Introduction
Lung cancer is one of the most common types of
cancer. Lung cancer is the leading cause of cancer mortalities
worldwide, including China. There have been certain small
improvements in the overall survival rate due to the advances in
treatment of lung cancer; however, the 5-year survival rate remains
low at ∼15% (1) as the majority of
lung cancer cases are diagnosed only when they advanced to more
malignant stages. Therefore, it is necessary to screen and diagnose
individuals at an earlier stage of lung cancer to increase the time
to manage the disease and improve clinical outcome.
A blood-based biomarker assay is a non-invasive
method to determine the presence of lung cancer during screening or
routine clinical visits, which can be useful in identifying
subjects that are most likely to have a malignant lesion in the
lung that requires further investigation (2). In nasopharyngeal carcinoma (NPC), a
blood-based gene signature (LDLRAP1, PHF20 and
LUC7L3) that accurately distinguished NPC patients from
controls and from patients with other diseases was identified
(3). In ovarian cancer, a signature of
genes involved in metastasis, invasion and inflammation were
identified in native unstimulated blood leukocytes from ovarian
cancer patients with a poor prognosis (4). The circulating STC1 mRNA is a
promising biomarker in the peripheral blood of non-small cell lung
cancer (5). A previous study
identified eight genes that can discriminate patients with lung
cancer from healthy controls with a high accuracy (6).
MALAT1 is a broadly expressed, long
non-coding RNA (∼8.7 kb in humans) in normal human and mouse
tissues and is overexpressed in numerous human carcinomas,
including cancer of the breast, pancreas, lung, colon, prostate and
liver, suggesting that MALAT1 dysregulation is indicated in
the development of numerous types of cancers (7,8). In the
present study, the expression of MALAT1 is reported in the
whole blood of lung cancer and this was lower compared to the
control. The expression of MALAT1 was stronger in the whole
blood of lung cancer with metastasis compared to non-metastasis.
Additionally, the whole blood with bone or brain metastasis
represented a higher expression of MALAT1 compared to the
blood with lymph node or pleura metastasis. Subsequently, a lower
expression of MALAT1 was detected in metastatic lymph node
tissues than that of the carcinoma in situ of lung. Taken
together, these results indicate that MALAT1 as a biomarker
to screen lung cancer may represent a host response to lung cancer.
The present study provides a novel perspective on the role of
MALAT1 in peripheral whole blood of lung cancer.
Materials and methods
Patients and blood samples
Blood samples of patients with lung cancer and
healthy volunteers (controls) were recruited at Tianjin Medical
University General Hospital (Tianjin, China) and Hebei General
Hospital (Shijiazhuang, China). Consent forms were obtained from
all the study participants according to protocols approved by the
hospitals' Research Ethics Board.
Gene expression analysis was performed on 105 blood
samples (Table I) and 35 tissue
samples obtained from patients with tumors confirmed as lung cancer
by hospital pathologists and 65 controls. All the samples were
collected between November 2011 and January 2013.
 | Table I.Characteristics of the patients and
control. |
Table I.
Characteristics of the patients and
control.
| Characteristics | Lung cancer | Control | P-value |
|---|
| No. of samples | 105 | 65 |
|
| Age, median
(range) | 51 (39–65) | 49 (35–63) | 0.75 |
| Gender |
|
|
|
| Male | 63 | 35 | 0.43 |
|
Female | 42 | 30 |
|
Blood collection, RNA isolation and
RNA quality control
Peripheral whole blood (10 ml) was collected from
patients in EDTA Vacutainer tubes and RNA was extracted with the
AxyPrep™ Blood Total RNA Miniprep kit (Axygen Scientific, Inc.,
Union City, CA, USA) in compliance with the manufacturer's
instructions. Total RNA was assessed spectrophotometrically using a
nucleic acid analyzer (Beckman Coulter, Inc., Brea, CA, USA). RNA
quality was determined by the absorbance at 260/280 nm quota and
quantity was measured as ng/µl.
cDNA synthesis and quantitative
polymerase chain reaction (qPCR) gene expression
One microgram of total RNA was transcribed into cDNA
in a 20 µl reaction volume using reverse transcriptase for
first-strand cDNA synthesis. Following reverse transcription of
RNA, all the qPCR reactions were performed using an ABI PRISM® 7000
Sequence Detection System (Applied Biosystems Life Technologies,
Foster City, CA, USA) with the designed primers for target genes
and an internal control gene, GAPDH. Each sample for each
gene was run in triplicate.
Statistical analysis
The gene expression levels in the whole blood of
lung cancer patients were compared to those in the control, or in
the lung cancer tissues compared to the metastatic lymph node
tissues with the use of the Wilcoxon test. The associations between
the gene expression levels and potential explanatory variables,
including lymph node metastasis, were evaluated with the
independent sample t-test. In addition, receiver operating
characteristic (ROC) analysis was generated by plotting the
sensitivity against the false positive rate (1-specificity) as the
discrimination between lung cancer patients and controls. Area
under the curve (AUC) was calculated. All the statistical analyses
were performed using SPSS, version 16.0 for Windows software (SPSS,
Inc., Chicago, IL, USA). Two-sided P-values were calculated and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of MALAT1 in the whole
blood of lung cancer and control patients
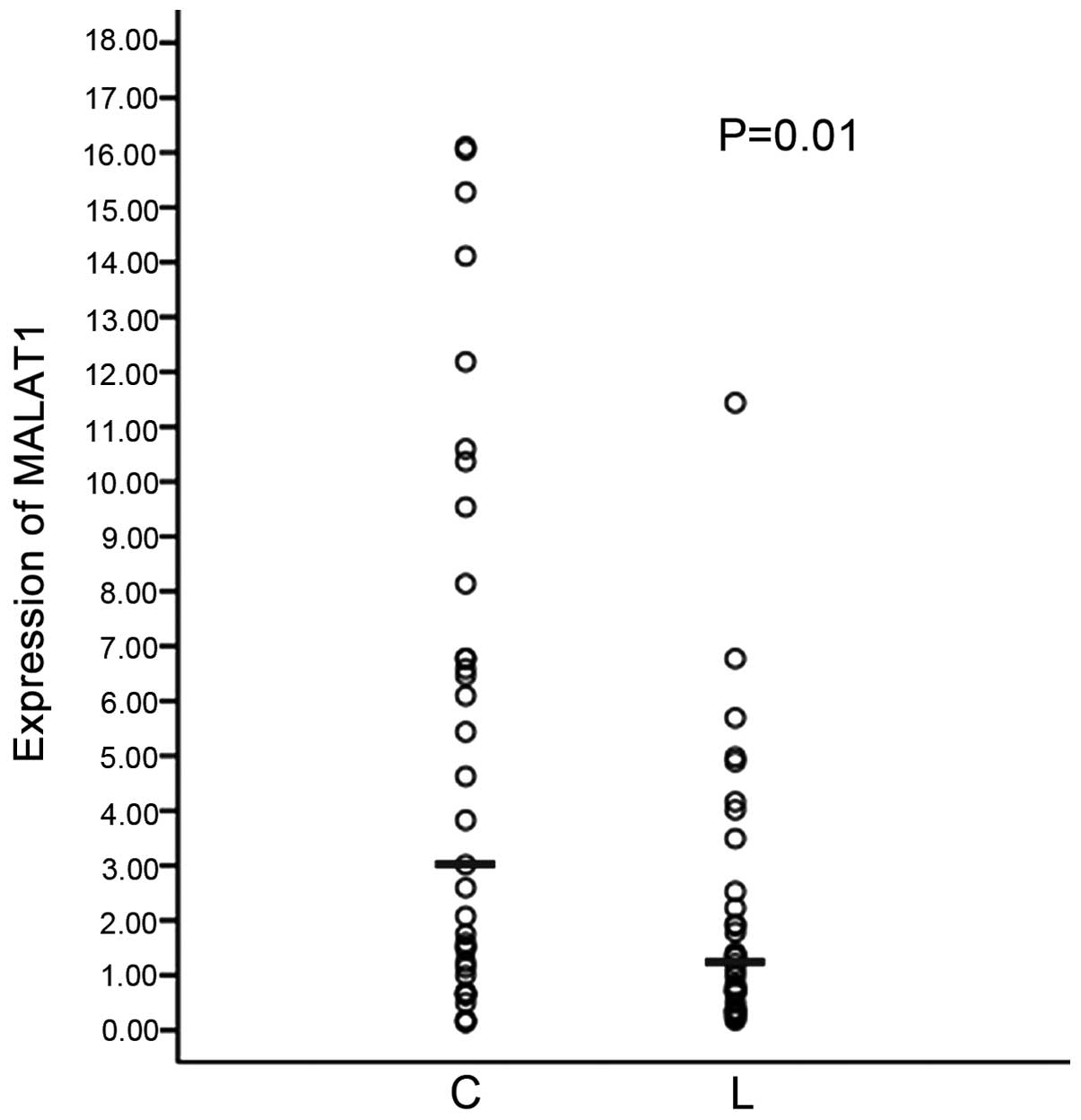
The expression level of the MALAT1 gene was
measured in the whole blood of lung cancer and control patients
using reverse transcription-qPCR. The expression of MALAT1
in the whole blood of lung cancer patients (n=105) was lower
compared to the control (n=65) (Fig.
1A). ROC curves show sensitivity versus specificity in
discriminating between lung cancer and control patients (Fig. 1B). The AUC was calculated as 0.718
(P<0.001). MALAT1 has the potential to distinguish
between cancer cases and controls with a cutoff value (10.3444) for
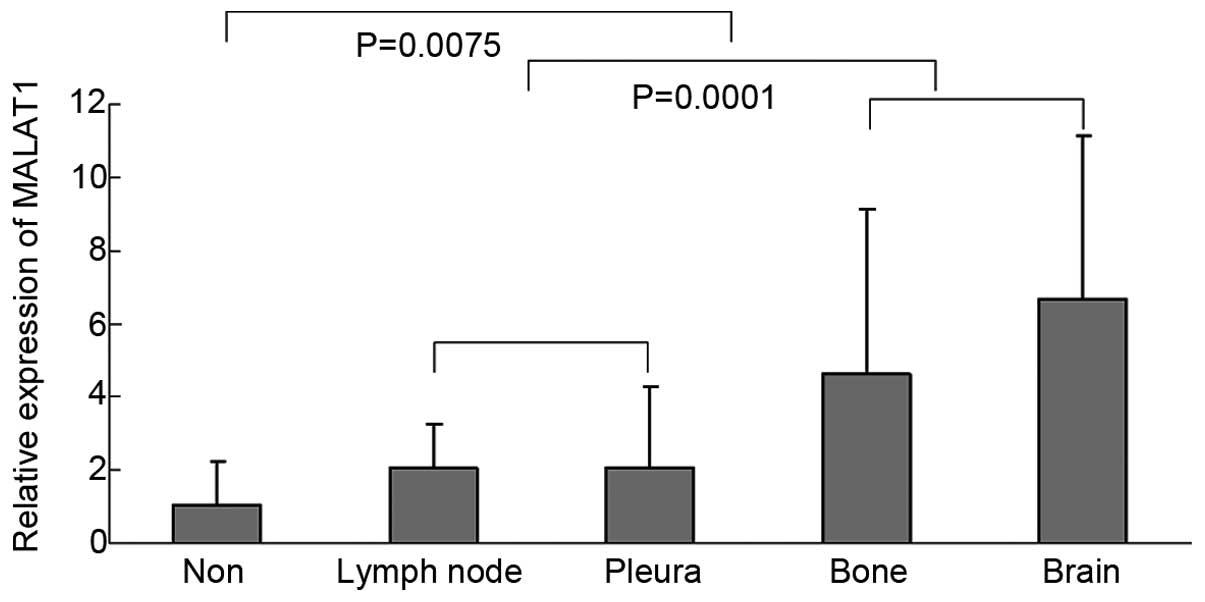
the clinical test. The correlations between MALAT1
expression and these clinicopathological features were analyzed and
the expression of MALAT1 was associated with metastasis
(lymph node, pleura, bone and brain) (Fig.
2). The expression of MALAT1 was stronger in the whole
blood of lung cancer patients with metastasis compared to weak
expression in non-metastasis. In addition, the whole blood with
bone or brain metastasis represented higher expression of
MALAT1 compared to the blood with lymph node or pleura
metastasis.
Expression of MALAT1 in lung cancer
and metastatic lymph node tissues
There is a small section of circulating tumor cells
(CTCs) that are released into blood from carcinoma in situ
in the peripheral whole blood of cancer patients and the expression
of MALAT1 is lowest in the blood with lymph node metastasis
compared to metastases of other sections. Whether CTCs with a low
MALAT1 expression metastasize to lymph nodes was questioned.
Subsequently, the expression of MALAT1 in lung cancer and
metastatic lymph nodes tissues was examined. As shown in Fig. 3, in the metastatic lymph node tissues,
the expression of MALAT1 is lower than that of the carcinoma
in situ of lung.
Discussion
MALAT1 lacks open reading frames and does not
translate MALAT1 in vitro to yield peptides,
suggesting that MALAT1 functions as a long non-coding RNA
(∼8.7 kb in humans) (7,9,10). The long
form of MALAT1 is subsequently localized to nuclear speckles
(11). MALAT1 is overexpressed
in cancer tissues. In a previous study of cervical cancer, the
inhibition of MALAT1 in CaSki human cervical cancer cells
suppressed cell proliferation and invasion (12). Another study identified that
MALAT1 enhanced cell motility of lung adenocarcinoma cells
(13). Lai et al (14) reported that MALAT1
overexpression predicted tumor recurrence of hepatocellular
carcinoma following liver transplantation. Certain studies have
reported that the long non-coding RNAs may be detected in cancers
and provide novel tumor biomarkers (15–17).
In the present study, the expression of
MALAT1 in the whole blood of lung cancer patients and
controls was explored. The expression of MALAT1 in the blood
of lung cancer was lower than that of the control. The expression
of MALAT1 in blood is contrary to that in tissues. In
tissues, the expression of MALAT1 in cancer was higher
compared to normal tissues (data not shown). Additionally, the
expression of MALAT1 was detected in carcinoma in
situ of lung and metastatic lymph node tissues. The expression
of MALAT1 in carcinoma in situ was higher compared to
that of the metastatic lymph node tissues. These results
demonstrate that MALAT1 may be able to identify lung cancer
using whole blood.
The mechanistic basis for the alterations in whole
blood MALAT1 in the presence of lung cancer is not clear.
The whole blood consists of several cell subtypes and subsets of
differentiated immune cells, such as neutrophils, lymphocytes and
monocytes, which may have different mRNA expression profiles
(18). The blood-based biomarkers
represent a host response to tumor. The changes in MALAT1
expression in blood show the body's systemic response to the
presence of lung cancer, including different clinical
characteristics. Controls (healthy patients) have a strong immune
system compared to lung cancer. MALAT1 may also be a marker
of the cells in immune system. There is a previous study reporting
that certain genes that are involved in cancer metastasis and
invasion were significantly downregulated in the native
unstimulated blood leukocytes from ovarian cancer patients with a
poor prognosis (4). Two previous
studies showed that the distinct whole-blood RNA expression
signatures identified can predict the severity of
castration-resistant prostate cancer (19,20).
The expression of MALAT1 in the whole blood
of lung cancer patients with metastasis was stronger compared to
non-metastasis, which showed that MALAT1 promotes the tumor
metastasis and additionally, the whole blood with lymph node
metastasis represented a lower expression of MALAT1 compared
to bone or brain metastasis. In addition to immunocytes, the
peripheral blood of cancer patients contains a small section of
CTCs, which are released into blood from carcinoma in situ.
The CTCs escape from the primary tumor and settle down at a
secondary site to cause metastasis. CTCs spread from the primary
tumor and colonize in the lymph node. The settlement is affected by
host and molecular characteristics. The expression of MALAT1
is lower in metastatic lymph node tissues than that of the
carcinoma in situ of the lungs. The CTCs with the signature
of MALAT1 expression are not suitable to locate in the lymph
nodes. This may suggest that the immune system, particularly for
inactive ones, expressed less of the MALAT1 gene in
accordance with the expression of MALAT1 in the whole blood
of lung cancer.
Acknowledgements
The present study was supported by grants from the
Tianjin Education Committee Foundation (no. 20110101), the National
Natural Science Foundation of China (no. 81201645), Doctoral
Program Foundation of Institutions of Higher Education (no.
20121202120008) to Dr FJ Guo; the National Natural Science
Foundation of China (no. 30973383) to Dr ZG Li; and the Key Project
from the National Natural Science Foundation of China (no.
30430300), National 863 Program (no. 2006AAOZA401), National 973
Program (no. 2010CB529405) and China-Sweden Cooperative Foundation
(no. 09ZCZDSF04100) to Dr QH Zhou.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patnaik SK, Yendamuri S, Kannisto E,
Kucharczuk JC, Singhal S and Vachani A: MicroRNA expression
profiles of whole blood in lung adenocarcinoma. PLoS One.
7:e460452012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zaatar AM, Lim CR, Bong CW, et al: Whole
blood transcriptome correlates with treatment response in
nasopharyngeal carcinoma. J Exp Clin Cancer Res. 31:762012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Isaksson HS, Sorbe B and Nilsson TK: Whole
blood RNA expression profiles in ovarian cancer patients with or
without residual tumors after primary cytoreductive surgery. Oncol
Rep. 27:1331–1335. 2012.PubMed/NCBI
|
|
5
|
Du YZ, Gu XH, Li L and Gao F: The
diagnostic value of circulating stanniocalcin-1 mRNA in non-small
cell lung cancer. J Surg Oncol. 104:836–840. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rotunno M, Hu N, Su H, et al: A gene
expression signature from peripheral whole blood for stage I lung
adenocarcinoma. Cancer Prev Res (Phila). 4:1599–1608. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ji P, Diederichs S, Wang W, et al:
MALAT-1, a novel noncoding RNA and thymosin beta4 predict
metastasis and survival in early-stage non-small cell lung cancer.
Oncogene. 22:8031–8041. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin R, Maeda S, Liu C, Karin M and
Edgington TS: A large noncoding RNA is a marker for murine
hepatocellular carcinomas and a spectrum of human carcinomas.
Oncogene. 26:851–858. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tripathi V, Shen Z, Chakraborty A, et al:
Long noncoding RNA MALAT1 controls cell cycle progression by
regulating the expression of oncogenic transcription factor B-MYB.
PLoS Genet. 9:e10033682013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Szymanski M, Barciszewska MZ, Erdmann VA
and Barciszewski J: A new frontier for molecular medicine:
Noncoding RNAs. Biochim Biophys Acta. 1756:65–75. 2005.PubMed/NCBI
|
|
11
|
Hutchinson JN, Ensminger AW, Clemson CM,
Lynch CR, Lawrence JB and Chess A: A screen for nuclear transcripts
identifies two linked noncoding RNAs associated with SC35 splicing
domains. BMC Genomics. 8:392007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo F, Li Y, Liu Y, Wang J, Li Y and Li G:
Inhibition of metastasis-associated lung adenocarcinoma transcript
1 in CaSki human cervical cancer cells suppresses cell
proliferation and invasion. Acta Biochim Biophys Sin (Shanghai).
42:224–229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tano K, Mizuno R, Okada T, Rakwal R,
Shibato J, Masuo Y, Ijiri K and Akimitsu N: MALAT-1 enhances cell
motility of lung adenocarcinoma cells by influencing the expression
of motility-related genes. FEBS Lett. 584:4575–4580. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lai MC, Yang Z, Zhou L, Zhu QQ, Xie HY,
Zhang F, Wu LM, Chen LM and Zheng SS: Long non-coding RNA MALAT-1
overexpression predicts tumor recurrence of hepatocellular
carcinoma after liver transplantation. Med Oncol. 29:1810–1816.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie H, Ma H and Zhou D: Plasma HULC as a
promising novel biomarker for the detection of hepatocellular
carcinoma. Biomed Res Int. 2013:1361062013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arita T, Ichikawa D, Konishi H, et al:
Circulating long non-coding RNAs in plasma of patients with gastric
cancer. Anticancer Res. 33:3185–3193. 2013.PubMed/NCBI
|
|
17
|
Sun Y, Wang Z and Zhou D: Long non-coding
RNAs as potential biomarkers and therapeutic targets for gliomas.
Med Hypotheses. 81:319–321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Passtoors WM, Beekman M, Deelen J, et al:
Gene expression analysis of mTOR pathway: Association with human
longevity. Aging Cell. 12:24–31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Olmos D, Brewer D, Clark J, et al:
Prognostic value of blood mRNA expression signatures in
castration-resistant prostate cancer: A prospective, two-stage
study. Lancet Oncol. 13:1114–1124. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ross RW, Galsky MD, Scher HI, et al: A
whole-blood RNA transcript-based prognostic model in men with
castration-resistant prostate cancer: A prospective study. Lancet
Oncol. 13:1105–1113. 2012. View Article : Google Scholar : PubMed/NCBI
|