
immunohistochemical features, given the overlap of clinical,
radiological and histological features with those of other CNS
tumors, particularly oligodendroglioma. SYN- and NSE-positive
staining suggests a diagnosis of neurocytoma; even NSE is not
specific for neurocytoma. A GFAP immunopositive reaction is usually
observed in gliomas, such as oligodendroglioma, while negative is
in the majority of EVNs. Zhu et al (10) found that neuronal nuclear antigen
(NeuN) was positive in almost all cases of CNC. Therefore, they
believed that NeuN can be applied as another reliable marker of
neurocytoma. From a genetic perspective, without the 1p/19q
deletion and the absence of p53 immunoexpression,
O6-methylguanine-DNA methyltransferase promoter methylation and low
frequency of epidermal growth factor receptor gene amplification
are the characteristics of EVNs, which differentiate it from
oligodendrogliomas (11). Electron
microscopic evaluation is necessary to confirm the diagnosis when
encountering an ambiguous immunophenotype. In the patient of the
present study, NSE and SYN stained positive, while GFAP, S-100,
nestin and EMA were negative, suggesting the diagnosis of a
neurocytoma. Expression of CGA suggests this entity was admixed
with ganglion cells. Absence of LH, FSH, GH, PRL, ACTH and TSH
excludes the diagnosis of a pituitary adenoma.
Radiotherapy and chemotherapy have played an
important role in the management of patients with EVNs. Rades et
al (12) have documented that
postoperative radiotherapy improved the local control and survival
rates of patients with incomplete resection. The optimal dose of
radiotherapy appears to be 54–60 Gy (3). Gamma-knife surgery delivers high-dose
radiation with minimal long-term side effects. Although the
experience in chemotherapy is not rich, certain investigators have
found it beneficial in recurrent EVNs that cannot be totally
resected and failed in radiotherapy. von Koch et al
(13) treated a 20-year-old female who
underwent four subtotal resections, but exhibited an enlarged
tumor, with procarbazine, lomustine and vincristine for
chemotherapy. The tumor size started decreasing subsequent to two
cycles of treatment and continued to shrink until it stabilized
after five cycles. In the present case, gross total resection was
performed and the patient did not receive radiotherapy or
chemotherapy. A long-term follow-up workshop should be
performed.
In conclusion, EVNs are relatively rare tumors.
Differential diagnosis with other tumors, particularly
oligodendrogliomas, is difficult as they share common clinical,
radiological and histological features. Total excision should be
the goal of treatment and adjuvant radiotherapy should be
considered in the case of subtotal resection.
References
|
1
|
Brat DJ, Scheithauer BW, Eberhart CG and
Burger PC: Extraventricular neurocytomas: Pathologic features and
clinical outcome. Am J Surg Pathol. 25:1252–1260. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sgouros S, Jackowski A and Carey MP:
Central neurocytoma without intraventricular extension. Surg
Neurol. 42:335–339. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharma MC, Deb P, Sharma S and Sarkar C:
Neurocytoma: A comprehensive review. Neurosurg Rev. 29:270–285.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferreol E, Sawaya R and de Courten-Myers
GM: Primary cerebral neuroblastoma (neurocytoma) in adults. J
Neurooncol. 7:121–128. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang GF, Wu SY, Zhang LJ, Lu GM, Tian W
and Shah K: Imaging findings of extraventricular neurocytoma:
Report of 3 cases and review of the literature. AJNR Am J
Neuroradiol. 30:581–585. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Tao R and Liu B: Response to:
Extraventricular neurocytoma of the sellar region. Br J Neurosurg.
27:551–552. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aralasmak A and Karaali K: Nonenhancing
hypovascular extraventricular neurocytoma. AJNR Am J Neuroradiol.
30:E117–E118. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ritz R, Roser F, Bornemann A, Hahn U and
Freudenstein D: Extraventricular neurocytoma presenting with
intratumoral hemorrhage. Clin Neuropathol. 24:101–105.
2005.PubMed/NCBI
|
|
9
|
Ueda F, Suzuki M, Matsui O and Uchiyama N:
Automated MR spectroscopy of intra- and extraventricular
neurocytomas. Magn Reson Med Sci. 6:75–81. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu P, Yan F, Ma Y and Ao Q:
Clinicopathological analysis of central and extraventricular
neurocytoma: A report of 17 cases. J Huazhong Univ Sci Technolog
Med Sci. 30:746–750. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Myung JK, Cho HJ, Park CK, Chung CK, Choi
SH, Kim SK and Park SH: Clinicopathological and genetic
characteristics of extraventricular neurocytomas. Neuropathology.
33:111–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rades D, Fehlauer F, Lamszus K, Schild SE,
Hagel C, Westphal M and Alberti W: Well-differentiated neurocytoma:
What is the best available treatment? Neuro Oncol. 7:77–83. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
von Koch CS, Schmidt MH, Uyehara-Lock JH,
Berger MS and Chang SM: The role of PCV chemotherapy in the
treatment of central neurocytoma: Illustration of a case and review
of the literature. Surg Neurol. 60:560–565. 2003. View Article : Google Scholar : PubMed/NCBI
|