Introduction
Bone remodeling is the main process in maintaining
the integrity of bone structure. Throughout life, bones undergo
three phases: Phase of rapid skeletal growth and increasing bone
mineral density, sustenance phase and phase of predominant bone
resorption causing bone loss. The bone remodeling process is
influenced by numerous factors, including estrogens and the
endocannabinoid system. Misbalance of bone remodeling mechanisms
causes one of the most common degenerative diseases in developed
countries, osteoporosis (1). It is
estimated that >200 million people worldwide suffer from
osteoporosis. The costs of health care services in the European
Union as consequences of osteoporotic complications are already
considerable and, if current trends continue, the costs are
predicted to double by 2050 (2).
Osteoblasts are influenced by estrogens at the
cellular and molecular level. Estrogens bind to nuclear estrogen
receptors (ERs) in osteoblasts, the ERs dimerize and act as
transcription factors modulating the expression of specific DNA
sequences (3). Estrogens increase
collagen I and osteoprotegerin gene expression and certain evidence
indicates inhibitory effects of estrogens on osteoblast apoptosis
(4,5).
Cannabinoids bind and activate cannabinoid receptors
1 and 2 (CB-1 and -2 receptors), as well as non-CB-1/CB-2 receptor
GPR55 and vanilloid type 1 receptor (TRPV1) (6–8). CB-1
receptors are predominantly located in the central nervous system,
whereas CB-2 receptors are expressed in the immune system,
cirrhotic liver, arteriosclerotic plaques, gastrointestinal mucosa
and during brain inflammation (9–11). CB-2
receptors have also been reported to have a significantly higher
expression in osteoblasts, osteoclasts and osteocytes compared to
CB-1 receptors (12–14). Therefore, specific CB-2
agonists/antagonists could be involved in the regulation of bone
remodeling as a result of their effects on osteoblasts and
osteoclasts. In addition, CB-2 receptor specific ligands do not
induce psychoactive adverse effects, making them more suitable for
potential clinical use (15). The CB1
and CB2 receptors inhibit adenylyl cyclase activity, which is
linked to a variety of secondary messengers, including p42/44
mitogen-activated protein kinase (16–18), p38
mitogen-activated protein kinase (19), c-Jun N-terminal kinase (20), activator protein 1 and Ca2+
transients (18,21). The role of GPR55 receptor in bone
formation has not been extensively studied and appears to be minor
(7). The TRPV1 receptor is also
considered to be important in the pathogenesis of osteoporosis
(22,23).
Increased expression of CB-2 receptors was found on
osteoclasts when they were treated with 17-β-estradiol (24). Our new hypothesis is that
17-β-estradiol could also influence the CB-2 receptor expression in
osteoblasts and is based on the 17-β-estradiol action on ERs and
changes in gene transcription.
Accordingly, a possible interaction between
17-β-estradiol and specific CB-2 agonist and/or antagonist was
considered and, to the best of our knowledge, for the first time
the hypothesis was experimentally tested in primary human
osteoblasts (HOB).
Materials and methods
Primary HOB growing and testing
Proliferating HOB were purchased from PromoCell
(Heidelberg, Germany). The donor was a healthy 60-year-old
Caucasian female. The cells were grown in phenol red-free cell
growth medium (PromoCell) at 37°C and 5% CO2
(CO2-incubator; Sanyo, Moriguchi, Japan). Trypsinization
was performed following the PromoCell's subcultivation
instructions. The experiment started with HOB in their 5th passage.
After 24 and 48 h of HOB exposure to different concentrations of
17-β-estradiol (1, 10 and 100 nM), to specific CB-2 agonist
(R,S)-AM1241 (1.0 and 7.5 µM), to specific CB-2 antagonist/inverse
agonist AM630 (10 µM) and to their selected combinations, the
colorimetric cell viability test WST-8 (PromoKine, Milpitas, CA,
USA; cat. no. PK-CA705-CK04-100) and alkaline phosphatase activity
colorimetric assay (BioVision, Milpitas, CA, USA; cat. no.
K412-500) were performed in triplicates, strictly following the
manufacturer's instructions. (R,S)-AM1241 (cat. no. A6478-5MG),
AM630 (cat. no. SML0327-5MG) and 17-β-estradiol (cat. no. E2758-1G)
were all obtained from Sigma (St. Louis, MO, USA). The samples with
HOB exposed to 1 nM concentration of 17-β-estradiol, which
approximately corresponds to physiological estrogen levels in
healthy females (25), were treated as
controls.
Statistical analysis
All the data are presented as means ± standard
deviation from three experimental samples. Statistical differences
between the groups were determined using SPSS version 18.0 software
(SPSS, Inc., Chicago, IL, USA), with independent samples t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Proliferation activity
In detail, the results on HOB proliferation activity
are presented in Figs. 1–3.
Influence of a combination of
17-β-estradiol and specific CB-2 antagonist/inverse agonist on HOB
proliferation activity
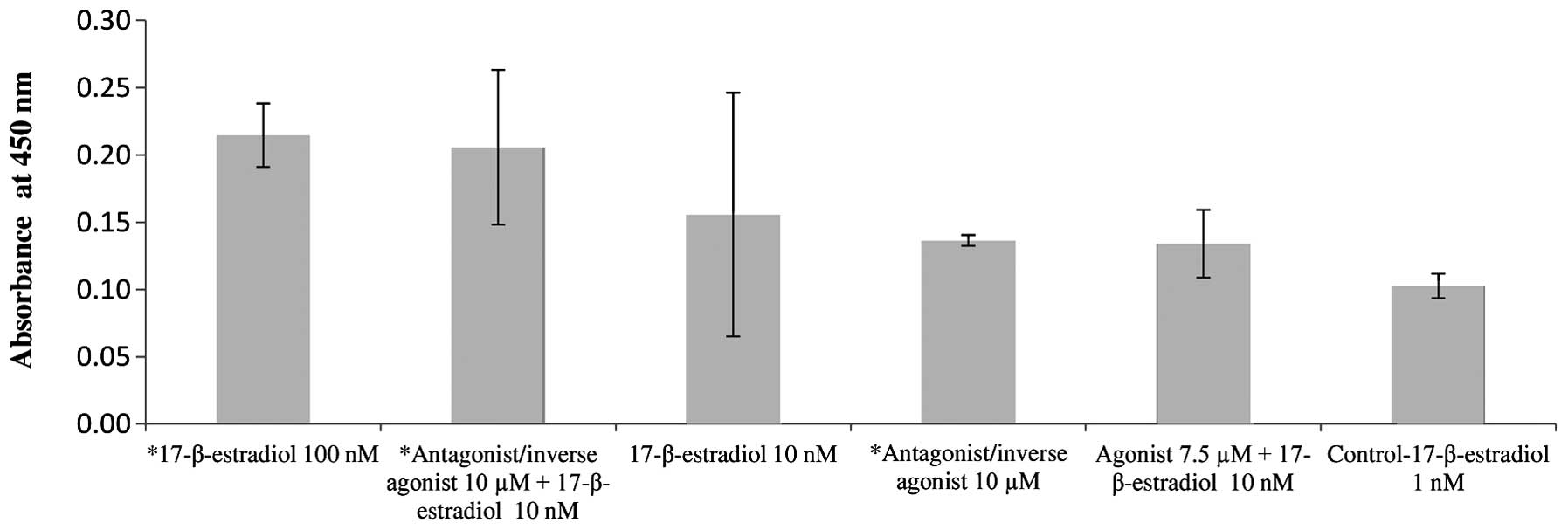
After 24 h, the highest proliferation activity was
observed in HOB exposed to the specific CB-2 agonist (7.5 µM;
Fig. 3), 100 nM concentration of 17-β
estradiol and in HOB exposed to a combination of 10 nM
concentration of 17-β estradiol with 10 µM of the specific CB-2
antagonist/inverse agonist. The interaction between 17-β-estradiol
and specific CB-2 antagonist/inverse agonist on HOB proliferation
activity thus appears to be synergistic.
Influence of 17-β-estradiol on HOB
proliferation activity
The influence of 17-β-estradiol (1, 10 and 100 nM)
on HOB proliferation activity was dose-dependent; HOB proliferation
activity increased with increasing 17-β-estradiol concentrations
(Figs. 1 and 2).
Influence of specific CB-2 agonist on
HOB proliferation activity
Similar dose-dependent findings were observed in HOB
treated with specific CB-2 agonist; higher concentration of
specific CB-2 agonist (7.5 µM) resulted in increased HOB
proliferation activity compared to the lower concentration of the
specific CB-2 agonist (1 µM) (Fig.
3).
Influence of a combination of
17-β-estradiol and specific CB-2 agonist on HOB proliferation
activity
HOB exposed only to specific CB-2 agonist (7.5 µM)
had higher proliferation activity compared to HOB exposed to a
combination of specific CB-2 agonist (7.5 µM) and 17-β-estradiol
(10 nM) (Figs. 1 and 3). HOB proliferation activity of the
combination was similar to that found in HOB treated only with 10
nM 17-β-estradiol. The interaction between 17-β-estradiol and
specific CB-2 agonist on HOB proliferation activity thus appears to
be antagonistic.
Influence of specific CB-2 on HOB
proliferation activity
Specific CB-2 agonist at 7.5 µM increased HOB
proliferation activity more than specific CB-2 antagonist/inverse
agonist at 10 µM. These results also show that HOB proliferation
activity was higher when the cells were concomitantly treated with
a combination of specific CB-2 agonist (7.5 µM) and specific CB-2
antagonist/inverse agonist (10 µM) compared to cell exposure solely
to specific CB-2 antagonist/inverse agonist (10 µM).
Similar findings on HOB proliferation activity were
also observed after 48 h of incubation; however, the differences
after 48 h were less prominent. In all the experiments, HOB
successfully passed the alkaline phosphatase differentiation
test.
Discussion
To the best of our knowledge, the present study
shows for the first time, a probable synergistic interaction
between 17-β-estradiol and the specific CB-2 antagonist/inverse
agonist AM630, as observed in primary HOB in vitro.
The influence of a combination of 17-β-estradiol and
specific CB-2 antagonist/inverse agonist on HOB proliferation
activity was examined. The results highlighted that the combination
of 17-β-estradiol (10 nM) with specific CB-2 antagonist/inverse
agonist (10 µM) increases HOB proliferation activity ~32% compared
to proliferation activity of HOB exposed solely to the same
concentration of 17-β-estradiol (10 nM). To achieve almost the same
effect on HOB proliferation activity as with 100 nM 17-β-estradiol,
10-times lower concentrations of 17-β-estradiol (10 nM) could be
used when co-administering 17-β-estradiol with specific CB-2
antagonist/inverse agonist (10 µM). A synergistic interaction
between estrogens and specific CB-2 antagonists/inverse agonists
could be proposed and explained by the possibility of the increase
in CB-2 receptor expression influenced by estrogens and/or by
interactions on other molecular levels. 17-β-Estradiol could be
assumed to increase the expression of CB-2 receptors, as has been
previously observed in osteoclasts in vitro (24), or it is possible that estrogens act
through other, non-genomic mechanisms (26). Recent studies also show that selective
estrogen modulators, raloxifene (27),
bazedoxifene and lasofoxifene, behave as CB-2 inverse agonists
(28), which may also be true for
17-β-estradiol and would correlate with the present results.
Previous studies showed that CB-2 receptor
antagonist/inverse agonist AM630 inhibited osteoclast formation and
bone resorption in vitro (12,29).
Furthermore, it has been shown that the administration of AM630 to
ovariectomized wild-type mice prevented ovariectomy-induced bone
loss (29). The present results do not
entirely support the above findings; HOB proliferation activity
after 24 h was higher compared to control cells when the tested
cells were exposed to specific CB-2 antagonist/inverse agonist as
the only testing substance.
Influence of 17-β-estradiol on HOB proliferation
activity. Estrogens have antiresorptive properties and have been in
clinical use for treatment of osteoporosis for a long time
(30). The results from the present
study confirm a dose-dependent estrogen response on HOB
proliferation activity. As expected, higher concentrations of
17-β-estradiol resulted in higher HOB proliferation activity
compared to lower 17-β estradiol concentrations.
The influence of specific CB-2 agonist on HOB
proliferation activity was investigated in the present study.
Previous studies have shown that specific CB-2 agonists directly
stimulate stromal cells/osteoblasts and simultaneously inhibit
monocyte/osteoclasts, directly and indirectly by inhibiting the
expression of RANKL in stromal cells/osteoblasts (13). Previous studies have also shown that
specific CB-2 agonists induce mitogenic effects in osteoblasts via
activation of a Gi protein-cyclin D1 and the extracellular
signal-regulated kinase 1/2 pathway (31,32). This
was indirectly confirmed in the present study by the increase in
proliferation activity of HOB when they were treated solely with
the specific CB-2 agonist. As hypothesized, HOB proliferation
activity was influenced by the specific CB-2 agonist in a
dose-dependent manner; higher concentrations of the specific CB-2
agonist increased HOB proliferation activity more than the lower
concentrations.
The influence of a combination of 17-β-estradiol and
specific CB-2 agonist were examined on HOB proliferation activity.
The results show that in the absence of 17-β-estradiol, the
specific CB-2 agonist had a stimulating effect on HOB proliferation
activity in vitro, whereas the combination of the two
substances appeared to have an antagonistic effect on HOB
proliferation activity. This result additionally confirms the
interplay between estrogens and cannabinoids, as already proposed
by a synergistic interaction between 17-β-estradiol and specific
CB-2 antagonist/inverse agonist.
Additional notable observations in the present study
are that 7.5 µM specific CB-2 agonist increases HOB proliferation
activity to a higher extent compared to 10 nM specific CB-2
antagonist/inverse agonist. Furthermore, in the presence of 7.5 µM
specific CB-2 agonist and 10 nM specific CB-2 antagonist/inverse
agonist, HOB proliferation activity is higher compared to cells
treated with 10 nM specific CB-2 antagonist/inverse agonist as the
only testing substance. Therefore, it could be speculated that in
the absence of estrogens, specific CB-2 agonists may overcome the
effects of specific CB-2 antagonists/inverse agonists on HOB
proliferation activity. However, further studies with different
CB-2 agonists and antagonists/inverse agonists in various
concentrations should be tested to clarify the above observations
and to evaluate their potential clinical importance.
Another factor in the present study is that all the
experiments and treatments, dose- and time-dependency, as well as
the controls, showed that HOB in cell cultures maintained their
alkaline phosphatase activity. In brief, HOB in vitro did
not de-differentiate in the controls or when treated with various
chemicals, but further quantitative studies should be performed in
order to evaluate the degree of differentiation exactly in each
single experiment setting.
There are possible clinical applications for the
results of the present study. The difference between the actions of
cannabinoid antagonists/agonists in the presence or absence of
estrogens may lead to specific drug treatment of osteoporosis in
elderly patients with specific CB-2 agonists, which are more
effective in the presence of low estrogen concentrations and even
better as a combination drug for females with CB-2
antagonists/inverse agonists and 17-β-estradiol, which would have a
synergistic effect on osteoblasts activity and could provide novel
treatment of postmenopausal induced osteoporosis in females. This
could lead to a new, possibly gender-dependent strategy in
osteoporosis prevention. Firstly, combination treatment of specific
CB-2 antagonists/inverse agonists and estrogens may lead to lower
doses of estrogens administered to patients with osteoporosis and
consequently fewer of their harmful side-effects and minimizing of
the serious complications. Notably, it is well-known that long-term
use of estrogens can promote carcinogenesis, particularly in breast
and endometrial cancer (32–34). Secondly, a number of studies indicate
that the endocannabinoid system plays protective roles against the
growth and the spreading of several types of carcinomas, including
endometrial and breast cancers (35,36).
Therefore, a combination of cannabinoids and estrogens may have
beneficial effects by inhibiting estrogen-induced carcinogenesis.
Thirdly, adding specific CB-2 antagonists/inverse agonists to
contraceptive pills could increase maximal bone mineral density in
females. Contraceptive pills are most commonly used before the age
of 30 years, which corresponds to the phase of rapid skeletal
growth and increasing bone mineral density; this makes it ideal for
preventing osteoporosis or delaying its development. Furthermore,
the involvement of the endocannaboid system is also indicated in
cardioprotection (37), which is a
particularly important concern in the postmenopausal era. These
benefits could potentially be increased by synergistic interactions
with estrogens.
Recent pharmacological improvements allow oral
application of specific CB-2 agonists (i.e., S-777469) and specific
CB-2 antagonists/inverse agonists (i.e., SR 144528); therefore
(38,39), their potential medical applications in
the treatment of various diseases are becoming even more
noteworthy. By contrast, safety and adverse effects of CB-2
agonists/antagonists/inverse agonists should be examined prior to
clinical use.
In conclusion, the results of the present study are
quite informative and represent the idea of a probable synergistic
interaction between 17-β-estradiol and specific CB-2
antagonist/inverse agonist in primary HOB. However, these
observations require further investigation in order to clarify and
evaluate the clinical applicability of the results. Mineralization,
specific osteoblast molecular biomarkers (i.e., runt-related
transcription factor 2, osteonectin, osteocalcin, bone sialoprotein
and collagen I), molecular pharmacodynamic interactions, expression
of ERs α and β, as well as the CB-1 and -2 receptors expression
should be performed. As indicated in the introduction, other
receptors are also involved in cannabinoid signaling pathways. For
this reason, an in depth understanding of how TRPV1 and GPR55
receptors in combination with CB-1 and -2 receptors interact to
modify the activity of osteoblasts could be helpful in predicting
the efficiency of cannabinoid pharmacotherapy.
Acknowledgements
The authors would like to thank Mr. Alojz Tapajner
for his useful suggestions in interpreting the study results and
Mr. Samo Lorber for helping to prepare the figures.
References
|
1
|
Raisz LG: Pathogenesis of osteoporosis:
concepts, conflicts, and prospects. J Clin Invest. 115:3318–3325.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reginster JY and Burlet N: Osteoporosis: a
still increasing prevalence. Bone. 38 (Suppl 1):S4–S9. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Centrella M and McCarthy TL: Estrogen
receptor dependent gene expression by osteoblasts - direct,
indirect, circumspect, and speculative effects. Steroids.
77:174–184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bilezikian JP, Raisz LG and Martin TJ:
Principles of bone biologyEstrogens and Progestins. Turner RT,
Rickard DJ, Iwaniec UT and Spelsberg TC: 3rd. Elsevier; Amsterdam:
pp. 855–885. 2008
|
|
5
|
Bradford PG, Gerace KV, Roland RL and
Chrzan BG: Estrogen regulation of apoptosis in osteoblasts. Physiol
Behav. 99:181–185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Howlett AC: The cannabinoid receptors.
Prostaglandins Other Lipid Mediat. 68–69:619–631. 2002. View Article : Google Scholar
|
|
7
|
Whyte LS, Ryberg E, Sims NA, Ridge SA,
Mackie K, Greasley PJ, Ross RA and Rogers MJ: The putative
cannabinoid receptor GPR55 affects osteoclast function in vitro and
bone mass in vivo. Proc Natl Acad Sci USA. 106:16511–16516. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van der Stelt M, Trevisani M, Vellani V,
De Petrocellis L, Schiano Moriello A, Campi B, McNaughton P, et al:
Anandamide acts as an intracellular messenger amplifying
Ca2+ influx via TRPV1 channels. EMBO J. 24:3026–3037.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Julien B, Grenard P, Teixeira-Clerc F, Van
Nhieu JT, Li L, Karsak M, Zimmer A, Mallat A and Lotersztajn S:
Antifibrogenic role of the cannabinoid receptor CB2 in the liver.
Gastroenterology. 128:742–755. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Steffens S, Veillard NR, Arnaud C, Pelli
G, Burger F, Staub C, Karsak M, Zimmer A, Frossard JL and Mach F:
Low dose oral cannabinoid therapy reduces progression of
atherosclerosis in mice. Nature. 434:782–786. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wright K, Rooney N, Feeney M, Tate J,
Robertson D, Welham M and Ward S: Differential expression of
cannabinoid receptors in the human colon: cannabinoids promote
epithelial wound healing. Gastroenterology. 129:437–453. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Idris AI, van't Hof RJ, Greig IR, Ridge
SA, Baker D, Ross RA and Ralston SH: Regulation of bone mass, bone
loss and osteoclast activity by cannabinoid receptors. Nat Med.
11:774–779. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ofek O, Karsak M, Leclerc N, Fogel M,
Frenkel B, Wright K, Tam J, Attar-Namdar M, Kram V, Shohami E, et
al: Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc
Natl Acad Sci USA. 103:696–701. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Whyte LS, Ford L, Ridge SA, Cameron GA,
Rogers MJ and Ross RA: Cannabinoids and bone: endocannabinoids
modulate human osteoclast function in vitro. Br J Pharmacol.
165:2584–2597. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hanus L, Breuer A, Tchilibon S, Shiloah S,
Goldenberg D, Horowitz M, Pertwee RG, Ross RA, Mechoulam R and
Fride E: HU-308: a specific agonist for CB2, a peripheral
cannabinoid receptor. Proc Natl Acad Sci USA. 96:14228–14233. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wartmann M, Campbell D, Subramanian A,
Burstein SH and Davis RJ: The MAP kinase signal transduction
pathway is activated by the endogenous cannabinoid anandamide. FEBS
Lett. 359:133–136. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Melck D, Rueda D, Galve-Roperh I, De
Petrocellis L, Guzman M and Di Marzo V: Involvement of the
cAMP/protein kinase A pathway and of mitogen-activated protein
kinase in the anti-proliferative effects of anandamide in human
breast cancer cells. FEBS Lett. 463:235–240. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu J, Gao B, Mirshahi F, Sanyal AJ,
Khanolkar AD, Makriyannis A and Kunos G: Functional CB1 cannabinoid
receptors in human vascular endothelial cells. Biochem J.
346:835–840. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Derkinderen P, Ledent C, Parmentier M and
Girault JA: Cannabinoids activate p38 mitogen-activated protein
kinases through CB1 receptors in hippocampus. J Neurochem.
77:957–960. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rueda D, Galve-Roperh I, Haro A and Guzmán
M: The CB1 cannabinoid receptor is coupled to the activation of
c-Jun N-terminal kinase. Mol Pharmacol. 58:814–820. 2000.PubMed/NCBI
|
|
21
|
Mombouli JV, Schaeffer G, Holzmann S,
Kostner GM and Graier WF: Anandamide-induced mobilization of
cytosolic Ca2+ in endothelial cells. Br J Pharmacol.
126:1593–1600. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rossi F, Siniscalco D, Luongo L, De
Petrocellis L, Bellini G, Petrosino S, Torella M, Santoro C, Nobili
B, Perrotta S, et al: The endovanilloid/endocannabinoid system in
human osteoclasts: possible involvement in bone formation and
resorption. Bone. 44:476–484. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rossi F, Bellini G, Luongo L, Torella M,
Mancusi S, De Petrocellis L, Petrosino S, Siniscalco D, Orlando P,
Scafuro M, et al: The endovanilloid/endocannabinoid system: a new
potential target for osteoporosis therapy. Bone. 48:997–1007. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rossi F, Bellini G, Luongo L, Mancusi S,
Torella M, Tortora C, Manzo I, Guida F, Nobili B, de Novellis V and
Maione S: The 17-beta-oestradiol inhibits osteoclast activity by
increasing the cannabinoid CB2 receptor expression. Pharmacol Res.
68:7–15. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu SF, Wang ZX, Yuan YE, Bing SM, Zhang
BZ, Wu JZ, Wu YE and Peng XY: Hormone changes during the menstrual
cycle of Chinese women. J Reprod Fertil. 76:43–52. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kousteni S, Chen JR, Bellido T, Han L, Ali
AA, O'Brien CA, Plotkin L, Fu Q, Mancino AT, Wen Y, et al: Reversal
of bone loss in mice by nongenotropic signaling of sex steroids.
Science. 298:843–846. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kumar P and Song ZH: Identification of
raloxifene as a novel CB2 inverse agonist. Biochem Biophys Res
Commun. 435:76–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kumar P and Song ZH: CB2 cannabinoid
receptor is a novel target for third-generation selective estrogen
receptor modulators bazedoxifene and lasofoxifene. Biochem Biophys
Res Commun. 443:144–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Idris AI, Sophocleous A, Landao-Bassonga
E, van't Hof RJ and Ralston SH: Regulation of bone mass, osteoclast
function, and ovariectomy-induced bone loss by the type 2
cannabinoid receptor. Endocrinology. 149:5619–5626. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Horst-Sikorska W and Wawrzyniak A: The
role of hormonal therapy in osteoporosis. Endokrynol Pol. 62:61–64.
2011.PubMed/NCBI
|
|
31
|
Ofek O, Attar-Namdar M, Kram V,
Dvir-Ginzberg M, Mechoulam R, Zimmer A, Frenkel B, Shohami E and
Bab I: CB2 cannabinoid receptor targets mitogenic Gi protein-cyclin
D1 axis in osteoblasts. J Bone Miner Res. 26:308–316. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sophocleous A, Landao-Bassonga E, Van't
Hof RJ, Idris AI and Ralston SH: The type 2 cannabinoid receptor
regulates bone mass and ovariectomy-induced bone loss by affecting
osteoblast differentiation and bone formation. Endocrinology.
152:2141–2149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chlebowski RT, Anderson GL, Gass M, Lane
DS, Aragaki AK, Kuller LH, Manson JE, Stefanick ML, Ockene J, Sarto
GE, et al: Estrogen plus progestin and breast cancer incidence and
mortality in postmenopausal women. JAMA. 304:1684–1692. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lépine J, Audet-Walsh E, Grégoire J, Têtu
B, Plante M, Ménard V, Ayotte P, Brisson J, Caron P, Villeneuve L,
et al: Circulating estrogens in endometrial cancer cases and their
relationship with tissular expression of key estrogen biosynthesis
and metabolic pathways. J Clin Endocrinol Metab. 95:2689–2698.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guida M, Ligresti A, De Filippis D,
D'Amico A, Petrosino S, Cipriano M, Bifulco G, Simonetti S, Orlando
P, Insabato L, et al: The levels of the endocannabinoid receptor
CB2 and its ligand 2-arachidonoylglycerol are elevated in
endometrial carcinoma. Endocrinology. 151:921–928. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qamri Z, Preet A, Nasser MW, Bass CE,
Leone G, Barsky SH and Ganju RK: Synthetic cannabinoid receptor
agonists inhibit tumor growth and metastasis of breast cancer. Mol
Cancer Ther. 8:3117–3129. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lagneux C and Lamontagne D: Involvement of
cannabinoids in the cardioprotection induced by lipopolysaccharide.
Br J Pharmacol. 132:793–796. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Odan M, Ishizuka N, Hiramatsu Y, Inagaki
M, Hashizume H, Fujii Y, Mitsumori S, Morioka Y, Soga M, Deguchi M,
et al: Discovery of S-777469: an orally available CB2 agonist as an
antipruritic agent. Bioorg Med Chem Lett. 22:2803–2806. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rinaldi-Carmona M, Barth F, Millan J,
Derocq JM, Casellas P, Congy C, Oustric D, Sarran M, Bouaboula M,
Calandra B, et al: SR 144528, the first potent and selective
antagonist of the CB2 cannabinoid receptor. J Pharmacol Exp Ther.
284:644–650. 1998.PubMed/NCBI
|