Introduction
Breast cancer is a pathologically and clinically
heterogeneous disease, and is the most frequent malignancy among
females (1). In addition, distant
metastases are the most common type of breast cancer recurrence and
are often the cause of fatality in breast cancer patients;
metastases result in >40,000 fatalities per year in the USA
alone (2). In a previous study, it was
demonstrated that most complications of breast cancer are
attributed to metastasis to distant organs, including lymph nodes,
bone, lung and liver (3).
Matrix metalloproteinases (MMPs) are a family of
zinc-dependent endoproteinases that digest components of the
extracellular matrix (ECM) and cell surface receptors for soluble
factors and junctional proteins involved in cell-cell and cell-ECM
interactions. Due to their matrix-degrading abilities and high
expression in advanced tumors, MMP activity has been shown to be
required for breast cancer cell invasion and angiogenesis (4,5). MMP-1, a
member of the MMP family, is upregulated in breast cancer cell
lines with an enhanced ability of tumor growth, invasion and
distant metastasis (6,7). However, the expression of MMP-1 in breast
cancer and cancer-adjacent tissues remains to be established. To
address this issue, immunohistochemical staining was used to detect
the MMP-1 expression in breast cancer and cancer-adjacent normal
breast tissue. An extended understanding of the expression of MMP-1
may provide a novel insight into the role of MMP-1 in pathological
process of breast tumorigenesis.
Materials and methods
Tissue microarray and
immunohistochemical analysis
Tissue microarray purchased from Chaoying
Bio-Technology Co., Ltd., (BR1002a; Xi'an, Shanxi, China). All the
specimens had detailed information including age, gender,
organization, pathological diagnosis, clinical grade,
tumor-node-metastasis classification, clinical stage, specimen type
and results.
Immunohistochemical analysis
To detect the expression of MMP-1,
immunohistochemical staining was performed. Sections were
heat-immobilized at 60˚C for 30 min and were deparaffinized in
xylene and rehydrated through a series of graded ethanol solutions.
Antigen retrieval was performed in a pressure cooker at 95˚C for 2
min using 0.01 M citrate buffer (pH 6.0). Endogenous peroxidase
activity was blocked by incubation with 3% hydrogen peroxide for 15
min at room temperature. Sections were subsequently incubated with
anti-MMP-1 mouse monoclonal antibody (1:50; sc-21731; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) for 60 min at 37˚C in a
humidified chamber. Subsequently, the sections were rinsed and
incubated with a biotinylated secondary antibody for 30 min,
followed by horseradish peroxidase-conjugated streptavidin for 30
min using the UltraSensitive™ Universal (anti-mouse/rabbit)
Detection reagent (Fuzhou Maixin Biotechnology Development Co.,
Ltd., Fuzhou, China). Finally, the tissue microarray sections were
stained with freshly prepared 3,3′-diaminobenzidine and
counterstained lightly with hematoxylin. As a negative control, the
primary antibody was replaced with normal rabbit or mouse
immunoglobulin G at the same dilution. MMP-1 stain intensity was
scored on a scale of 0 (negative) to 2+ (intense
staining).
Results
Expression of MMP-1 in breast cancer
and cancer-adjacent normal tissue
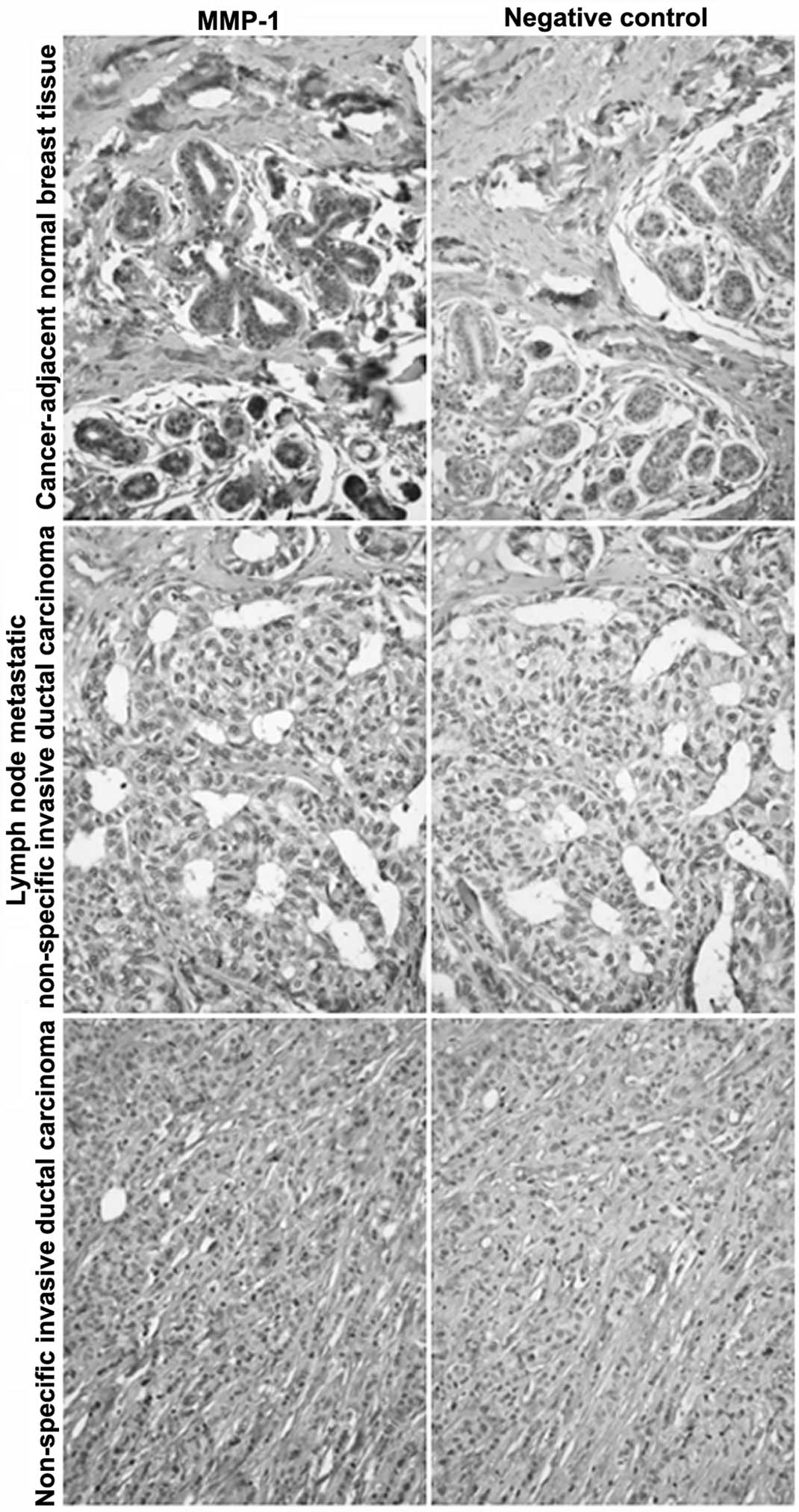
The MMP-1 expression in breast cancer and
cancer-adjacent normal tissue was detected by immunohistochemical
staining. As shown in Table I, and
Figs. 1 and 2, MMP-1 had a positive expression in normal
lymph node tissue and lymph node metastatic non-specific invasive
ductal carcinoma. MMP-1 had partially negative expression in
non-specific invasive ductal carcinoma of the breast and
cancer-adjacent normal breast tissue. The MMP-1 negative expression
rate was only 6.1% in non-specific invasive ductal carcinoma of the
breast and 2.9% in cancer-adjacent normal breast tissue,
respectively.
 | Table I.Matrix metalloproteinase-1 (MMP-1)
expression in breast cancer and cancer-adjacent normal tissue. |
Table I.
Matrix metalloproteinase-1 (MMP-1)
expression in breast cancer and cancer-adjacent normal tissue.
| Organ | Pathology
diagnosis | No. of tumors | MMP-1 (-, +, ++) |
|---|
| Breast | Non-specific invasive
ductal carcinoma grade I | 13 | 1, 6, 6 |
| Breast | Non-specific invasive
ductal carcinoma grade II | 12 | 0, 4, 8 |
| Breast | Non-specific invasive
ductal carcinoma grade III | 8 | 1, 3, 4 |
| Breast | Cancer-adjacent
normal breast tissue | 34 | 1, 26, 7 |
| Lymph node | Lymph node metastatic
non specific invasive ductal carcinoma | 9 | 0, 3, 6 |
| Lymph node | Normal lymph node
tissue | 9 | 0, 9, 0 |
MMP-1 expression is higher in
non-specific invasive ductal carcinoma compared to cancer-adjacent
normal breast tissue
The MMP-1 expression rate in non-specific invasive
ductal carcinoma and cancer-adjacent normal breast tissue, lymph
node metastatic non-specific invasive ductal carcinoma and normal
lymph node tissue was analyzed. As shown in Table II, the MMP-1 positive expression rate
(+ and ++%) in non-specific invasive ductal
carcinoma was 39.4 and 54.5%, respectively. The MMP-1 positive
expression rate (+ and ++%) in
cancer-adjacent normal breast tissue was 76.5 and 20.6%,
respectively. The MMP-1 positive expression rate (++%)
was significantly higher in non-specific invasive ductal carcinoma
compared to cancer-adjacent normal breast tissue. In addition, the
MMP-1 expression rate (++%) in lymph node metastatic
non-specific invasive ductal carcinoma was 66.7%, significantly
more than in the normal lymph node tissue.
 | Table II.Different matrix metalloproteinase-1
(MMP-1) expression rates in non-specific invasive ductal carcinoma
and cancer-adjacent normal breast tissue. |
Table II.
Different matrix metalloproteinase-1
(MMP-1) expression rates in non-specific invasive ductal carcinoma
and cancer-adjacent normal breast tissue.
| Pathology
diagnosis | No. of tumors | MMP-1 expression rate
(-, +, ++%) |
|---|
| Non-specific invasive
ductal carcinoma | 33 | (6.1, 39.4,
54.5) |
| Cancer-adjacent
normal breast tissue | 34 | (2.9, 76.5,
20.6) |
| Lymph node metastatic
non-specific invasive ductal carcinoma | 9 | (0, 33.3, 66.7) |
| Normal lymph node
tissue | 9 | (0, 100, 0) |
Discussion
MMPs are expressed in nearly all tumors, where they
facilitate tumor growth, invasion and metastasis (4,5). The study
by Kang et al (8) used
microarray analysis to show that overexpression of MMP-1 enhances
MDA-MB-231 cell bone metastasis. Additionally, in breast cancer,
short hairpin RNA-mediated stable knockdown of MMP-1 or induction
of MMP-1 by overexpression of δ-crystallin enhancer factor 1
significantly regulated the invasive ability of MDA-MB-231
(6,7).
Since MMP-1 has been confirmed as a factor that facilitates breast
cancer metastasis, the present study provides a novel discovery
that MMP-1 expression is higher in non-specific invasive ductal
carcinoma and lymph node metastatic non-specific invasive ductal
carcinoma compared to cancer-adjacent normal breast tissue and
normal lymph node tissue. The high expression of MMP-1 in breast
cancer may be closely associated with its invasion and
metastasis.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81302323), the
Natural Science Foundation of Hebei province (nos. C2014209140 and
C2013209024), General Higher Education Young Talents Program of
Hebei province (no. BJ2014027), Science and Technology Support
Program of Tangshan city (no. 14120208a) and Students' Innovation
and Entrepreneurship Training Program of Hebei United University
(no. X2014083) and the Educational Reform Project of Tangshan
Normal University (no. 2011001012).
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rugo HS: The importance of distant
metastases in hormone-sensitive breast cancer. Breast. 17 (Suppl
1):S3–S8. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nagaiah G and Abraham J: Circulating tumor
cells in the management of breast cancer. Clin Breast Cancer.
10:209–216. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chabottaux V and Noel A: Breast cancer
progression: insights into multifaceted matrix metalloproteinases.
Clin Exp Metastasis. 24:647–656. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu H, Kato Y, Erzinger SA, et al: The
role of MMP-1 in breast cancer growth and metastasis to the brain
in a xenograft model. BMC Cancer. 12:5832012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu F, Wang C, Guo S, et al: δEF1 promotes
osteolytic metastasis of MDA-MB-231 breast cancer cells by
regulating MMP-1 expression. Biochim Biophys Acta. 1809:200–210.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kang Y, Siegel PM, Shu W, et al: A
multigenic program mediating breast cancer metastasis to bone.
Cancer Cell. 3:537–549. 2003. View Article : Google Scholar : PubMed/NCBI
|