Introduction
The skin is the largest organ in the body that is
divided into two anatomically distinct regions, the dermis and
epidermis. The normal structure and function of this organ is
dependent on the intact epidermis anchored to its vascular, elastic
dermis (1,2). Fibroblasts are the most prevalent cell
types in the dermis, which produce different growth factors that
induce proliferation of keratinocytes in vivo and in
vitro (3). The principal cell type
of the epidermis is the keratinocyte (1,4), which is a
small epithelial cell, located at the top of the epidermal basal
membrane and characterized by a low division rate (5,6).
Thus far, various enzymatic methods for
dermal-epidermal separation have been applied (6–8). For
instance, trypsin separates suprabasal hemidesmosomes that causes
the basal layer cells to attach to the dermal layer (6). Thermolysin is another enzyme that
selectively separates desmosomes (5,9) and is able
to separate the epidermis at the basal membrane zone level
(5,10).
Although the dissociation method of keratinocytes in
primary culture is well-established, attempts to acquire purified
adult stem-cell like⁄progenitor keratinocytes from whole human skin
are still ongoing. In particular, different techniques are
currently being applied to achieve high purity or homogeneous
primary cultures enriched in keratinocyte progenitor⁄precursor
cells. These include filtration, density gradient centrifugation
and fluorescence-activated cell sorting using cell surface
antibodies, as well as differential adhesion to enrich the cells
that rapidly attach to particular substrates (11).
A previous study showed that when
keratinocytes/precursor cells separate from the epidermal layer
using enzymatic treatment, a number of these cells will not be able
to develop colonies, due to their state of differentiation. Only
3–4% of the keratinocytes can form colonies, even under optimal
culture conditions. The formation and confluence of the colonies
are directly associated with the keratinocyte layer produced, which
will be used as a graft (9).
Numerous studies have been performed on the foreskin
of children, whose cells have an extremely different replicative
profile to keratinocytes in comparison to older skin (1,12,13). Previous studies have shown that the
keratinocytes appear to migrate out from whole skin explants over
the first few days in culture (14).
Additionally, certain studies have shown that these early migrating
cells originate from the basal layer of the epidermis, and
fibroblasts do not grow out from adult human skin explants until
several days after the appearance of keratinocytes (14,15). This
time lag between the migration of keratinocytes versus fibroblasts
can be used to reach the pure keratinocytes. Explant-derived
keratinocytes can be grown rapidly to multiple passages using the
current methods of culture, and notably, the original explants can
be recycled and used as a continuing source of keratinocytes. Using
previous data associated with the explants culture of children's
foreskin, the present study aimed to establish a feasible method in
the separation and growth of differentiating keratinocytes in
culture medium.
Materials and methods
Tissue collection
Immediately following circumcision of children (age
range, 8 day-2 year old), and obtaining parental consent, the
foreskin samples were collected from a private clinic and were kept
in the bottle containing transfer medium on ice during
transportation. The transfer medium was phosphate-buffered saline
(PBS) containing 0.5 µg/ml amphotericin B, 100 IU/ml gentamycin,
100 IU/ml penicillin and 100 µg/ml streptomycine (all from Gibco
Life Technologies, Grand Island, NY, USA). In total, 20 samples
were collected during 2012.
Tissue preparation
Method I: The procedure was started immediately in
the cell culture room of the Cellular and Molecular Research Center
(CMRC; Ahvaz, Iran). The samples were placed in 70% ethanol for 10
sec and washed 3–5 times with PBS. The whole hypodermis layer and
associated blood vessels were discarded. Subsequently, the samples
were cut into 2–3 mm pieces and put in 0.25% trypsin (Gibco Life
Technologies) at 4°C overnight. During the overnight treatment, the
epidermal layer was separated from the dermis. The isolated
epidermal layer was placed in a petri dish containing trypsin at
37°C for 15 min until the epidermis became loose and the
keratinocytes were released easily. Subsequently, the suspended
cells were centrifuged at 1,400 × g for 15 min and the cell pellet
was placed in a 25 cm2 flask and divided into two
groups: i) With no feeder layer and ii) onto a type I collagen
scaffold (Sigma-Aldrich, St. Louis, MO, USA).
Method II: When the dissociation of the epidermis
from the dermis was performed using trypsin, explants of isolated
epidermis layer were prepared. The size of the explant pieces were
2–3 mm and 10–15 pieces were placed in each flask, which were
divided into two groups: i) With no feeder layer and ii) onto a
type I collagen scaffold. One drop full of medium was added to each
piece of explant. The medium components contained DMEM/F12, 10%
fetal bovine serum, 100 IU/ml penicillin, 100 µg/ml streptomycin,
25 µg/ml gentamycin, 2 mM L-glutamine, 1 µg/ml amphotericin B, 0.4%
bovine pituitary extract, 0.125 ng/ml epidermal growth factor human
recombinant, 5 µg/ml human insulin, 0.33 µg/ml hydrocortisone, 10
µg/ml human transferrin, 0.39 µg/ml epinephrine and 0.15 mM
CaCl2. After one day, 1 ml of medium was added to each
flask and the cells began to separate from the explants. During the
4th day, the sections of the non-digested explants were removed and
1 ml of medium was added to the flask. Subsequently, the cells in
the flasks were maintained in this condition until the 6th day.
During the 6th day, the whole medium was removed and replaced by
fresh medium. Cells were checked daily to observe the normal
growth.
Immunocytochemistry (ICC)
For characterization of the keratinocytes, cultured
cells with 50% confluency were fixed in 2% paraformaldehyde and
permeabilized by 0.1% Triton X-100 in PBS. Following three washes
in PBS, the cells were incubated in a common type of monoclonal
antibody, anti-human anti-pan cytokeratin (0.001 µg/ml), produced
by the C11 hybrid cells (C11, sc-8018; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) at 4°C overnight. After washing three times
with PBS, anti-mouse-immunoglobulin-fluorescein isothiocyanate
(cat. no. sc-2010; Santa Cruz Biotechnology) was used as a
secondary conjugated antibody (16).
The cultures were stained with
4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI; blue
fluorescence) for 30 min to show the cell nuclei of the
keratinocytes (17). Subsequently, the
cells were washed with PBS three times and the positive cells were
detected using a fluorescence microscope.
Results
Enzymatic method
In the enzymatic method, trypsin was applied for
separation of the keratinocytes from the epidermal layer, and after
~7–10 days no attached cells were found in the two cell culture
dishes.
Keratinocyte outgrowth from skin
explants
Keratinocytes were separated from the tissue
explants after ~24 h. However, to prevent the appearance of
fibroblasts at ~5 days, the epidermal cells were removed after 4
days as an alternative method to obtain keratinocyte primary
cultures free of fibroblasts. In the first few days (1–2 days), an
early migration phase of keratinocytes from explants were observed
and the population of keratinocytes increased due to keratinocyte
migration and proliferation.
For generating organotypic co-cultures, epidermal
keratinocytes were plated onto the upper surface of the collagen
scaffold, where they attached rapidly and formed big colonies. In
the absence of a feeder layer, small colonies developed with a
rapid loss of proliferation within 2–3 days.
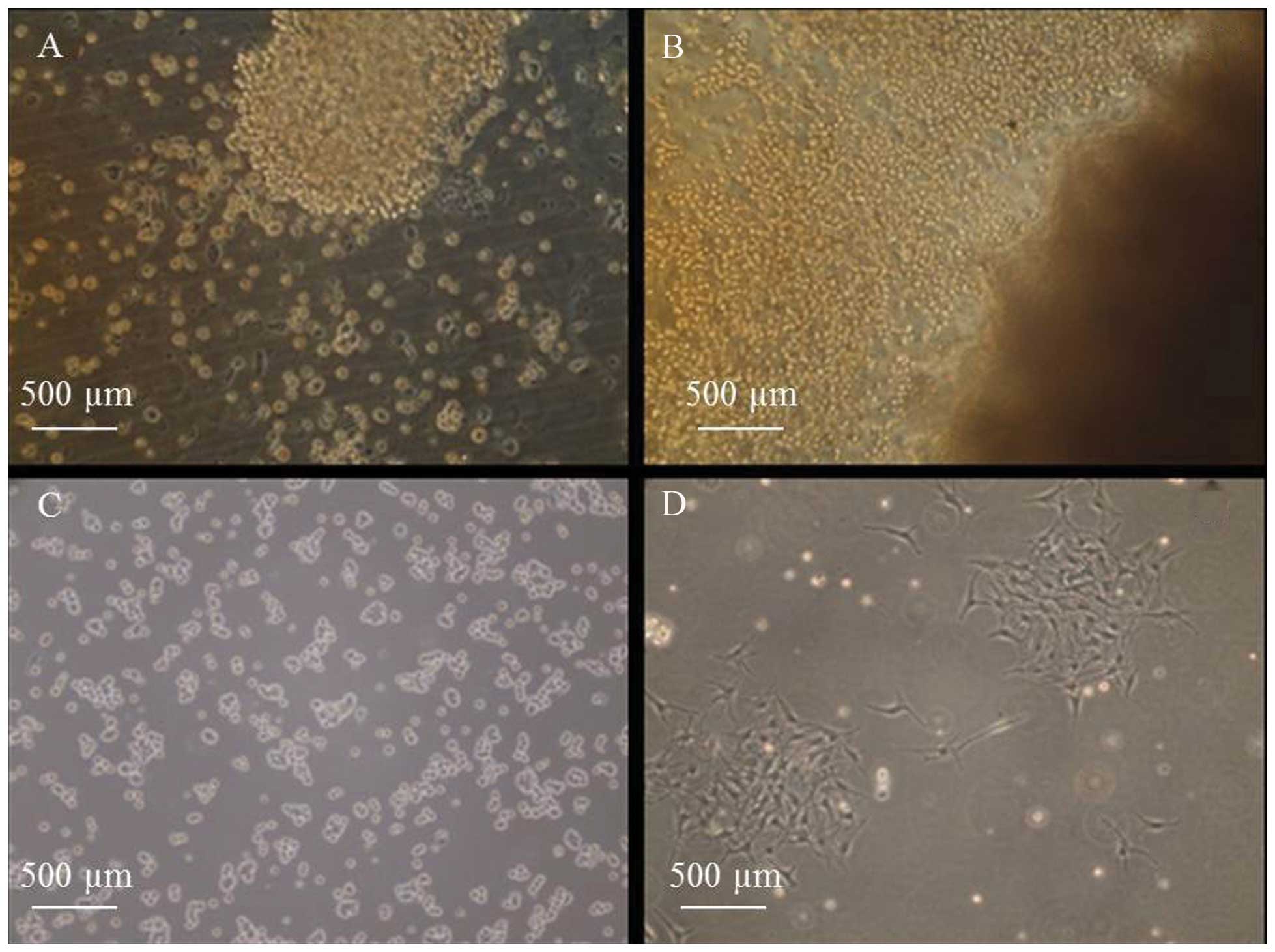
In the collagen-coated flask, keratinocytes were
first observed growing out from the explants in a continuous sheet
between 24 and 36 h. By day 2, the outgrowth resembled a ring
surrounding the explants (Fig. 1A) and
during days 3 and 4, this outgrowth continued to expand (Fig. 1B). During day 6, colonies of cells
started to form (Fig. 1C) and on day
12, large colonies of keratinocytes appeared (Fig. 1D).
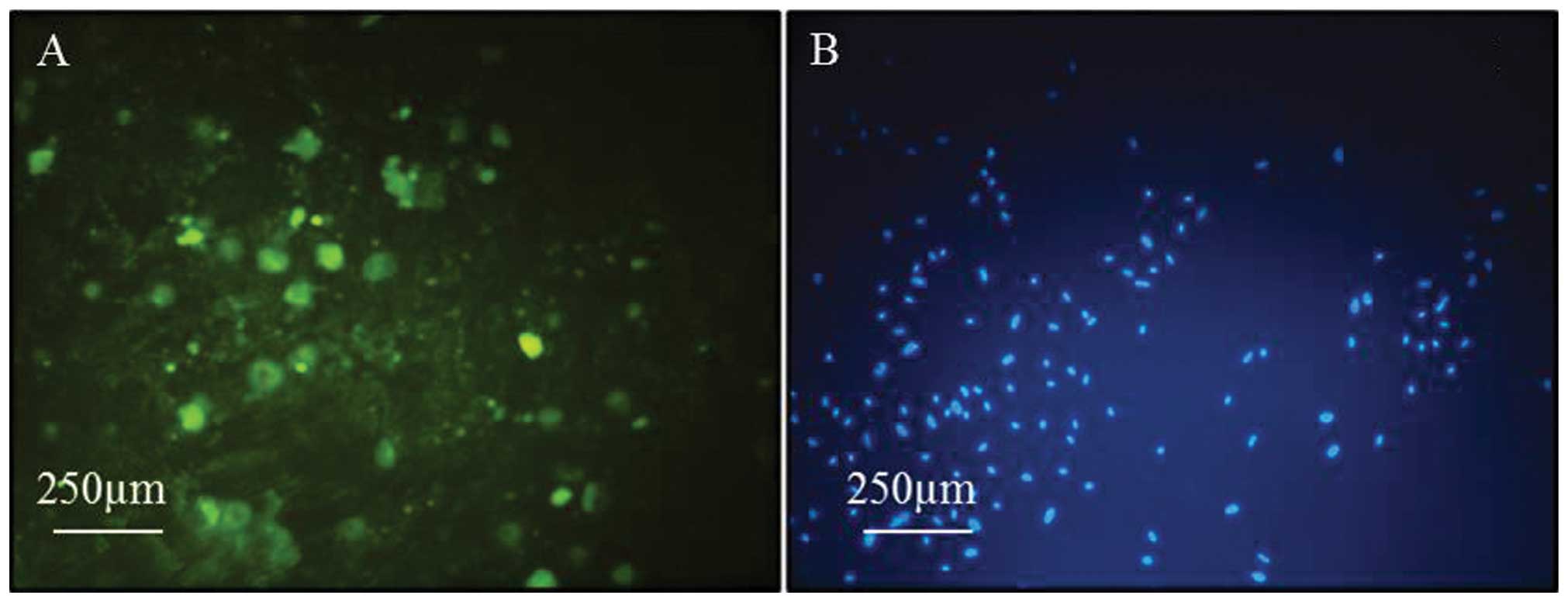
ICC
The specific marker that cells express as they grow
out from the explants was examined. When the cells reached 50%
confluence, dual staining was carried out. Cells were immunostained
for pan-cytokeratin, which is a known standard marker for
keratinocytes. The keratinocytes population showed strong positive
immunoreactivity for the pan-cytokeratin marker (Fig. 2A).
Extremely clear blue staining of the keratinocyte
nuclei were observed when the growing cells were stained with the
DAPI staining method (Fig. 2B).
Discussion
In the present study, an explant method was
developed for the primary keratinocyte precursor cell culture
derived from the foreskin of children. The method was rapid, simple
and reliable for the generation and differentiation of
keratinocytes without fibroblast contamination. Using this method,
the cells showed clear growth characteristics and a typical cell
profile presentation.
Two different methods were compared for the
separation of keratinocytes from the epidermal layer: Enzymatic
method using trypsin and the explant method. In the enzymatic
treatment after ~7–10 days, no attached cells were found in both
cell culture dishes, but using the explant method, the cells showed
clear growth characteristics and typical cell profile presentation.
Consistent with the present study, Leigh et al (9) showed that when keratinocytes are
separated from the epidermal layer by the enzymatic method, even
under excellent culture conditions, only 3–4% of the keratinocytes
form colonies. Guo et al (13)
investigated the explant method for keratinocyte culture using a
serum-free medium known as Epilife™. The study showed that serial
outgrowths of keratinocytes could be harvested from the same
explant, which further enhances the utility of this method as it
provides the possibility of obtaining much larger numbers of
keratinocytes from a single source. Regardless of the present
study, McHeik et al (18)
isolated keratinocytes following a double-enzymatic digestion
(dispase and trypsin) and harvested on average of 11.5 million
cells from 4 cm2 of foreskin tissue.
The explant culture method is where a small piece of
skin will settle on a culture dish and produce a sizeable outgrowth
of cells.. This method has long been employed as a model of wound
healing or adult skin epidermal outgrowth rather than a source of
keratinocytes for clinical or experimental purposes (19–24). The
major limitation of the explant method is due to the fact that
fibroblasts also grow out from the same explants and will
eventually outgrow the keratinocytes. In the present study,
keratinocytes were separated from explants after ~24 h, which is
consistent with the previous study by Guo et al (13). As no fibroblast outgrowth was observed
until at least 5 days, it was possible to obtain fibroblast-free
populations of keratinocytes by removing the explants from the dish
after 4 days.
In the present study, in the first few days (1–2
days) an early migration phase of keratinocytes from explants was
observed and the population of keratinocytes increased due to
keratinocyte migration and proliferation. Consistent with this, the
studies by Stoll et al (21)
and Clark (25) explained that
epidermal reepithelialization in skin wounds can be divided into
two phases: i) Early migration lasting 1–2 days during which
keratinocytes do not proliferate but migrate rapidly in order to
close the wound, and ii) a second phase, beginning at day 3, which
is characterized by strong keratinocyte proliferation. A similar
process was observed in explant cultures of human skin.
For generating organotypic co-cultures, epidermal
keratinocytes were plated onto the upper surface of the collagen
scaffold, where they rapidly attached and formed large colonies. In
the absence of a feeder layer, small colonies were developed with a
rapid loss of proliferation within 2–3 days. The co-cultured
fibroblasts can be demonstrated to produce growth factors in an
in vitro system, which are essential for epidermal
morphogenesis, as previously explained by Witte and Kao (3). Pajoum Pajoum et al (1) showed that the growth of isolated human
skin keratinocytes in modified medium was increased compared to
serum-free medium. The different evaluations of the
collagen-chitosan scaffold showed that it is important for
keratinocyte growth and has a good flexibility in the manipulation
of living skin equivalents.
There are numerous studies that have focused on the
development of nutritionally optimized, readily defined,
reproducible media and culture conditions for keratinocyte cells
(26–29). The aims of the present study were not
to establish a new medium, and therefore, the Rheinwald and Green
(30) protocol was applied. The
present results showed that a new combination worked well. The
latter protocol utilized DMEM supplemented with growth factors that
are mentioned in the present study.
Although Coolen et al (29) showed that keratinocytes can be cultured
without a fibroblast feeder layer and fetal calf serum, the present
results demonstrated that in the modified medium and onto a type I
collagen scaffold (as a feeder layer), keratinocyte cell growth was
greater than the condition without a feeder layer, and this is
consistent with certain previous studies by Gingras et al
(31) and Arpornmaeklong et al
(32) in 2007. However, a long-term
follow-up study is required to more precisely evaluate its fate
following proper grafting.
In conclusion, the keratinocytes obtained from the
explant culture were shown to exhibit a specific marker and grow
for multiple passages when plated onto the upper surface of the
collagen scaffold. From a clinical and practical standpoint, this
method provides a useful and simple method for growing large
numbers of keratinocytes from only a small biopsy quickly.
Finally, this practical method would be useful for
separation and growth of the keratinocyte precursor cell, which is
a suitable choice for tissue engineering and cell therapy in a
number of skin diseases.
Acknowledgements
The present study was financially supported by Ahvaz
Jundishapur University of Medical Sciences (grant no. CMRC-60), and
experimental studies were performed at CMRC. The authors wish to
thank Dr Amir Homayon Zandvakili for the preparation of the
foreskin samples.
References
|
1
|
Pajoum Shariati SR, Shokrgozar MA,
Vossoughi M and Eslamifar A: In vitro co-culture of human skin
keratinocytes and fibroblasts on a biocompatible and biodegradable
scaffold. Iran Biomed J. 13:169–177. 2009.PubMed/NCBI
|
|
2
|
Yannas IV: What criteria should be used
for designing artificial skin replacements and how well do the
current grafting materials meet these criteria. J Trauma. 24 Suppl
9:S29–S39. 1984.PubMed/NCBI
|
|
3
|
Witte RP and Kao WJ:
Keratinocyte-fibroblast paracrine interaction: The effects of
substrate and culture condition. Biomaterials. 26:3673–3682. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parenteau NL, Nolte CM, Bilbo P, Rosenberg
M, Wilkins LM, Johnson EW and Watson S: Mason VS and Bell E:
Epidermis generated in vitro: Practical considerations and
applications. J Cell Biochem. 45:245–251. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Green H: Cultured cells for the treatment
of disease. Sci Am. 265:96–102. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gragnani A, Sobral CS and Ferreira LM:
Thermolysin in human cultured keratinocyte isolation. Braz J Biol.
67:105–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tompkins RG and Burke JF: Alternative
wound coveringsTotal Burn Care. Herndon D.N: W.B. Saunders Company
Ltda; pp. 164–172. 1996
|
|
8
|
Morgan JR and Yarmush M: Bioengineered
skin substitutes. Sci Med. 4:6–15. 1997.
|
|
9
|
Leigh IM, Lane EB and Watt FM: The
Keratinocyte Handbook. 1st edition. Cambridge University Press;
Cambridge: pp. 5661995
|
|
10
|
Walzer C, Benathan M and Frenk E:
Thermolysin treatment: A new method for dermo-epidermal separation.
J Invest Dermatol. 92:78–81. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kaur P and Li A: Adhesive properties of
human basal epidermal cells: An analysis of keratinocyte stem
cells, transit amplifying cells, and postmitotic differentiating
cells. J Invest Dermatol. 114:413–420. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barrandon Y and Green H: Three clonal
types of keratinocyte with different capacities for multiplication.
Proc Natl Acad Sci USA. 84:2302–2306. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo A and Jahoda CA: An improved method of
human keratinocyte culture from skin explants: Cell expansion is
linked to markers of activated progenitor cells. Exp Dermatol.
18:720–726. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Taylor JR, Halprin KM, Levine V and
Woodyard C: Effects of methotrexate in vitro on epidermal cell
proliferation. Br J Dermatol. 108:45–61. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Van Der Schueren B, Cassiman J-J and Van
Den Berghe H: Morphological characteristics of epithelial and
fibroblastic cells growing out from biopsies of human skin. J
Invest Dermatol. 74:29–35. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen TA, Halliwell RE and Hill PB: Failure
of extracts from Malassezia pachydermatis to stimulate canine
keratinocyte proliferation in vitro. Vet Dermatol. 13:323–329.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fischer TW, Zbytek B, Sayre RM, Apostolov
EO, Basnakian AG, Sweatman TW, Wortsman J, Elsner P and Slominski
A: Melatonin increases survival of HaCaT keratinocytes by
suppressing UV-induced apoptosis. J Pineal Res. 40:18–26. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McHeik JN, Barrault C, Bernard FX and
Levard G: Quantitative and qualitative study in keratinocytes from
foreskin in children: Perspective application in paediatric burns.
Burns. 36:1277–1282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Halprin KM, Lueder M and Fusenig NE:
Growth and differentiation of postembryonic mouse epidermal cells
in explant cultures. J Invest Dermatol. 72:88–98. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hammar H: Stimulated mouse ear epidermis
in explant culture- The effect of retinoic acid and hexadecane.
Arch Dermatol Res. 270:469–481. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stoll SWK, Kansra S and Elder JT:
Keratinocyte outgrowth from human skin explant cultures is
dependent upon p38 signaling. Wound Repair Regen. 11:346–353. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Karasek MA: In vitro culture of human skin
epithelial cells. J Invest Dermatol. 47:533–540. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koeper L-M, Schulz A, Ahr HJ and Vohr H-W:
In vitro differentiation of skin sensitizers by cell signaling
pathways. Toxicology. 242:144–152. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mutasim DF, Vaughan A, Supapannachart N
and Farooqui J: Skin explant culture: A reliable method for
detecting pemphigoid antibodies in pemphigoid sera that are
negative by standard immunofluorescence and immunoblotting. J
Invest Dermatol. 101:624–627. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Clark R and Henson PM: The Molecular and
Cellular Biology of Wound Repair. Springer; Berlin: 1996
|
|
26
|
Rosdy M and Clauss L: Complete human
epidermal cell differentiation in chemically defined medium at the
air-liquid interface on inert filter substrates. J Invest Dermatol.
95:409–414. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Barreca A, De Luca M, Del Monte P,
Bondanza S, Damonte G, Cariola G, Di Marco E, Giordano G, Cancedda
R and Minuto F: In vitro paracrine regulation of human keratinocyte
growth by fibroblast-derived insulin-like growth factors. J Cell
Physiol. 151:262–268. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matsumoto K, Hashimoto K, Yoshikawa K and
Nakamura T: Marked stimulation of growth and motility of human
keratinocytes by hepatocyte growth factor. Exp Cell Res.
196:114–120. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Coolen NA, Verkerk M, Reijnen L, Vlig M,
van den Bogaerdt AJ, Breetveld M, Gibbs S, Middelkoop E and Ulrich
MM: Culture of keratinocytes for transplantation without the need
of feeder layer cells. Cell Transplant. 16:649–661. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rheinwald JG and Green H: Serial
cultivation of strains of human epidermal keratinocytes: The
formation of keratinizing colonies from single cells. Cell.
6:331–343. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gingras M, Paradis I and Berthod F: Nerve
regeneration in a collagen-chitosan tissue-engineered skin
transplanted on nude mice. Biomaterials. 24:1653–1661. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Arpornmaeklong P, Suwatwirote N,
Pripatnanont P and Oungbho K: Growth and differentiation of mouse
osteoblasts on chitosan-collagen sponges. Int J Oral Maxillofac
Surg. 36:328–337. 2007. View Article : Google Scholar : PubMed/NCBI
|