Introduction
Cancer cells rely on critical signaling pathways for
their proliferation, invasion and metastasis. Critical signaling
molecules, which function particularly at points where several of
these pathways crosstalk, provide valuable targets for the
development of novel anticancer drugs (1).
Rho GDP-dissociation inhibitor α (RhoGDIα), which is
a member of a family of GDIs that include D4-GDI, RhoGDI-3 and
RhoGDIα, functions mainly by affecting the cellular distribution
and activity of Rho GTPases (2–4). RhoGDIα can
negatively modulate Rho proteins by three methods: i) By suspending
their interaction with guanine nucleotide exchange factors, thereby
inhibiting GTPase activation; ii) by shielding the
membrane-anchoring domain of the GTPases, thereby restricting them
to a cytosolic localization; and iii) by blocking the binding to
downstream target molecules (5). The
expression of RhoGDIα is upregulated in diverse types of human
cancer, including lung cancer, breast cancer and melanoma (6,7).
Overexpression of RhoGDIα is associated with tumor progression and
poor prognosis (8). The critical role
of RhoGDIα in cancer cell function and their role in cancer
etiology highlights the importance of RhoGDIα as a novel target for
anticancer treatment.
In our previous study, a specific RhoGDIα inhibitor
(no. SKLB-163) was developed via computer-aided drug design and
de novo synthesis in our laboratory. Our data exhibited that
SKLB-163 had good anticancer activities in vitro and in
vivo (9,10). The molecular mechanism is involved as
follows: SKLB-163 inhibited the upstream RhoGDIα protein and
activated that c-Jun N-terminal kinase 1 signaling pathway that
could contribute to the activation of caspase-3, decreased level of
phosphorylated mitogen-activated protein kinase and AKT. To fully
explore the potential of SKLB-163, the antitumor efficacy of the
combination of SKLB-163 and paclitaxel was evaluated in the LL/2
mice model in the present study. The findings showed that the
combination therapy clearly inhibited cell proliferation and
induced apoptosis of LL/2 in vitro. The LL/2 mice model also
showed that the combination therapy inhibited tumor growth in
vivo. Proliferative cell nuclear antigen (PCNA)
immunohistochemistry and terminal deoxynucleotidyl transferase dUTP
nick end-labeling (TUNEL) showed that combination therapy inhibited
cell proliferation and increased apoptosis compared to the
treatment with SKLB-163 or paclitaxel alone. The data suggests that
the combination therapy exerted synergistic antitumor effects,
providing a novel way to augment the antitumor efficacy of
cytotoxic chemotherapy.
Materials and methods
Synthesis of SKLB-163
The route adapted for the synthesis of the SKLB-163
compound was performed as previously described (10). SKLB-163 was dissolved in dimethyl
sulfoxide (DMSO) as a stock solution and was stored at 4°C. In the
in vitro study, the stock solution was diluted in cell
culture medium at a final DMSO concentration of 0.05% (v/v). The
formulation used in the in vivo study contains SKLB-163,
0.5% carboxymethylcellulose and 1% glycerin.
Cell culture
The LL/2 murine Lewis lung cancer cell line, CT26
murine colon adenocarcinoma cell line, B16 murine melanoma cell
line, HB1 human bronchial epithelial cell line, LO2 human liver
cell line and HEK293 human embryonic kidney cell line were grown in
RPMI-1640 (Invitrogen Life Technologies, Bedford, MA, USA) or
Dulbecco's modified Eagle's medium (Invitrogen Life Technologies)
containing 10% heat-inactivated fetal bovine serum, 100 U/ml
penicillin and 100 U/ml streptomycin in a humidity chamber at 37°C
under a 5% CO2 in atmosphere.
Cell viability assay
Cells were seeded at 4–5×103 cells/well
in 96-well plates and were allowed to attach overnight at 37°C.
Subsequently, medium containing agents were added to each well and
cells were further cultured at 37°C for 48 h. Cell viability was
estimated using the MTT assay. The absorbance was measured at 570
nm with a microplate reader (Bio-Rad, Berkeley, CA, USA).
Quantitative assessment of
apoptosis
Apoptotic cells treated with corresponding agents
were further analyzed by a flow cytometer. Collected cells were
stained with 1 ml hypotonic fluorochrome solution containing 50
µg/ml propidium iodide in 0.1% sodium citrate plus 0.1% Triton
X-100. Subsequently, flow cytometric analysis was performed to
identify apoptotic cells or sub-G1 cells to measure the
percentage of sub-G1 cells (ESP Elite; Beckman Coulter,
Brea, CA, USA). Apoptotic cells had less DNA content than that of
the G1 cells in the cell cycle distribution and the
results were estimated with list mode software.
In vivo tumor experiment
The study received approval from the Ethics
Committee of Sichuan University (Sichuan, China). The animal
studies were approved by the Institutional Animal Care and
Treatment Committee of Sichuan University. C57BL/6 mice, 6 to 8
weeks old, were obtained from the Experimental Animal Center of
Sichuan University and were housed in our animal research facility.
LL/2 cells (~1×106) in 0.1 ml of phosphate-buffered
saline (PBS) were injected subcutaneously into the right oxter of
each mouse. When the diameter of the tumors reached up to 6–8 mm,
animals were randomized into one of the following 4 groups:
Control, normal saline-treated group; SKLB-163 group, 100 mg/kg by
intragastric administration once daily; paclitaxel group, 10 mg/kg
by intraperitoneal (i.p.) injection once a week; and the
SKLB-163+paclitaxel group, SKLB-163 (100 mg/kg by intragastric
administration once daily) and paclitaxel (10 mg/kg by i.p.
injection once a week). Tumor growth was evaluated every 3 days by
measurement of tumor diameters and the volume of the tumor was
determined using the formula: Volume (mm3) = length ×
width2 × 0.52, as previously described (11). After all the mice from each group were
sacrificed, the tumor net weight of each mouse was measured.
PCNA immunohistochemistry
The tumor sections were stained by the
EnVision™+System-horseradish peroxidase method (DakoCytomation,
Carpinteria, CA, USA), according to the manufacturer's
instructions. The primary antibody for PCNA was purchased from
Santa Cruz Biotechnology, Inc., (Dallas, TX, USA; rabbit
anti-mouse; cat. no. sc-7907).
TUNEL assay
The presence of apoptotic cells within the tumor
sections was evaluated by the TUNEL technique using the DeadEnd™
Fluorometric TUNEL System (Promega, Madison, WI, USA) following the
manufacturer's instructions. Percent apoptosis was determined by
counting the number of apoptotic cells and dividing by the total
number of cells in the field (5 high power fields/slide).
Results
Cytotoxicity effect of SKLB-163
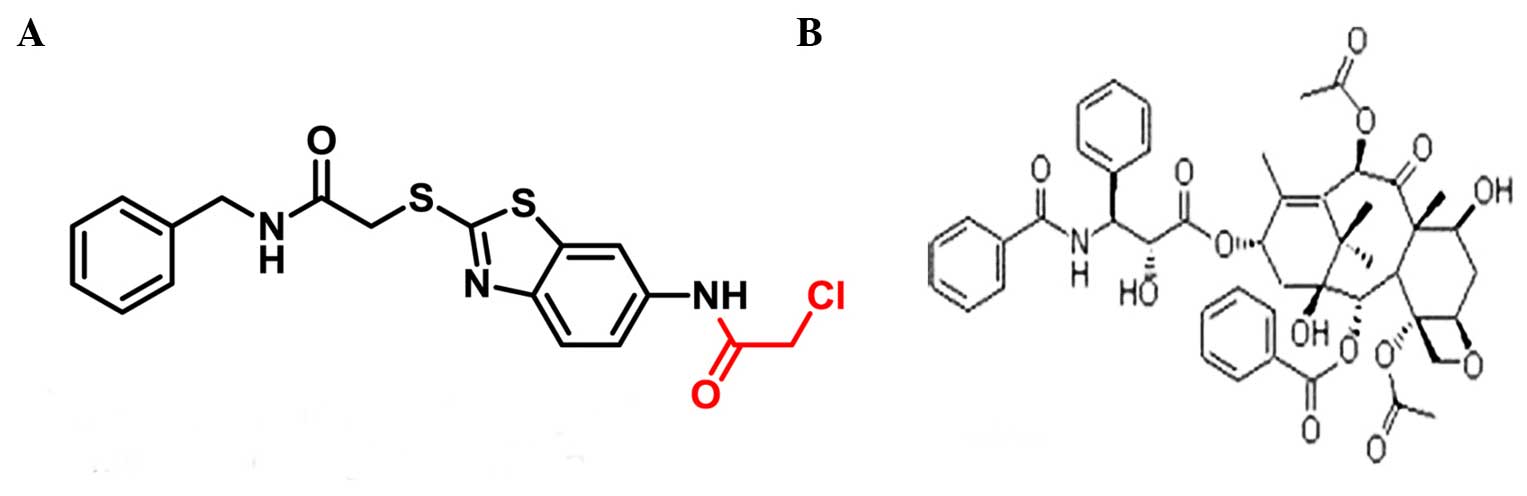
SKLB-163 was synthesized in our laboratory (Fig. 1A). In the present study, 3 murine
cancer cell lines (LL/2, CT26 and B16) and normal cell lines (HB1,
LO2 and HEK293) were used to investigate the cytotoxicity of
SKLB-163. After 48 h treatment, SKLB-163 inhibited the viability of
all the murine cancer cell lines. The IC50 is shown in
Table I. Additionally, no apparent
toxicity on normal cells was observed (Table I).
 | Table I.Cytotoxicity effect of SKLB-163. |
Table I.
Cytotoxicity effect of SKLB-163.
| Tumor cell line | Cell type | IC50,
µmol/l |
|---|
| LL/2 | Murine Lewis lung
cancer | 2.45±0.67 |
| CT26 | Murine colon
adenocarcinoma | 7.56±1.34 |
| B16 | Murine melanoma | 6.31±1.27 |
| HB1 | Human bronchial
epithelial | >40 |
| LO2 | Human liver | >40 |
| HEK293 | Human embryonic
kidney | >40 |
SKLB-163 enhances LL/2 cell
sensitivity to paclitaxel in vitro
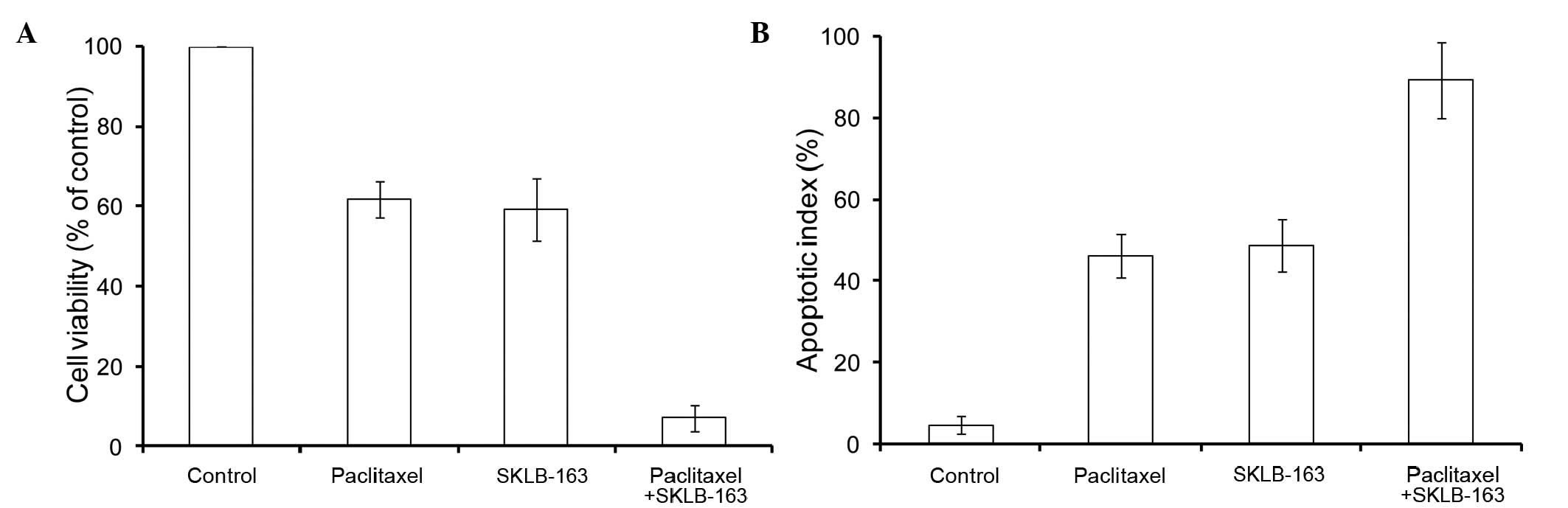
In order to investigate whether SKLB-163 could
affect LL/2 cell sensitivity to paclitaxel (Fig. 1B), the MTT assay and flow cytometry
were carried out. Cell viability of LL/2 cells was significantly
reduced in the SKLB-163 plus paclitaxel treated group compared to
the treatment with paclitaxel alone (1.5 nM), SKLB-163 alone (2
µmol/l) and PBS control (Fig. 2A). In
addition, apoptosis was markedly enhanced in the SKLB-163 plus
paclitaxel-treated group compared to the treatment with paclitaxel
alone (1.5 nM), SKLB-163 alone (2 µmol/l) and PBS control (Fig. 2B).
Antitumor effect of combination
therapy in vivo
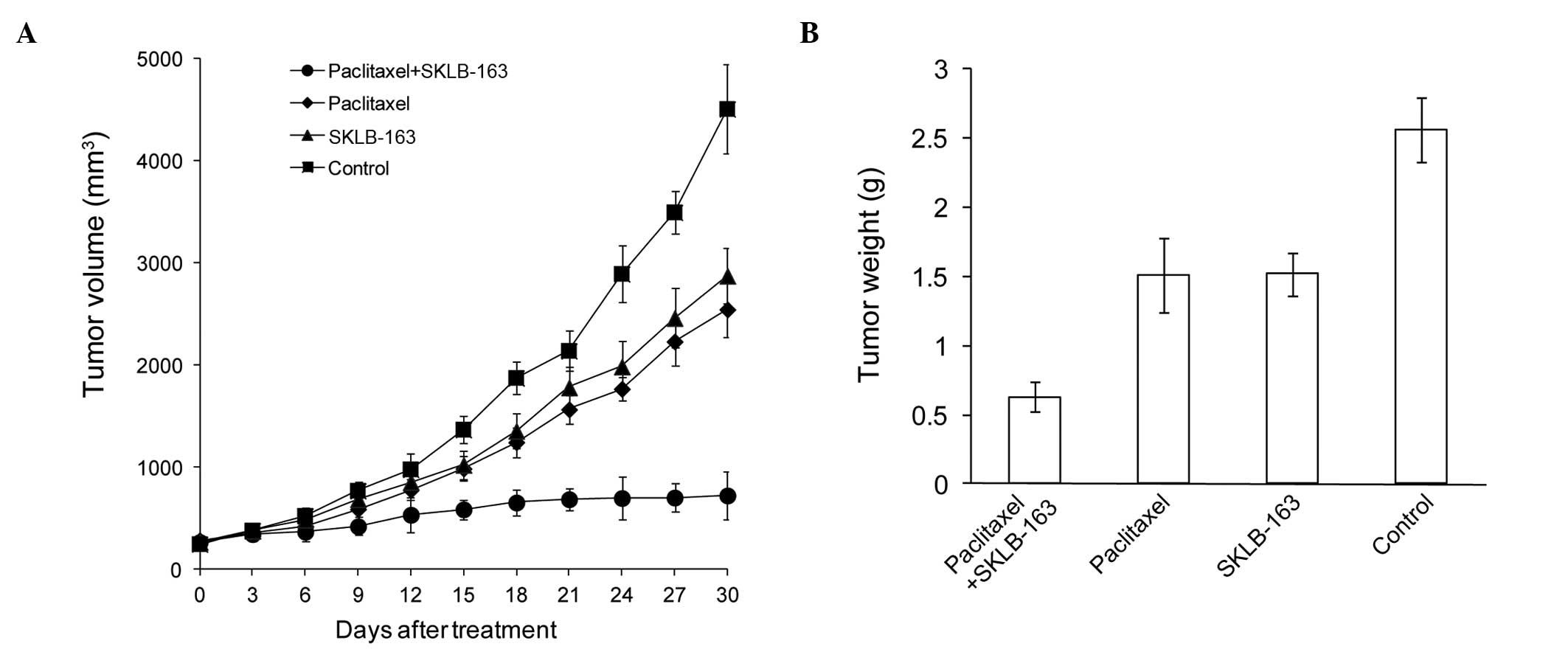
In order to study the antitumor effect of
combination therapy, the LL/2 mice model was established. As shown
in Fig. 3A, single paclitaxel or
SKLB-163 treatment suppressed tumor growth and the tumor inhibition
rate was 43 and 36%, respectively, in the LL/2 model. However, the
combination therapy significantly decreased the tumor volume and
resulted in 84% tumor regression. Similar results were also found
for the tumor weight (Fig. 3B). All
the data showed that SKLB-163 increased the suppression of tumor
growth induced by paclitaxel.
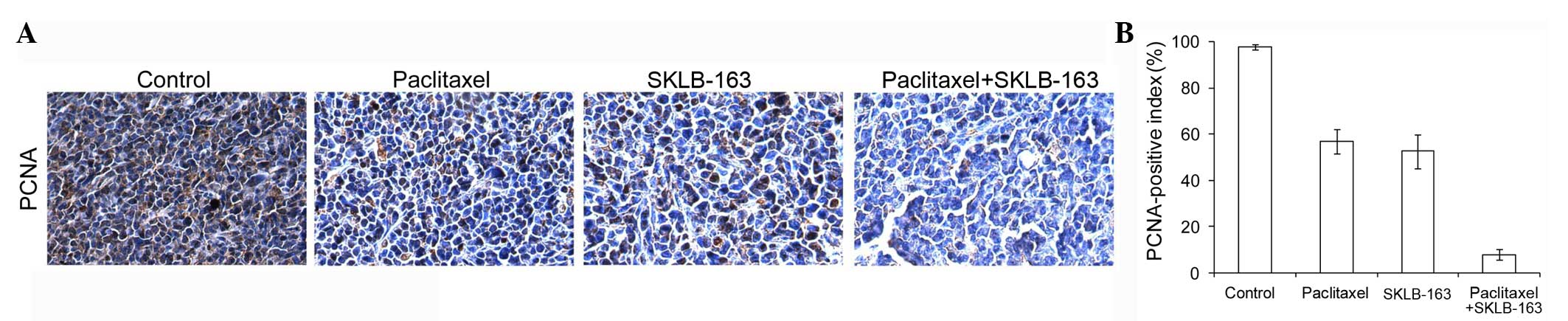
Combination therapy inhibited
proliferation in vivo
To obtain additional insight into the in vivo
effects, tumor cell proliferation was assessed by PCNA
immunoreactivity analysis. As shown in Fig. 4, combination therapy clearly reduced
percentages of PCNA-positive nuclei in LL/2 tumor models when
compared with the tumors from the control group.
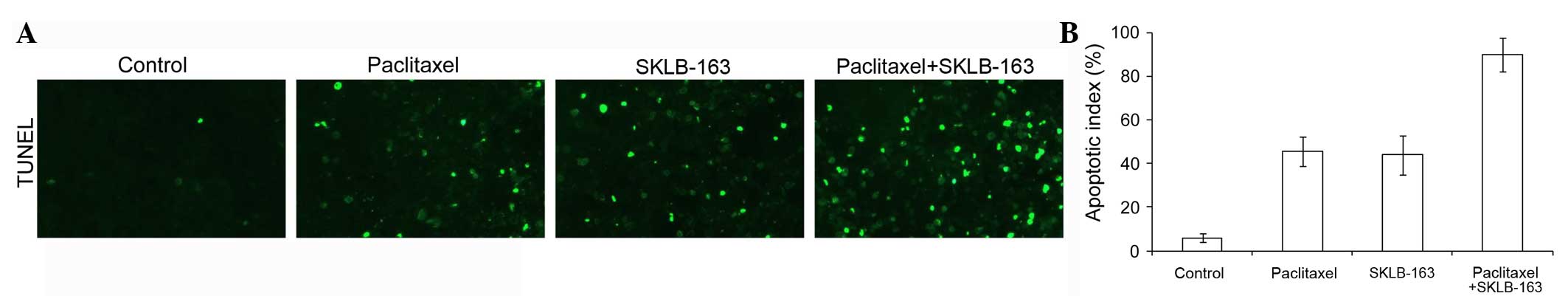
Combination therapy increased
apoptosis in vivo
In the TUNEL assay to evaluate apoptosis in vivo, a
significantly greater percentage of TUNEL-positive nuclei could be
observed in the combination therapy group when compared with the
tumors from the control groups (Fig.
5).
Discussion
Due to the high degree of cancer clonal
heterogeneity and cell signal complexity, downregulation of a
single target does not necessarily eradicate the cancer. Therefore,
traditional chemotherapy combined with targeted agents may be the
most effective way to improve treatment efficacy and overcome
resistance in oncotherapy (1).
The present study investigated the synergistic
antitumor effects of SKLB-163 used in combination with paclitaxel
in the murine LL/2 model. SKLB-163, a new benzothiazole-2-thiol
derivative, was developed via a computer-aided drug design and
de novo synthesis. SKLB-163 showed significant cytotoxicity
against various murine cancer cells by the MTT assay. A clear
increased suppression of tumor cell proliferation and increased
induction of apoptosis were evidenced in the combination therapy
group by the MTT assay and flow cytometry. The in vivo
effects were explored in LL/2 mice models. Single paclitaxel or
SKLB-163 suppressed tumor growth and the inhibition rate of the
tumor was 43 and 36%, respectively, in the LL/2 model. However, the
combination therapy significantly decreased the tumor volume and
resulted in 84% tumor regression. To obtain additional insight into
the in vivo effects, tumor cell proliferation and apoptosis
were assessed by PCNA immunoreactivity analysis and the TUNEL
assay. Data exhibited that combination therapy clearly reduced the
percentages of PCNA-positive nuclei and increased the percentages
of TUNEL-positive nuclei.
Benzothiazole derivatives have been known for
diverse biological functions, including antitubercular,
antimalarial, antihelmintic, analgesic, anticonvulsant,
anti-inflammatory and antitumor activities (12,13). The
benzothiazole moiety modified with certain functional groups, such
as imidazole and aryl, can significantly inhibit the growth of
certain cancer cell lines (14–16).
However, the majority of studies focused on designing new
benzothiazole compounds by substituting 2-aminobenzothiazoles or
2-arylbenzothiazoles; only a few investigators employed the
benzothiazole-2-thiol as a functional group. In the present study,
SKLB-163, a new benzothiazole-2-thiol derivative, was developed via
computer-aided drug design and de novo synthesis.
SKLB-163 has numerous advantages. Firstly, the
synthetic route is easy to handle and cost is low. Secondly, oral
administration is usually safe and convenient, which patients can
easily accept and adopt. Finally, SKLB-163 has broad-spectrum
antitumor activity as RhoGDI is overexpressed in multiple types of
human cancer.
In conclusion, the data suggests that SKLB-163
combined with paclitaxel exerted synergistic antitumor effects,
providing a novel way to augment the antitumor efficacy of
cytotoxic chemotherapy.
Acknowledgements
The present study was supported by the National
Natural Sciences Foundation of China (grant no. 81402494) and tbe
Science and Technology Support Program of Sichuan province (grant
nos. 2015SZ0076 and 2014HH0063).
References
|
1
|
Li F, Zhao C and Wang L:
Molecular-targeted agents combination therapy for cancer:
Developments and potentials. Intl J Cancer. 134:1257–1269. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Olofsson B: Rho guanine dissociation
inhibitors: Pivotal molecules in cellular signalling. Cell Signal.
11:545–554. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sasaki T and Takai Y: The Rho small G
protein family-Rho GDI system as a temporal and spatial determinant
for cytoskeletal control. Biochem Biophys Res Commun. 245:641–645.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Golovanov AP, Chuang TH, DerMardirossian
C, Barsukov I, Hawkins D, Badii R, Bokoch GM, Lian LY and Roberts
GC: Structure-activity relationships in flexible protein domains:
Regulation of rho GTPases by RhoGDI and D4 GDI. J Mol Biol.
305:121–135. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang B, Zhang Y, Dagher MC and Shacter E:
Rho GDP dissociation inhibitor protects cancer cells against
drug-induced apoptosis. Cancer Res. 65:6054–6062. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Poland J, Schadendorf D, Lage H, Schnolzer
M, Celis JE and Sinha P: Study of therapy resistance in cancer
cells with functional proteome analysis. Clin Chem Lab Med.
40:221–234. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sinha P, Kohl S, Fischer J, Hütter G, Kern
M, Köttgen E, Dietel M, Lage H, Schnölzer M and Schadendorf D:
Identification of novel proteins associated with the development of
chemoresistance in malignant melanoma using two-dimensional
electrophoresis. Electrophoresis. 21:3048–3057. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao L, Wang H, Li J, Liu Y and Ding Y:
Overexpression of Rho GDP-dissociation inhibitor alpha is
associated with tumor progression and poor prognosis of colorectal
cancer. J Proteome Res. 7:3994–4003. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng X, Xie G, Wang Z, Lin H, Zhou T,
Xiang P, Jiang Y, Yang S, Wei Y, Yu L, et al: SKLB-163, a new
benzothiazole-2-thiol derivative, exhibits potent anticancer
activity by affecting RhoGDI/JNK-1 signaling pathway. Cell Death
Dis. 5:e11432014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Z, Shi XH, Wang J, Zhou T, Xu YZ,
Huang TT, Li YF, Zhao YL, Yang L, Yang SY, et al: Synthesis,
structure-activity relationships and preliminary antitumor
evaluation of benzothiazole-2-thiol derivatives as novel apoptosis
inducers. Bioorg Med Chem Lett. 21:1097–1101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi W, Tang Q, Chen X, Cheng P, Jiang P,
Jing X, Chen X, Chen P, Wang Y, Wei Y, et al: Antitumor and
antimetastatic activities of vesicular stomatitis virus matrix
protein in a murine model of breast cancer. J Mol Med Berl.
87:493–506. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hutchinson I, Chua MS, Browne HL, Trapani
V, Bradshaw TD, Westwell AD and Stevens MF: Antitumor
benzothiazoles. 14. Synthesis and in vitro biological properties of
fluorinated 2-(4-aminophenyl) benzothiazoles. J Med Chem.
44:1446–1455. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kok SH, Gambari R, Chui CH, Yuen MC, Lin
E, Wong RS, Lau FY, Cheng GY, Lam WS and Chan SH: Synthesis and
anti-cancer activity of benzothiazole containing phthalimide on
human carcinoma cell lines. Bioorg Med Chem. 16:3626–3631. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Trapani G, Franco M, Latrofa A, Reho A and
Liso G: Synthesis, in vitro and in vivo cytotoxicity, and
prediction of the intestinal absorption of substituted
2-ethoxycarbonyl-imidazo[2,1-b] benzothiazoles. Eur J Pharm Sci.
14:209–216. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song EY, Kaur N, Park MY, Jin Y, Lee K,
Kim G, Lee KY, Yang JS, Shin JH, Nam KY, et al: Synthesis of amide
and urea derivatives of benzothiazole as Raf-1 inhibitor. Eur J Med
Chem. 43:1519–1524. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Srimanth K, Rao VR and Krishna DR:
Synthesis and evaluation of anticancer activity of some
imidazothiazolyl, imidazobenzothiazolyl and dihydroimidazothiazolyl
coumarins. Arzneimittelforschung. 52:388–392. 2002.PubMed/NCBI
|