Introduction
Hypertension is an important risk factor for
cardiovascular disease events (1) and
left ventricular hypertrophy exists in approximately one-third of
hypertensive patients (2). Left
ventricular hypertrophy resulting from hypertension is recognized
as an independent risk factor for cardiovascular complications
(3). Cellular proliferations are
closely associated with the progression of hypertension, myocardial
hypertrophy and apoptosis. In the early stage of hypertension,
there are more myocardial proliferations than apoptosis. With the
development of hypertension, apoptosis becomes the dominant
position (4).
Since the caspase family was first identified in
1992, 15 members of this family in mammalian cells have been found.
Certain members of the caspase family play a vital role in cytokine
maturation and inflammation, while some participate in apoptosis
(5). For those apoptosis-related
caspases, they were divided into two groups; executioner and
initiator caspases (6). Initiator
caspases possess a death effectors domain or the
caspase-recruitment domain, which are responsible for receiving
death-inducing signaling complexes. Executioner caspases carry out
the program of apoptosis, causing changes in cell morphology
(7). In the caspase-dependent
apoptotic pathway, caspase-3 is the core of the executioner
caspases, while caspase-8 and −9 are the representatives of the
initiator caspases. In general, there are two pathways that can be
activated through the caspase. One is the death receptor-mediated
pathway (such as the caspase-8-dependent pathway) and another is
the mitochondrion-mediated pathway (such as the caspase-9-dependent
pathway) (8).
The aims for hypertension treatment are not only to
lower blood pressure, but also to reverse the left ventricular
hypertrophy and to delay the occurrence and development of heart
failure. Valsartan, an angiotensin II antagonist, is widely used in
the control of hypertension. It has been shown that valsartan can
inhibit myocardial apoptosis (9) and
improve left ventricular remodeling (10). However, it is not known that valsartan
inhibits myocardial apoptosis through the death receptor-mediated
extrinsic pathway or mitochondrial-mediated intrinsic pathway.
The main aims of the present study were to observe
the effect of valsartan on left ventricular hypertrophy, systolic
blood pressure (SBP) and myocardial apoptosis in spontaneously
hypertensive rats (SHRs), as well as to clarify the involved
caspase-mediated apoptosis signal pathways.
Materials and methods
Animal care
Sixteen-week-old male SHRs and Wistar-Kyoto (WKY)
rats (280–320 g) provided by the Animal Center of Central South
University (Changsha, China) were housed at a constant temperature
(22°C) with a 12-h light/dark cycle. Animal care was in compliance
with the Guidelines for the Care and Use of Laboratory Animals.
Animal treatment
SHRs were randomly divided into two groups: i) The
valsartan group (SHR + valsartan, n=8), rats were fed with
valsartan (Novartis Pharma Ltd., Beijing, China) using a stomach
tube (30 mg/kg/day intragastrically) for 8 weeks; and ii) the SHR
group (n=7), rats were fed with distilled water. In addition, 8 WKY
rats served as the control group, and they were also fed with
distilled water. At the end of the experiments, the left
ventricular myocardial tissues were collected. Some were embedded
with paraffin and some were stored at −80°C for further
studies.
SBP and left ventricular mass
index
The weight and caudal artery SBP of the rats were
recorded at the end of the experiments. The left ventricles were
isolated, washed with normal saline solution and weighed.
Hematoxylin and eosin (H&E)
staining
The left ventricular tissues were fixed in 4%
paraformaldehyde and embedded in paraffin. The slices underwent
H&E staining according to protocol. Following H&E staining,
the results as images were captured by microscope to assess the
morphological changes among the experimental groups.
Tunel assay
Three paraffin sections randomly picked up from the
left ventricular tissue randomly were analyzed by a terminal
deoxynucleotidyl transferase-mediated dUTP nick end labeling
(TUNEL) assay according to the manufacturer's instructions. The
TUNEL index (%) was calculated as the ratio of the number of
TUNEL-positive cells divided by the total number of cells. At least
3 representative fields were evaluated for each experimental group,
from which an average value was calculated.
Activities of caspase-3, −8 and
−9
Frozen myocardial tissues were rinsed in cold
phosphate-buffered saline and crushed in liquid nitrogen. Tissue
samples were homogenized in lysis buffer and protein concentrations
in the supernatant were determined by the Bradford assay.
Supernatant (50 µl) together with 5 µl of caspase-3, −8 or −9
substrates was incubated at 37°C for 4 h in darkness. The optical
density (OD) was determined at 405 nm by a microplate reader and
the activity of caspase was presented by calculating the ratio of
OD (sample)/OD (negative).
Statistical analysis
All the data are presented as mean ± standard error.
Comparisons between the valsartan and SHR groups, and SHR and
control groups were assessed by independent-samples t-test.
Comparisons between the valsartan and SHR groups were assessed by
paired-samples t-test. Pearson correlation coefficients were
calculated when indicated. P<0.05 was considered to indicate a
statistically significant difference. For statistical analysis, the
SPSS version 19.0 software (IBM, Corp., Armonk, NY, USA) was
used.
Results
SBP and left ventricular mass
index
There was no significant difference in body weight
between the SHR and valsartan groups. The SBP was significantly
elevated in the SHR group compared with that in the control group,
which was evidently attenuated by valsartan. The left ventricular
remodeling was evaluated by comparing the left ventricular mass
index, which was the ratio of the left ventricular weight to body
weight. The index was significantly increased in the SHR group
compared with that in the control group, which was clearly reversed
in the presence of valsartan (Table
I).
 | Table I.Effects of valsartan on SBP and LV
mass index. |
Table I.
Effects of valsartan on SBP and LV
mass index.
| Group | No. | SBP, mmHg | BW, g | LV weight/BW,
mg/g |
|---|
| Control | 8 | 118.3±4.5 | 368±19.5 | 3.2±0.28 |
| SHR | 7 |
192.4±6.1a | 342±22.7a | 4.6±0.31a |
| Valsartan | 8 |
161.0±5.7a,
b | 345±21.8a | 3.9±0.30a, b |
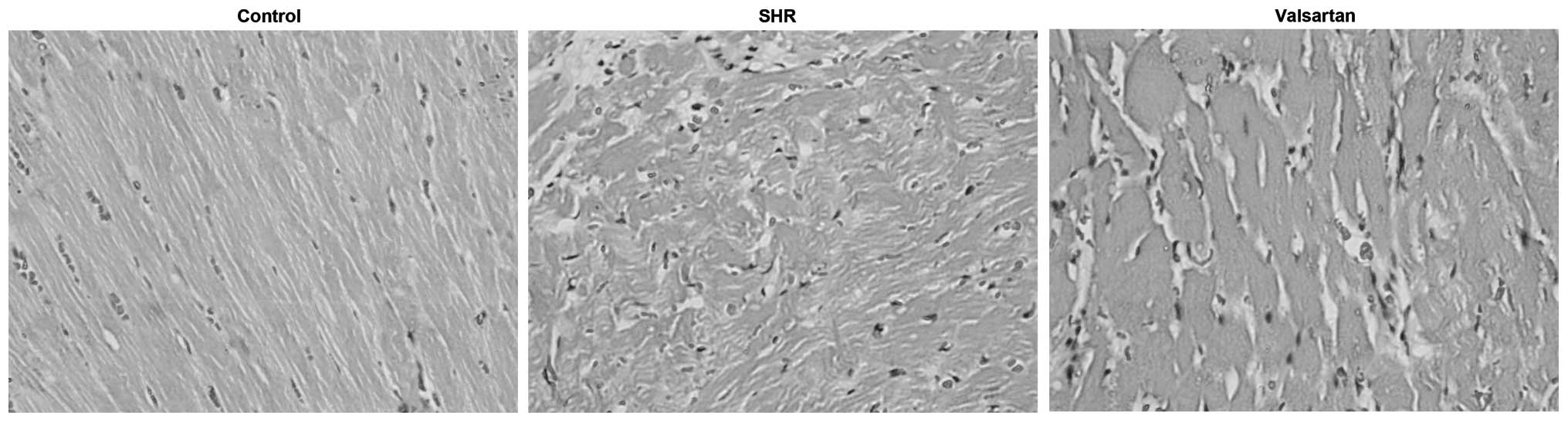
Morphological changes of myocardial
tissues
In the control group, the left ventricular
myocardial fibers were arranged in neat rows and intact borders,
with little congestion, edema and inflammatory cell infiltration.
By contrast, myocardial tissues in the SHR group showed
disorganized myocardial cells, deep-dye nucleus, swelling
myocardial interstitium and capillary hyperemia. These phenomena
were significantly improved following valsartan treatment (Fig. 1).
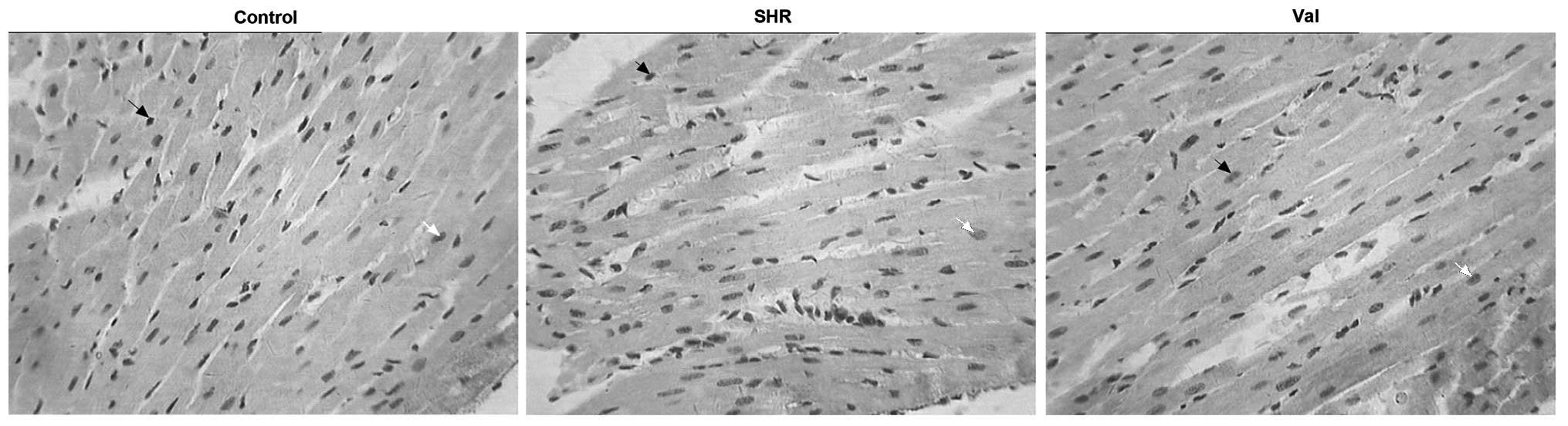
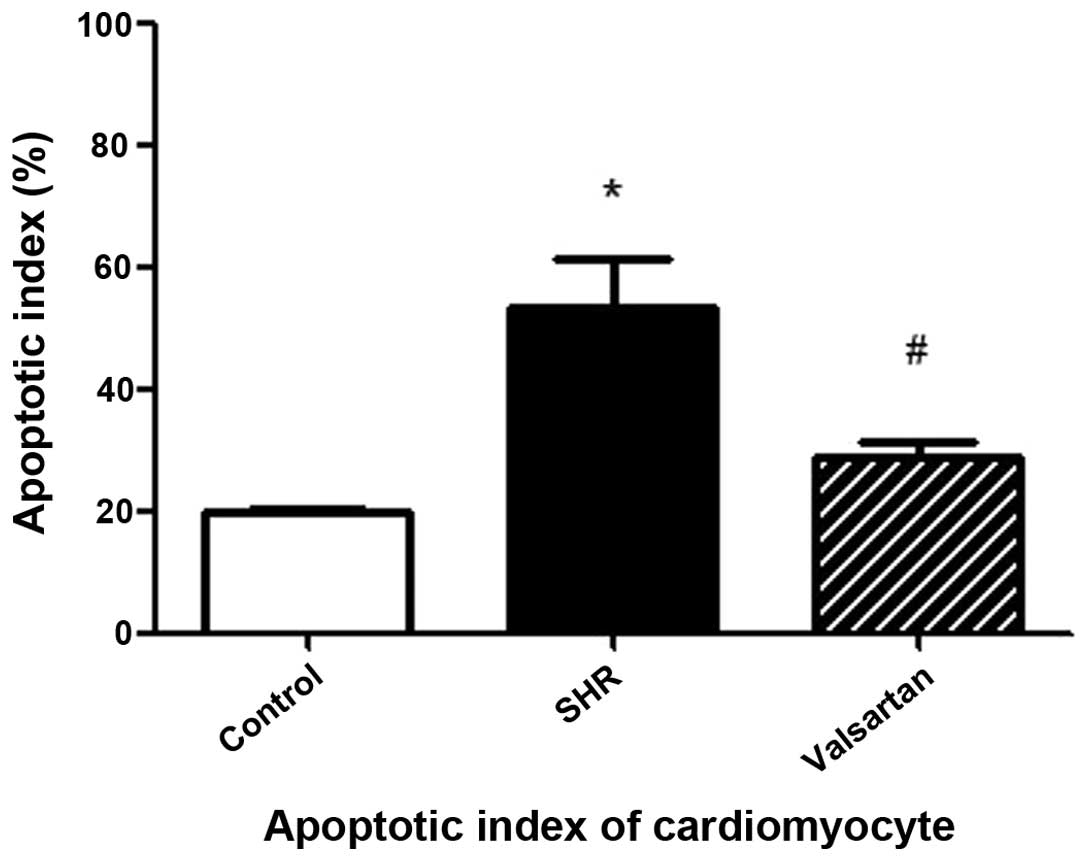
Cellular apoptosis in myocardial
tissues
As shown in Fig. 2, the
black arrows indicate the non-apoptotic cells while the white
arrows indicate the apoptotic cells. Compared to the control group,
the apoptotic index in the SHR group was significantly increased.
This increase was evidently attenuated in the presence of valsartan
(Fig. 3).
Activities of caspase-3, −8 and
−9
The activities of caspase-3, −8 and −9 were
determined by calculating the ratio of OD (sample)/OD (negative).
As shown in Table II, the activities
of caspase-3, −8 and −9 in the SHR group were dramatically elevated
compared to that in the control group, which were markedly reduced
by valsartan treatment.
 | Table II.Activity of caspase-3, −8 and −9 among
the different groups. |
Table II.
Activity of caspase-3, −8 and −9 among
the different groups.
| Group | No. | Caspase-3 | Caspase-8 | Caspase-9 |
|---|
| Control | 8 | 5.10±0.68 | 5.43±0.84 | 5.23±0.41 |
| SHR | 7 |
11.21±1.05a |
10.92±0.74a |
11.50±0.80a |
| Valsartan | 8 |
7.86±1.18a,
b |
7.63±0.66a,
b |
7.84±0.87a,
b |
Discussion
Hypertension is an important cause and risk factor
for various cardiovascular diseases (11). In recent years, numerous studies
demonstrated that high blood pressure induced myocardial apoptosis
(9,12,13). The
caspase is involved in apoptosis, and therefore it was used in the
present study as a marker of apoptosis. Previous studies showed
that valsartan, an angiotensin II antagonist, could exert an effect
on apoptosis and left ventricular remodeling (10,14,15). However, the underlying mechanisms
remain unclear.
The caspase family is involved in two major
functions including inflammation and apoptosis. However, recent
studies focus more on their role in apoptosis. Caspases can be
classified into initiator and executioner caspases (16). Caspases-8 and −9 are typical initiator
caspases, with an extremely long and functionally pro-domain, to
initiate and regulate apoptosis. However, the pro-domain of
executioner caspases is extremely short. They act downstream in the
common pathway, amplifying signals from intrinsic or extrinsic
pathways, carrying out the biochemical changes in apoptosis
(17). Morphological changes of
apoptosis appeared several hours previously, followed by the
activation of downstream caspase-3, −6 and −7, the cell death would
be inevitable (18). Caspase-3, −6 and
−7, typical executioner caspases, must rely on the activation of
initiator caspase, subsequent to performing their activities
(19). There are two pathways in
caspase-dependent apoptosis. One is the death signal-induced, death
receptor-mediated pathway (such as the caspase-8-dependent
pathway). Another is the stress-induced, mitochondrion-mediated
pathway (such as the caspase-9-dependent pathway). In the two
pathways, the downstream caspases, such as caspase-3, always play a
central role in apoptosis (20). In
the present study, the activity of caspase-3 in the left ventricle
from SHR was significantly increased compared with the control
rats, which was reversed by valsartan. The results indicated that
hypertension-induced apoptosis was associated with caspases and
valsartan can inhibit the caspase-dependent apoptosis pathway.
The activation of the extrinsic death receptor
pathway is mediated by caspase-8 (21)
and the activation of the intrinsic mitochondrial pathway is
mediated by caspase-9 (16,22). The present results showed that the
activities of caspase-8 and −9 in the left ventricle from the
hypertensive rats were evidently elevated compared with the control
rats. Valsartan treatment significantly reversed the increase of
caspase-8 and −9 activities. Together with the abovementioned
results, the results suggest that hypertension-induced myocardial
cell apoptosis is associated with the extrinsic death receptor
pathway and intrinsic mitochondrial pathway.
In conclusion, valsartan is able to inhibit
hypertension-induced left ventricular remodeling, which is
associated, at least in part, to its inhibitory effect against
myocardial apoptosis in the death receptor-mediated extrinsic, as
well as the mitochondrial-mediated intrinsic pathways.
Acknowledgements
The present study was supported by the Chinese
Medical Association (grant no. 08010008).
References
|
1
|
Franklin SS, Gokhale SS, Chow VH, Larson
MG, Levy D, Vasan RS, Mitchell GF and Wong ND: Does low diastolic
blood pressure contribute to the risk of recurrent hypertensive
cardiovascular disease events? The Framingham Heart Study.
Hypertension. 65:299–305. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giles TD, Berk BC, Black HR, Cohn JN,
Kostis JB, Izzo JL Jr and Weber MA: Expanding the definition and
classification of hypertension. J Clin Hypertens (Greenwich).
7:505–512. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mathew J, Sleight P, Lonn E, Johnstone D,
Pogue J, Yi Q, Bosch J, Sussex B, Probstfield J and Yusuf S: Heart
Outcomes Prevention Evaluation (HOPE) Investigators: Reduction of
cardiovascular risk by regression of electrocardiographic markers
of left ventricular hypertrophy by the angiotensin-converting
enzyme inhibitor ramipril. Circulation. 104:1615–1621. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiao Y and Hong X: Effect of heart
remodeling and cardiac myocyte apoptosis in juvenile spontaneously
hypertensive rats under the intervention of valsartan, enalapril
and propranolol. Jiangsu Med J. 29:255–257. 2003.
|
|
5
|
Yamamoto-Tanaka M and Hibino T: Caspase-14
protocols. Methods Mol Biol. 1133:89–100. 2014.PubMed/NCBI
|
|
6
|
McStay GP and Green DR: Detection of
caspase activity using antibody-based techniques. Cold Spring Harb
Protoc. 2014:783–788. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marani M, Tenev T, Hancock D, Downward J
and Lemoine NR: Identification of novel isoforms of the BH3 domain
protein Bim which directly activate Bax to trigger apoptosis. Mol
Cell Biol. 22:3577–3589. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fan TJ, Han LH, Cong RS and Liang J:
Caspase family proteases and apoptosis. Acta Biochim Biophys Sin
(Shanghai). 37:719–727. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li W, Sun N, Liu W, Chen Y and Yu Y:
Influence of Valsartan on myocardial apoptosis in spontaneously
hypertensive rats. Chin Med J (Engl). 115:364–366. 2002.PubMed/NCBI
|
|
10
|
Zhi-Bin H, Chang F, Mao-Huan L, Gui-Yi Y,
Shu-Xian Z and Wei W: Valsartan improves the electrophysiological
characteristics of left ventricular hypertrophic myocardium in
spontaneously hypertensive rats. Hypertens Res. 37:824–829. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gaziano TA: Reducing the growing burden of
cardiovascular disease in the developing world. Health Aff
(Millwood). 26:13–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Correia-Pinto J, Henriques-Coelho T,
Roncon-Albuquerque R Jr, Lourenço AP, Melo-Rocha G, Vasques-Nóvoa
F, Gillebert TC and Leite-Moreira AF: Time course and mechanisms of
left ventricular systolic and diastolic dysfunction in
monocrotaline-induced pulmonary hypertension. Basic Res Cardiol.
104:535–545. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kumar V, Bhandari U, Tripathi CD and
Khanna G: Evaluation of antiobesity and cardioprotective effect of
Gymnema sylvestre extract in murine model. Indian J Pharmacol.
44:607–613. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Der Sarkissian S, Tea BS, Touyz RM, de
Blois D and Hale TM: Role of angiotensin II type 2 receptor during
regression of cardiac hypertrophy in spontaneously hypertensive
rats. J Am Soc Hypertens. 7:118–127. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Akashiba A, Ono H, Ono Y, Ishimitsu T and
Matsuoka H: Valsartan improves L-NAME-exacerbated cardiac fibrosis
with TGF-β inhibition and apoptosis induction in spontaneously
hypertensive rats. J Cardiol. 52:239–246. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu X, Kim CN, Yang J, Jemmerson R and
Wang X: Induction of apoptotic program in cell-free extracts:
Requirement for dATP and cytochrome c. Cell. 86:147–157. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Singh SS and Kang PM: Mechanisms and
inhibitors of apoptosis in cardiovascular diseases. Curr Pharm Des.
17:1783–1793. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brunet CL, Gunby RH, Benson RS, Hickman
JA, Watson AJ and Brady G: Commitment to cell death measured by
loss of clonogenicity is separable from the appearance of apoptotic
markers. Cell Death Differ. 5:107–115. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim NH and Kang PM: Apoptosis in
cardiovascular diseases: Mechanism and clinical implications.
Korean Circ J. 40:299–305. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Haunstetter A and Izumo S: Apoptosis:
Basic mechanisms and implications for cardiovascular disease. Circ
Res. 82:1111–1129. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen G and Goeddel DV: TNF-R1 signaling: A
beautiful pathway. Science. 296:1634–1635. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li P, Nijhawan D, Budihardjo I,
Srinivasula SM, Ahmad M, Alnemri ES and Wang X: Cytochrome c and
dATP-dependent formation of Apaf-1/caspase-9 complex initiates an
apoptotic protease cascade. Cell. 91:479–489. 1997. View Article : Google Scholar : PubMed/NCBI
|