Introduction
The coronary sinus (CS) is a small tubular structure
just above the posterior left atrioventricular junction and has
several main branches (1,2). It accepts blood following myocardial
contraction and guides the blood into the right atrium. The blood
of the CS contains information that directly or indirectly reflects
cardiac metabolism and physical condition (3,4). In heart
failure (HF), the information possessed by the CS may be more
significant than that possessed by other anatomical locations
(5,6).
The results of studies on myocardial oxygen
consumption in the setting of different cardiac pathological states
have varied (7–9). HF increases myocardial oxygen
consumption. Therefore, the blood in the CS has a lower oxygen
saturation (SO2) in the setting of worsening cardiac
function (10). Blood gas analysis
results from the CS indirectly reflect myocardial oxygen
consumption. The association between cardiac oxygen consumption and
cardiac ejection fraction (EF) has not been definitively elucidated
by previous studies and control groups composed of patients with
normal cardiac function have been absent from these studies. By
establishing a control group, the present study aimed to determine
the difference in cardiac oxygen consumption between patients with
normal cardiac function and patients with abnormal cardiac
function, and identify the association between myocardial oxygen
consumption and EF.
Patients and methods
Patients
Between June 2013 and December 2013, 78 patients met
the inclusion criteria of the study, signed an informed consent
form and were subsequently included in the study. Of these
patients, 34 had normal cardiac function and were placed in the
control group and 44 had abnormal cardiac function and were placed
in the experimental group. The inclusion criteria for the
experimental group were as follows: Cardiomyopathy; a left
ventricular EF (LVEF) <50%; a history of HF >6 months; New
York Heart Association (NYHA) stage ≥II; and agreement by the
patient to provide blood samples from the CS, aortic root and
peripheral vein. The exclusion criteria for the experimental group
were as follows: Absence of cardiopathy; a LVEF ≥50%; and refusal
of the patient to provide blood samples from the CS, aortic root
and peripheral vein. The inclusion criteria of the control group
were as follows: LVEF ≥50% and agreement by the patient to provide
blood samples from the CS, aortic root and peripheral vein. The
exclusion criteria for the control group were as follows: LVEF
<50%; a history of HF; a history of myocardial infarction or
disease causing increased myocardial oxygen consumption
(hyperthyroidism and anemia); and refusal of the patient to provide
blood samples from the CS, aortic root and peripheral vein. The
experimental group consisted of 31 males and 13 females. The
control group consisted of 16 males and 18 females. The average
ages of the individuals in the experimental and control group were
60.27±10.65 and 54.97±11.96 years, respectively. The experimental
group included older patients and a greater proportion of male
patients. Baseline information for the two groups is shown in
Table I.
 | Table I.Baseline characteristics. |
Table I.
Baseline characteristics.
| Characteristics | Experimental
group | Control group | P-value |
|---|
| Number | 44 | 34 |
|
| Gender, m/f | 31/13 | 16/18 | <0.001 |
| Age, years | 60.27±10.65 | 54.97±11.96 | <0.001 |
| DCM, n | 14 | 0 |
|
| ICM, n | 24 | 0 |
|
| HCM, n | 4 | 0 |
|
| VDH, n | 2 | 0 |
|
| PSVT, n | 0 | 17 |
|
| RVOT, n | 0 | 2 |
|
| CHD, n | 24 | 9 |
|
| PE, n | 0 | 5 |
|
| HP, n | 18 | 12 |
|
| DM, n | 12 | 4 |
|
| AF, n | 6 | 1 |
|
| History of HF,
years | 2.49±3.22 | 0 |
|
| SBP, mmHg | 118.82±21.26 | 126.59±14.47 | 0.710 |
| DBP, mmHg | 73.41±14.21 | 76.71±10.79 | 0.264 |
| Weight, kg | 70.68±13.15 | 70.38±16.43 | 0.299 |
| HB, g/l | 134.02±17.31 | 137.88±16.47 | 0.322 |
| CR, mmol/l | 89.55±25.28 | 63.97±15.19 | <0.001 |
| ALT, U | 29.26±20.77 | 29.23±21.07 | 0.994 |
| CK-MB | 15.52±9.44 | 13.37±4.96 | 0.233 |
| LVED, mm | 62.64±7.97 | 47.71±3.24 | <0.001 |
| LAD, mm | 42.30±4.69 | 33.68±4.33 | <0.001 |
| EF, % | 39.25±5.67 | 63.21±4.63 | <0.001 |
Method of extracting blood
samples
All the patients were prepared to undergo either
coronary angiography or radiofrequency catheter ablation. Two
sheathes were placed in the femoral artery and femoral vein and
blood samples were extracted from the aortic root, CS and femoral
vein using a coronary angiographic catheter. All the patients with
HF were treated with anti-HF drugs and their cardiac function
improved to a classification of NYHA II. Prior to extracting blood
from the CS, the X-ray position was 30° relative to the left
anterior oblique position. Under fluoroscopy, the catheter reached
the upper right atrium and turned toward the spine. Subsequently,
the operator slowly pulled back the catheter. Jumping of the
catheter signified that the catheter's tip had potentially reached
the CS. The operator used a contrast agent during angiography to
ensure that the catheter was within the CS.
Blood gas analysis
Following the withdrawal of blood samples from three
different places, the samples were twice subjected to blood gas
analysis. The result of each blood gas analysis is expressed as an
average number. The machine used for blood gas analysis was the
Abbott i-STAT EG7+ (Abbott, Abbott Park, IL, USA).
Statistical analysis
Statistical analyses were performed using SPSS 17.0
(SPSS, Inc., Chicago, IL, USA). Continuous variables were expressed
as the mean ± standard deviation and analyzed using independent
sample t-tests. Correlation analyses and regression analyses were
also performed. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patients
As each of the groups had a different
pathophysiological baseline, each group's baseline information was
different. The experimental group included patients with HF and was
older and consisted of more males than the control group. The major
diseases characterizing the experimental group were ischemic
cardiomyopathy and dilated cardiomyopathy. By contrast, the major
diseases of the control group were arrhythmia and coronary heart
disease, but not myocardial infarction. Additionally, five healthy
patients were enrolled in the control group as they were suspected
of having coronary artery disease and required a coronary angiogram
to determine their diagnoses. Due to cardiac dysfunction, the
experimental group had poor kidney function, larger left
ventricular end diastolic diameters and larger left atrial sizes.
However, the blood pressures, weights, hemoglobin values and liver
function test results of the two groups were not significantly
different. All the clinical data are shown in Table I.
Blood gas analysis
The partial pressure of oxygen (PO2) and
SO2 in the peripheral veins of the experimental group
were lower than those in the control group. Similar results were
obtained for the CS, as the PO2 and SO2 of
the CSs of the experimental group were also lower than those in the
control group. The blood within the coronary artery was derived
from the aortic root. As a result, the blood gas analysis of the
coronary artery yielded results similar to those of the aortic
root. The coronary artery and CS are the sole cardiac arterial and
venous blood supply. Therefore, the blood flow volume of the
coronary artery should be the same as the blood flow volume of the
CS. Therefore, the SO2 difference was used between the
aortic root and the CS to represent myocardial oxygen consumption.
Myocardial oxygen consumption in patients with HF is greater than
that in patients without HF. The detailed results of the blood gas
analyses are shown in Table II and
Fig. 1.
 | Table II.Blood gas analysis results. |
Table II.
Blood gas analysis results.
| Characteristics | Experimental
group | Control group | P-value |
|---|
| pH (A) | 7.41±0.45 | 7.40±0.434 | 0.368 |
| PCO2 (A),
mmHg | 34.98±4.84 | 37.92±5.05 | 0.011 |
| PO2 (A),
mmHg | 68.73±11.06 | 70.26±11.43 | 0.550 |
| SO2 (A),
% | 93.09±2.93 | 93.35±3.20 | 0.708 |
| BE (A), mmol/l | -2.48±3.22 | -1.21±2.33 | 0.056 |
| HCO3− (A),
mmol/l | 22.08±2.99 | 23.29±2.28 | 0.054 |
| pH (V) | 7.37±0.38 | 7.38±0.27 | 0.079 |
| PCO2 (V),
mmHg | 44.29±6.27 | 42.06±5.54 | 0.106 |
| PO2 (V),
mmHg | 31.57±5.12 | 34.76±6.33 | 0.016 |
| SO2 (V),
% | 56.98±11.73 | 63.44±11.85 | 0.019 |
| BE (V), mmol/l | -0.20±3.12 | 0.00±2.82 | 0.766 |
|
HCO3− (V),
mmol/l | 25.16±2.96 | 25.13±2.73 | 0.959 |
| pH (CS) | 7.35±0.63 | 7.36±0.40 | 0.445 |
| PCO2
(CS), mmHg | 48.25±6.66 | 47.89±5.48 | 0.079 |
| PO2
(CS), mmHg | 20.20±3.43 | 24.18±3.25 | <0.001 |
| SO2
(CS), % | 29.16±7.33 | 35.76±7.60 | <0.001 |
| BE (CS),
mmol/l | 1.18±3.39 | 1.88±2.97 | 0.382 |
| HCO3−
(CS), mmol/l | 27.12±3.31 | 27.24±2.71 | 0.863 |
| SO2
(A-CS), mmol/l | 63.93±8.16 | 57.58±7.44 | 0.001 |
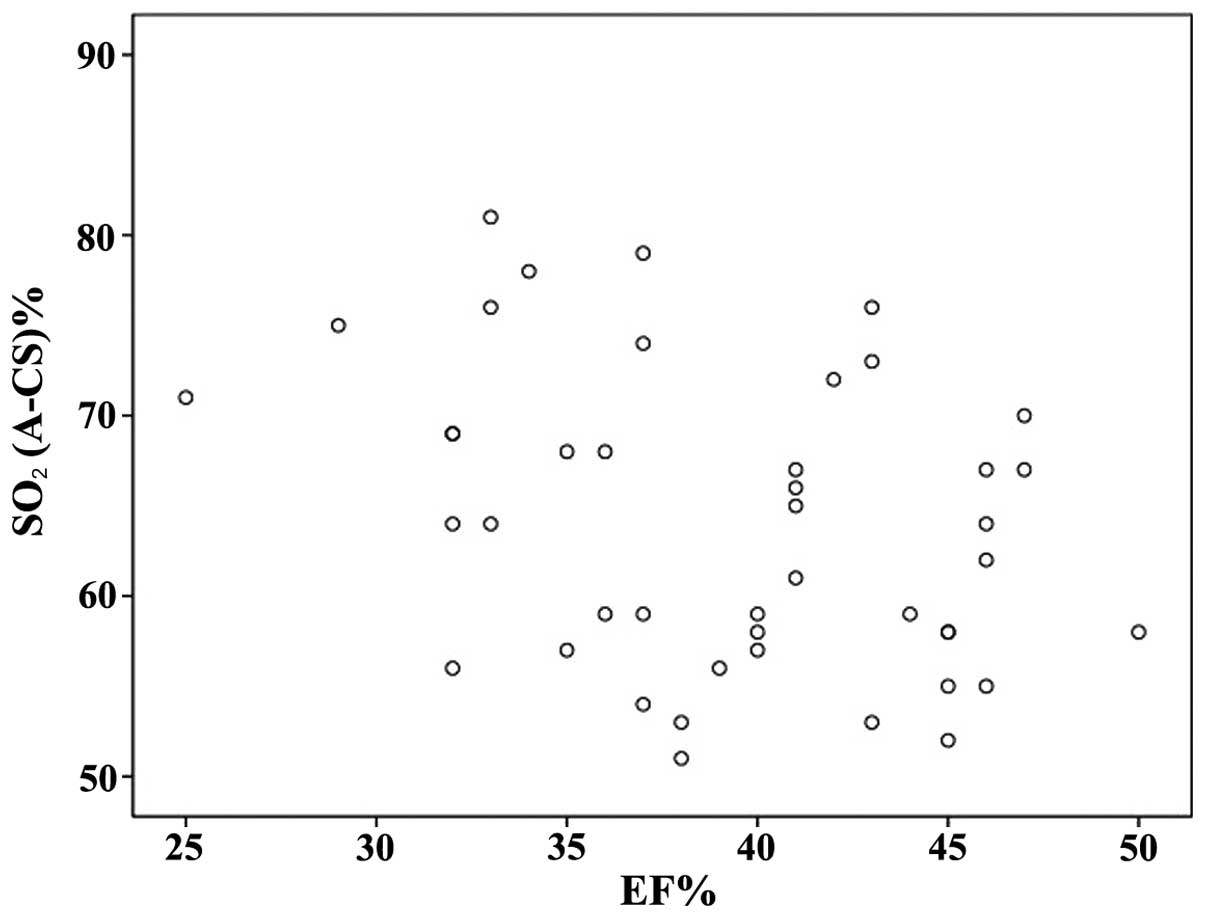
Correlation and regression
analysis
Using linear correlation analysis, the association
between cardiac EF and myocardial oxygen consumption was examined
in the experimental group. There is a significant correlation
between cardiac EF and myocardial oxygen consumption (R=-0.336,
P=0.026). The association between cardiac EF and cardiac oxygen
consumption was also examined using linear regression analysis. The
F value of the regression model was 5.333 (P=0.026), indicating
statistical significance. The scatter diagram depicting
SO2 and EF is shown in Fig.
2. The correlation between cardiac EF and myocardial oxygen
consumption was statistically significant. The regression equation
was y = 82.906–0.483×. Thus, cardiac EF not only correlates with
cardiac oxygen consumption, but is also likely to influence
myocardial oxygen consumption.
Discussion
Previous studies have suggested that arterial
SO2 is associated with mortality and rehospitalization,
and has prognostic implications for patients with HF (11,12). These
studies demonstrated that lower arterial SO2 indicates a
worse prognosis. Arterial SO2 reflects the body's supply
of oxygen; however, it does not reflect cardiac oxygen consumption.
Therefore, to a certain degree, this conclusion is controversial
(13).
The CS contains only venous blood as a result of
cardiac metabolism. The SO2 of the blood is the result
of myocardial oxygen consumption. Therefore, the difference in
SO2 between the aortic root and CS can be used to
represent myocardial oxygen consumption. The difference directly
reflects myocardial oxygen consumption.
A previous study has shown that worsening cardiac
function is associated with lower CS SO2 (10). However, few investigations of blood gas
in the CS have featured a control group composed of patients with
normal cardiac function. In the present study, by comparing
patients with normal cardiac function with those with abnormal
function, patients with abnormal cardiac function have a lower
SO2 and PO2 in the peripheral vein and CS.
Previous studies have suggested that the body's oxygen consumption
may increase in the setting of HF and is an independent predicator
of mortality due to HF (14,15). The results of the present study
indicate that myocardial oxygen metabolism is similar to the body's
oxygen metabolism in the setting of HF. A conclusion regarding the
association between myocardial oxygen consumption and the prognosis
of HF was not achieved due to an insufficient time to reach an
endpoint.
Previous studies have suggested that CS
SO2 and PO2 levels are associated with
cardiac function and the prognosis of patients with HF is
associated with the oxygen content of CS blood (10,16).
However, Boehrer et al (17)
disagreed with these results. In their study, the content of the CS
was not associated with the extent of coronary artery disease or
left ventricular dysfunction. In the present study, it was found
via correlation and regression analyses that cardiac EF and
myocardial oxygen consumption have a linear association, as well as
that cardiac EF influences myocardial oxygen consumption. A
significant correlation index (R), −0.336, was obtained from the
data. However, this correlation index is relatively small,
indicating that the correlation is not extremely strong and
indicates that there are other factors that impact myocardial
oxygen consumption, such as medications, autonomic nervous tension
and physical functions (18–20). Compared with other unstable factors,
cardiac EF may be the most stable factor that influences myocardial
oxygen consumption in patients with HF.
Certain limitations of the present study should be
considered. First, the members of the control group were not
completely healthy. Although patients in the control group had
normal cardiac function, preexisting diseases in the group, such as
arrhythmia and coronary artery disease, may have had an effect on
myocardial oxygen consumption (9,21).
Additionally, as no follow-up occurred with the experimental group,
whether myocardial oxygen consumption is a significant prognostic
indicator of HF was not determined.
In the present study, myocardial oxygen consumption
in individuals with HF was greater compared to those with normal
cardiac function. Cardiac EF is associated with myocardial oxygen
consumption and influences myocardial oxygen consumption. More
studies are required to determine whether myocardial oxygen
consumption can predict the prognosis of HF.
References
|
1
|
Serova EV: Surgical anatomy of the
coronary venous sinus. Grudn Khir. 5:24–26. 1963.(In Russian).
PubMed/NCBI
|
|
2
|
Gilard M, Mansourati J, Etienne Y, Larlet
JM, Truong B, Boschat J and Blanc JJ: Angiographic anatomy of the
coronary sinus and its tributaries. Pacing Clin Electrophysiol.
21:2280–2284. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kagaya Y, Otani H, Tanikawa T, Namiuchi S,
Isoyama S and Shirato K: Concentrations of angiotensin II,
endothelin–1 and BNP in the coronary sinus and ascending aorta of
patients with heart disease. Heart. 81:102–103. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yasuda T, Sawa S, Ishikawa N and Tanaka N:
Simultaneous measurement of coronary sinus oxygen saturation and
blood flow during terminal warm blood cardioplegia in coronary
artery bypass grafting. Ann Thorac Cardiovasc Surg. 4:271–274.
1998.PubMed/NCBI
|
|
5
|
Tsutamoto T, Sakai H, Nishiyama K, Tanaka
T, Fujii M, Yamamoto T and Horie M: Direct comparison of
transcardiac increase in brain natriuretic peptide (BNP) and
N-terminal proBNP and prognosis in patients with chronic heart
failure. Circ J. 71:1873–1878. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Watson CJ, Ledwidge MT, Phelan D, Collier
P, Byrne JC, Dunn MJ, McDonald KM and Baugh JA: Proteomic analysis
of coronary sinus serum reveals leucine-rich ɑ2-glycoprotein as a
novel biomarker of ventricular dysfunction and heart failure. Circ
Heart Fail. 4:188–197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vlasov IuA and Okuneva GN: Specific oxygen
consumption in the myocardium of patients with congenital and
acquired heart defects. Fiziol Cheloveka. 29:133–137. 2003.[(In
Russian)]. PubMed/NCBI
|
|
8
|
Vlasov IuA, Okuneva GN, Litasova EE and
Nikolaeva TM: Correlation between specific oxygen consumption in
the myocardium and myocardial mass in healthy people and in
patients with heart defects. Fiziol Cheloveka. 21:92–99. 1995.(In
Russian). PubMed/NCBI
|
|
9
|
Kumon K, Kuwabara M, Hirata T, Tanaka K,
Kawazoe K, Kitoh Y, Nakajima N and Fujita T: Continuous measurement
of coronary sinus oxygen saturation after cardiac surgery. Crit
Care Med. 15:595–597. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
White M, Rouleau JL, Ruddy TD, De Marco T,
Moher D and Chatterjee K: Decreased coronary sinus oxygen content:
A predictor of adverse prognosis in patients with severe congestive
heart failure. J Am Coll Cardiol. 18:1631–1637. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Milo-Cotter O, Cotter G, Kaluski E, Rund
MM, Felker GM, Adams KF, O'Connor CM and Weatherley BD: Rapid
clinical assessment of patients with acute heart failure: First
blood pressure and oxygen saturation - is that all we need?
Cardiology. 114:75–82. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Masip J, Gaya M, Paez J, Betbese A,
Vecilla F, Manresa R and Ruiz P: Pulse oximetry in the diagnosis of
acute heart failure. Rev Esp Cardiol (Engl Ed). 65:879–884. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Minana G, Nunez J, Banuls P, Sanchis J,
Nunez E, Robles R, Mascarell B, Palau P, Chorro FJ and Llacer A:
Prognostic implications of arterial blood gases in acute
decompensated heart failure. Eur J Intern Med. 22:489–494. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van den Broek SA, van Veldhuisen DJ, de
Graeff PA, Landsman ML, Hillege H and Lie KI: Comparison between
New York Heart Association classification and peak oxygen
consumption in the assessment of functional status and prognosis in
patients with mild to moderate chronic congestive heart failure
secondary to either ischemic or idiopathic dilated cardiomyopathy.
Am J Cardiol. 70:359–363. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cohn JN, Johnson GR, Shabetai R, Loeb H,
Tristani F, Rector T, Smith R and Fletcher R: Ejection fraction,
peak exercise oxygen consumption, cardiothoracic ratio, ventricular
arrhythmias and plasma norepinephrine as determinants of prognosis
in heart failure. The V-HeFT VA Cooperative Studies Group.
Circulation. 87 (Suppl 6):VI5–V16. 1993.PubMed/NCBI
|
|
16
|
Foex P and Ryder WA: Effect of
CO2 on the systemic and coronary circulations and on
coronary sinus blood gas tensions. Bull Eur Physiopathol Respir.
15:625–638. 1979.PubMed/NCBI
|
|
17
|
Boehrer JD, Lange RA, Willard JE and
Hillis LD: Lack of relation of coronary sinus oxygen content to
extent of coronary artery disease or left ventricular dysfunction.
Am J Cardiol. 70:1623–1625. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hagl S, Froer W, Heimisch W, Braun E,
Gebhardt K and Mendler N: The effect of nitrates on the function of
intact and ischemic myocardium (author's transl). Thoraxchir Vask
Chir. 25:219–229. 1977.(In German). PubMed/NCBI
|
|
19
|
Kumakura S and Oshima T: Effects of beta
blockers on cardiac function and myocardial oxygen consumption in
the isolated supported heart preparation of the dog. Jpn Heart J.
16:592–602. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alders DJ, Cornelussen RN, Prinzen FW,
Specht PA, Noble MI, Drake-Holland AJ, de Kanter FJ and van Beek
JH: Regional sympathetic denervation affects the relation between
canine local myocardial blood flow and oxygen consumption. Exp
Physiol. 92:541–548. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miwa K, Fujita M, Ejiri M and Sasayama S:
Biphasic changes (initial increase and late decrease) in coronary
sinus venous oxygen saturation during anginal attacks induced by
intracoronary acetylcholine in patients with variant angina.
Cardiology. 81:221–232. 1992. View Article : Google Scholar : PubMed/NCBI
|