Introduction
The use of arsenic in China can be traced back
thousands of years. Arsenic and arsenic salts, including orpiment,
realgar and arsenolite, were fundamental ingredients in certain
cancer treatments (1). The Fowler's
solution (1% potassium arsenite) was used in the treatment of
malignant diseases, such as leukemia and Hodgkin's disease, by
numerous physicians in the 19–20th centuries (1). Arsenic trioxide
(As2O3) has emerged as the first-line therapy
in the treatment of acute promyelocytic leukemia (APL); however,
the toxicity hinders its clinical applications (2,3).
Realgar contains >90% arsenic sulfide and is
widely used externally and internally in a number of traditional
medicine recipes in China (4). For
example, realgar in combination with Indigo naturalis and
Salvia miltiorrhiza is effective against APL (5), and targets RING-type E3 ligase c-CBL to
induce degradation of BCR-Abl in chronic myelogenous leukemia
(6). Realgar also enhances the
antitumor effects of imatinib (7).
Results from multicenter clinical trials using oral realgar
formulations plus intravenous As2O3 against
hematological malignancy are promising (8).
However, realgar is insoluble in water, resulting in
poor bioavailability. The poor solubility of realgar limits its
clinical applications (9) and presents
a major challenge in cancer chemotherapy (10). The realgar nanoparticles (11–13) and
realgar bioleaching solution (14) are
more effective compared to crude realgar in antitumor studies,
indicative of smaller sizes of realgar being preferable to achieve
a more efficient bioavailability (9).
Thus, realgar quantum dots (RQDs; 5.48±1.09 nm) were prepared in
our previous study using a modified method (15). The cytotoxic effects in vitro of
realgar nanoparticles and RQDs on human ovarian (CI80-13S, OVCAR,
OVCAR-3) and cervical (HeLa) cancer cell lines was comparable to
As2O3 (11,15);
however, their toxicities towards normal cells were to a smaller
extent (15).
Arsenic compounds have also been investigated for
their effects against solid tumors, including hepatocellular
carcinoma (HCC), in animal models and in humans (3,9,16). Our recent study investigated the
effects of RQDs in animal models and identified that RQDs can
effectively inhibit the uterine cervix U14 tumor xenografts in
tumor-bearing mice, without producing overt toxicity (17). The present study further investigated
the effects of RQDs against human hepatocellular (HepG2) cells,
focusing on induction of apoptosis and necrosis through endoplasmic
reticulum (ER) stress.
Materials and methods
Cell lines and culture conditions
The tumor HepG2 cell line and normal L02 cell line
(3) were purchased from the Cell Bank
of Shanghai Institute of Biological Science, Chinese Academy of
Sciences (Shanghai, China). The cells were maintained in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 10% fetal bovine
serum (FBS), 100 µg/ml streptomycin and 100 IU/ml penicillin at
37°C in a humidified atmosphere of 5% CO2. Cells in
their exponential growth phase were used.
Materials
High-glucose DMEM, FBS and trypsin were purchased
from Gibco Life Technologies (Carlsbad, CA, USA). purchased from
Gibco Life Technologies (Carlsbad, CA, USA). The antibodies for
β-actin, Bcl-2 and Bax were purchased from Sigma (St. Louis, MO,
USA). Secondary antibodies, goat anti-rabbit immunoglobulin
G-horseradish peroxidase (IgG-HRP) (SC-2004) and goat anti-mouse
IgG-HRP (SC-2005), were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA).
Preparation of RQDs
RQDs were prepared by Dr Jin-Zhu Wu from Harbin
Institute of Technology (Harbin, China) by modifying a previous
method (5), with a size of 5.48±1.09
nm. A total of 2.0 g course bulk realgar powder was added into 50
ml ethanolamine under bubbling argon gas and an ultrasonic method
was used for dissolving. The supernatant was obtained following
centrifugation, heated at 80°C for 12 h, and subsequently, citric
acid was added until pH 8.0±0.2 was reached. The determination of
the photoluminescence (PL) quantum yield of the synthesized RQDs
was estimated by a previous method (15).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
assay to measure cell proliferation
HepG2 and L02 cells were seeded in 96-well plates at
a density of 8×103 cells/well and cultured at 37°C in an
incubator with an atmosphere of 5% CO2 for 24 h. Cells
were subsequently treated with phosphate-buffered saline (PBS,
control) or different concentrations of (7.5, 15, 30 and 60 µg/ml)
RQDs. After 6 h, 10 µl of MTT solution (5 mg/ml was dissolved in
PBS) was added and the plate was incubated at 37°C. Four hours
later, 100 µl of dimethylsulfoxide was added to each well to
dissolve the formazan. The amount of colored formazan metabolite
formed was determined by measuring its absorbance at 570 nm in
Microplate Reader (Molecular Devices Inc., Sunnyvale, CA, USA).
Experiments were performed in 6 replicates in 96-well flat-bottomed
culture plates. The half maximal inhibitory concentration
(IC50) was calculated using SPSS software, version 17
(SPSS, Inc., Chicago, IL, USA) and visualized via graphic
plotting.
Flow cytometric analysis of
apoptosis
HepG2 cells were seeded in 6-well plates at a
density of 1×106 cells/well and cultured at 37°C in an
incubator with an atmosphere of 5% CO2 for 24 h. HepG2
cells were treated with PBS (control) or different concentrations
(7.5, 15 and 30 µg/ml) of RQDs for 6 h and were trypsinized and
collected by centrifugation at 72 × g for 5 min. Subsequent to
washing 3 times with cold PBS (0.15 M, pH 7.2), the precipitates
were resuspended in 100 µl of binding buffer and stained with 5 µl
of Annexin V-fluorescein isothiocyanate solution together with 10
µl of propidium iodide (PI) solution for 20 min at room temperature
in the dark. To each sample tube, 500 µl of 1X binding buffer was
added and the samples were analyzed using the FACSCalibur and
CellQuest software (Beckman Coulter, Inc., Fullerton, CA, USA). All
the experiments were performed in triplicate.
Western blot analysis
HepG2 cells were seeded in 6-well plates at a
density of 1×106 cells/well and incubated for 24 h.
Cells were treated with PBS (control) or different concentrations
(3.75, 7.5, 15 and 30 µg/ml) of RQDs for 6 h at 37°C. The cells
were lysed for 10 min with lysis buffer (radioimmunoprecipitation
assay) containing protease inhibitor phenylmethylsulfonyl fluoride.
The extracts were subsequently centrifuged at 800 × g for 10 min at
4°C to remove debris. Following boiling with loading buffer,
protein samples were separated by 12% SDS-PAGE and were transferred
onto polyvinylidene difluoride membranes, which were blocked with
5% non-fat milk for 2 h. Subsequently, membranes were incubated
with the primary antibodies at 4°C overnight. Following washing 5
times, the membranes were incubated with the HRP-conjugated
secondary antibodies at 37°C for 2 h. Western blots were developed
with ECL (Bio-Rad, Hercules, CA, USA). Normalization was ensured by
β-actin and each band was quantified using ImageJ software.
Reverse transcription-polymerase chain
reaction (RT-PCR)
HepG2 cells were seeded in 6-well plates at a
density of 1×106 cells/well and incubated for 24 h. When
HepG2 cells were treated with PBS (control) or RQDs (3.75, 7.5, 15
and 30 µg/ml) for 6 h, the total RNA was isolated using TRIzol
reagent (Solarbio Science & Technology Co., Ltd., Beijing,
China). The RT was performed with M-MuLV RT (Takara Bio, Inc.
Shiga, Japan) according to the manufacturer's instructions.
Amplification was performed on the CFX96 Touch Detection System
(Bio-Rad) according to the manufacturer's instructions. The primer
sequences are given in Table I.
Finally, the CT (2−ΔΔCT) method was used to
calculate the relative concentration of the amplified products.
 | Table I.Primers used in the reverse
transcription-polymerase chain reaction. |
Table I.
Primers used in the reverse
transcription-polymerase chain reaction.
| Gene | Primer sequences
(5′-3′) |
|---|
| Bax | F:
AGAGGATGATTGCCGCCGT |
|
| R:
CAACCACCCTGGTCTTGGATC |
| Bcl-2 | F:
ATGTGTGTGGAGAGCGTCAA |
|
| R:
ACAGTTCCACAAAGGCATCC |
| GADD153 | F:
CCACTCTTGACCCTGCTTCT |
|
| R:
ACCACTCTGTTTCCGTTTCC |
| GRP78 | F:
TAATGGAACGACAGGCACAC |
|
| R:
TGAGGGCAGGATTTCAAGAC |
| β-actin | F:
TGACGTGGACATCCGCAAAG |
|
| R:
CTGGAAGGTGGACAGCGAGG |
Confocal microscopy
HepG2 cells (1×105) were seeded in 35-mm
dishes for 24 h, and were subsequently treated with PBS (control)
or 30 µg/ml RQDs for 6 h at 37°C. Cells were incubated with
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl-carbocyanine
iodide (JC-1) (Qcbio Science & Technologies Co., Ltd.,
Shanghai, China) staining liquid for 20 min at 37°C, washed 3 times
with JC-1 staining buffer and examined under confocal microscope
(Leica, Mannheim, Germany).
Statistical analysis
All the data were analyzed using the SPSS 7.5
software for Windows, Student's version. For all the measurements,
one-way analysis of variance followed by Tukey's test was used to
assess the statistical significance between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Characterization of RQDs
The synthesized RQDs were characterized by
ultraviolet (UV)-visible spectrophotometry absorption and PL
emission spectra exhibiting classic band-edge emission of QDs. The
PL emission peak at 475 nm is in accordance with blue-cyan
fluorescence observed under 365 nm UV light. The mean diameter of
the RQDs was 5.48±1.09 nm
HepG2 cells are more sensitive when
compared with L02 cells to RQDs cytotoxicity
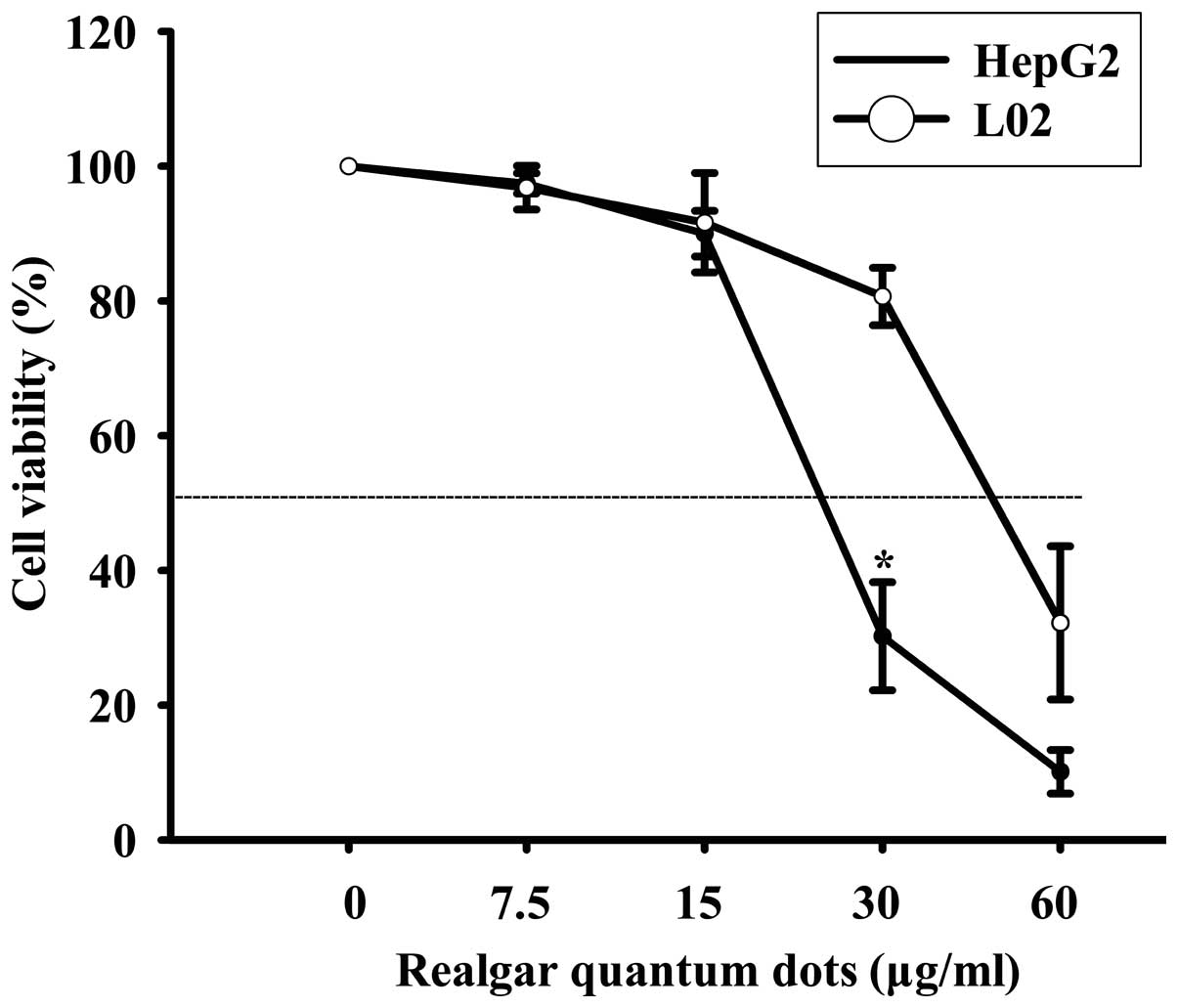
The MTT method was used to evaluate the inhibitory
effects of RQDs on the growth of HepG2 and L02 cells. The results
demonstrated that the cytotoxicity of RQDs was dose-dependent in
the HepG2 and L02 cells (Fig. 1). The
RQDs at 20, 40 and 80 µg/ml were also investigated. There was a
sharp decline from 20 to 40 µg/ml and no significant difference
between 40 and 80 µg/ml (data not shown). The IC50 for
HepG2 and L02 cells after 6 h were 23 and 50 µg/ml, respectively.
RQDs exhibited a significantly higher cytotoxicity in HepG2 cells
compared with the L02 cells.
RQD induces apoptosis and
necrosis
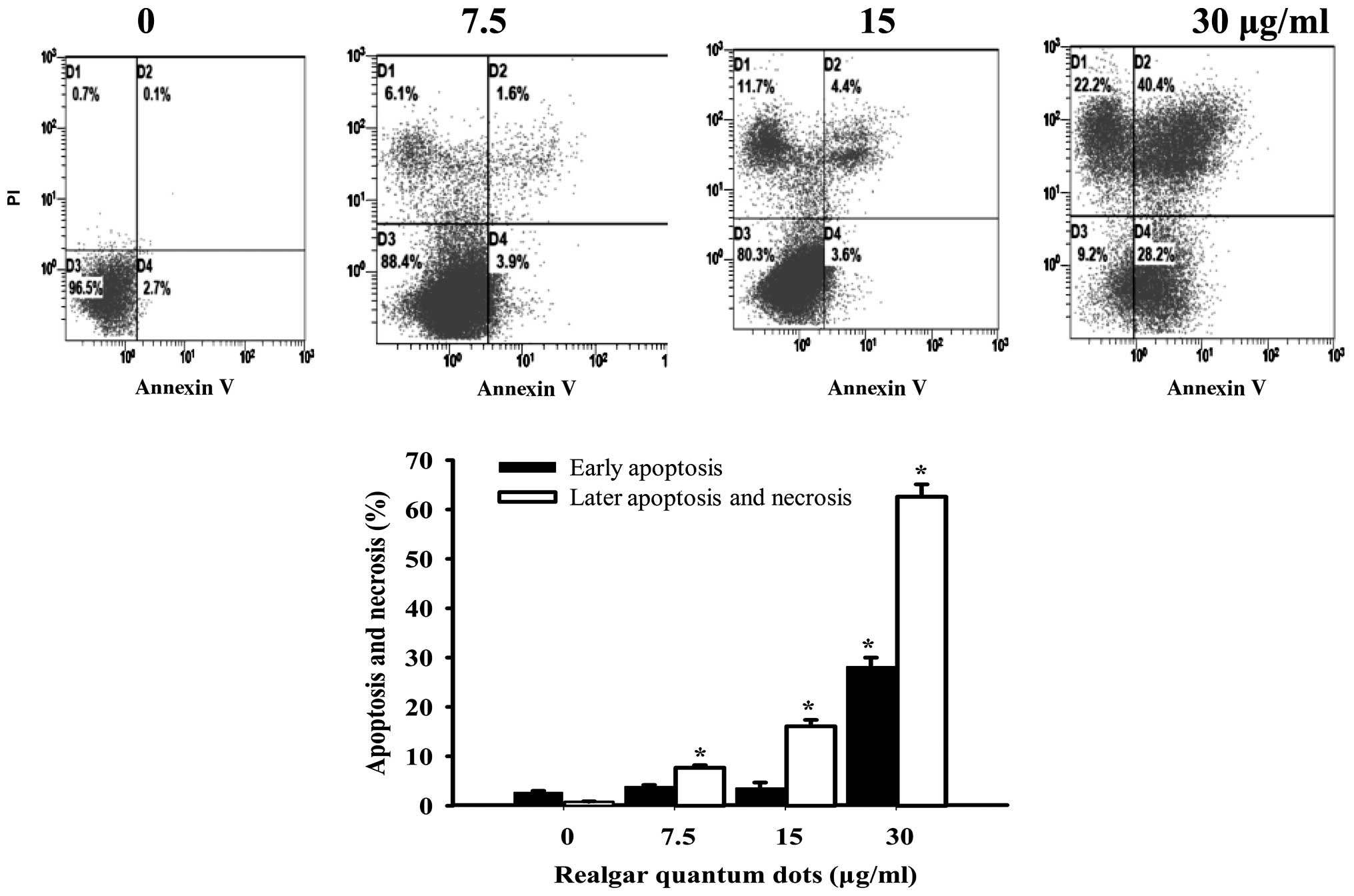
The apoptotic effect of RQDs on HepG2 cells was
detected by flow cytometry with Annexin V/PI staining. The early
apoptotic cells were determined by the sum of the cells in D2 and
the late apoptosis and necrosis cells appeared mainly in the D4
region (Fig. 2). The percentages of
early apoptotic cells following treatment with different RQD
concentrations (0, 7.5, 15 and 30 µg/ml) were 2.7±0.3, 3.9±0.3,
3.6±1.1 and 28.2±1.8%. The percentages of necrotic and late
apoptotic cells were 0.8±0.1, 7.7±0.5, 16.1±0.3 and 62.6±2.5%,
respectively.
Bax and Bcl-2 protein and mRNA
expression
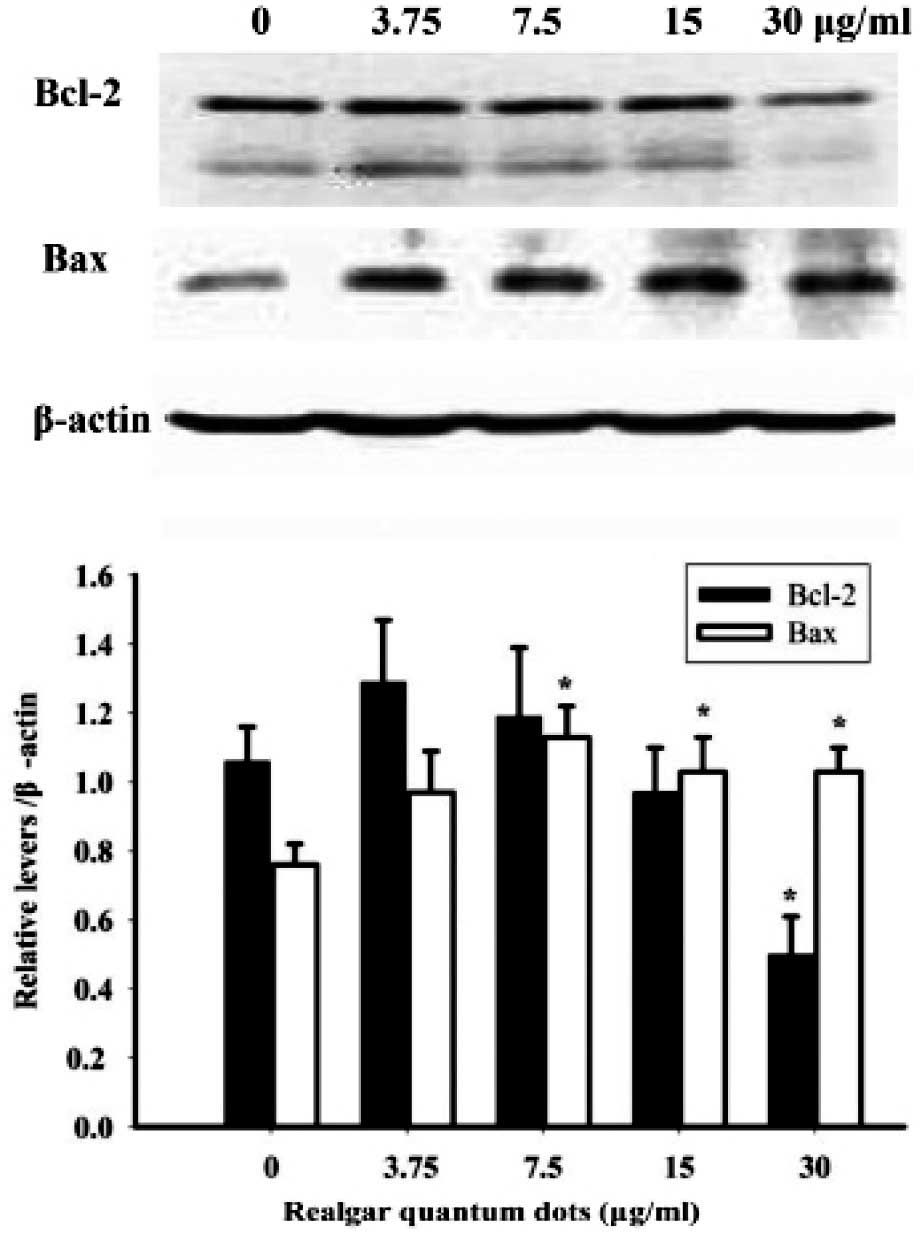
Bax and Bcl-2 are apoptosis-related proteins. The
expression of Bax and Bcl-2 was determined in RQD-treated HepG2
cells (Fig. 3). Compared with the
control cells, RQDs (30 µg/ml) induced the downregulated expression
of the anti-apoptotic Bcl-2 protein by 50%. By contrast, the
expression of the pro-apoptotic Bax protein was upregulated by 36%
following RQD (3.75, 7.5, 15 and 30 µg/ml) treatments.
The mRNA expression of the apoptosis-related genes,
Bcl-2 and Bax, was examined (Table II). The mRNA expression of
Bcl-2 showed a decrease with increasing RQD concentrations,
while the mRNA expression of Bax increased by 30% at 30
µg/ml compared with untreated cells. The mRNA expression of
Bcl-2 and Bax is consistent with their protein
levels.
 | Table II.mRNA expression of apoptosis-related
genes following RQD treatment. |
Table II.
mRNA expression of apoptosis-related
genes following RQD treatment.
|
| Concentration of RQD,
µg/ml |
|---|
|
|
|
|---|
| Gene | 0 | 3.75 | 7.5 | 15 | 30 |
|---|
| Bcl-2 |
2.93±0.91 |
1.25±0.26 |
1.47±0.59 |
1.59±0.24 |
0.76±0.19a |
| Bax |
7.44±0.72 |
7.06±0.80 |
9.04±1.17 |
11.8±0.34a |
10.6±0.71a |
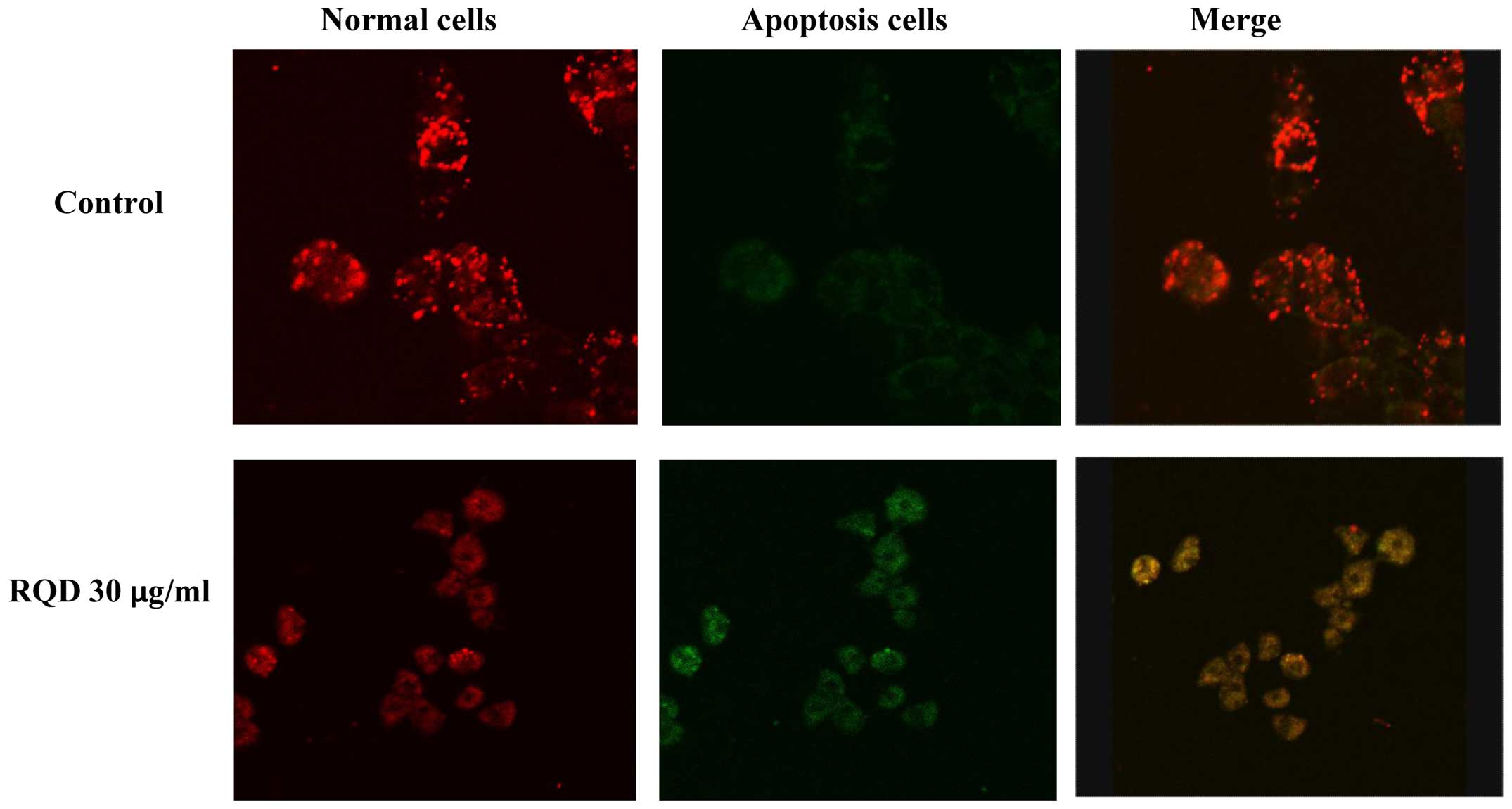
Mitochondrial membrane potential
Mitochondria, as the major energy generators in
cells, have a vital role in cell apoptosis induced by stimuli. The
JC-1 probe is capable of entering selectively into the mitochondria
and reversibly changes its color from red to green as the membrane
potential decreases. The results showed that RQDs caused a decrease
in the red fluorescence (JC-1 aggregates) and an increase in the
green fluorescence (JC-1 monomers), indicative of a loss of
mitochondrial membrane potential (Fig.
4).
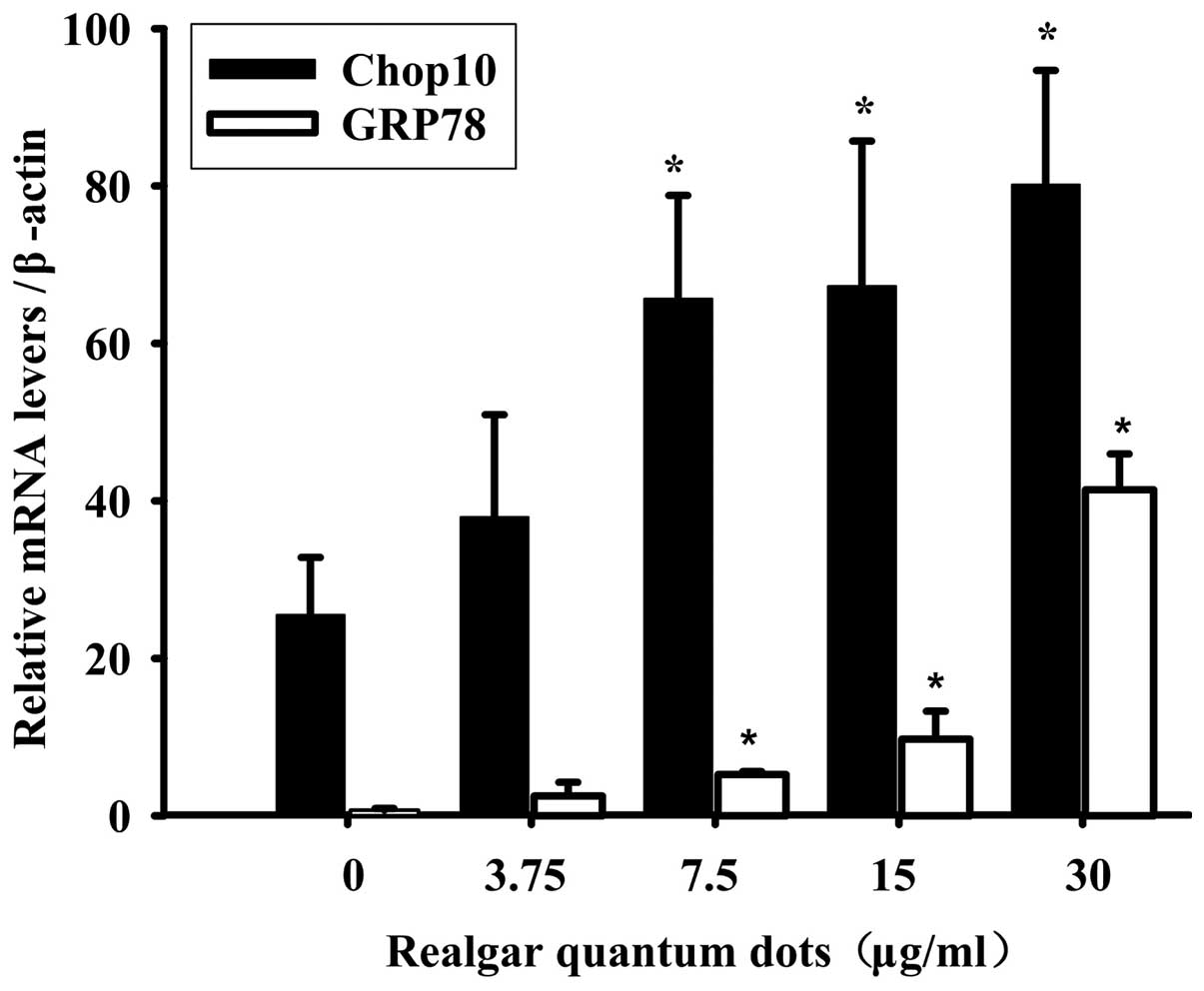
mRNA expression of ER stress
genes
The mRNA expression of ER stress genes,
C/EBP-homologous protein 10 (Chop10) (also known as GADD153) and
glucose-regulated protein 78 (GRP78) were further examined
in RQD-treated HepG2 cells (Fig. 5).
The mRNA expression of Chop10 and GRP78 increased with
increasing RQD concentrations. Compared with the control group,
RQDs (30 µg/ml) induced the expression of Chop10 by 3.14-fold and
GRP78 by ~30-fold.
Discussion
HCC was reported to be the third most common cause
of cancer-related fatality worldwide. The most difficult problem
for HCC treatment is the lack of effective chemotherapeutics.
Realgar and realgar nanoparticles have been demonstrated to inhibit
the proliferation of several cancer cell lines, including
hepatocellular HepG2 cells (3,15), and in clinical trials for HCC (2). To improve the bioavailability of realgar
through smaller sizes would be an advantageous strategy against
solid tumors (9,10). In this regard, RQDs with a size of ~6
nm were prepared by a chemical method and were revealed to be
effective in inhibiting the uterine cervix U14 tumor xenografts
without overt toxicity in tumor-bearing mice (17). The present study further demonstrated
that it is also effective in inhibiting HepG2 cell growth. At low
concentrations (7.5 and 15 µg/ml), RQDs induced tumor cell
apoptosis and at the high concentration (30 µg/ml) necrosis was
induced. The study clearly demonstrates that RQDs are effective
against inhibiting proliferation in HepG2 cells.
The MTT assay showed that HepG2 cells were more
sensitive when compared to the normal L02 cells for RQD treatments,
similar to the differential induction of apoptosis by parthenolide
towards HepG2 cells as compared to the normal cells (3), suggestive of HepG2 cell sensitivity to
RQDs cytotoxicity.
The results of flow cytometry analysis, as shown in
Fig. 2, clearly demonstrated that the
percentages of early apoptosis cells increased to 28.2% and the
necrosis cells increased to 62.6% when treated with 30 µg/ml of
RQDs. Dysregulation of apoptosis is linked to the development of
the majority of cancers. Thus, induction of apoptosis in cancer
cells is a crucial way to induce tumor cell death (3,18). The
apoptosis-related protein and gene expression was further examined
by western blot analysis and RT-PCR. The results clearly
demonstrated that RQDs induced Bax and decreased Bcl-2 expression.
The ratio of Bax/Bcl-2 increased ≤3-fold at an RQD dose of 30
µg/ml. Dysregulation of apoptosis-related expression of Bax and
Bcl-2 is the major mechanism for numerous anticancer agents, such
as rice bran phytic acid (19),
deoxycholic acid (20) and
Saccharina japonica (21), and
is a major mechanism for RQDs to induce HepG2 cell death.
The Bcl-2 family is also involved in the regulation
and performance of mitochondrial outer membrane permeability, and
alteration of the mitochondrial membrane potential leads to cell
apoptosis and necrosis. The JC-1 probe is widely used for
mitochondrial membrane potential loss and apoptosis detection
(22). The destruction of the
mitochondrial membrane potential is a sign of early apoptosis.
Apoptotic cells usually have membrane permeability changes. The
increase of membrane permeability results in mitochondrial protein
release, including cytochrome c and apoptosis-inducing
factor from the mitochondrial matrix into cytoplasm. The release of
cytochrome c with complete loss of membrane potential
triggers an apoptosis enzyme cascade effects. The mitochondrial
membrane potential decreased significantly when cells were treated
with 30 µg/ml RQDs, indicative that the loss of mitochondrial
membrane permeability is associated with RQD-induced apoptosis in
HepG2 cells.
ER stress is associated with the loss of
mitochondrial membrane potential and increased Bax/Bcl-2 ratio,
leading to cell apoptosis (4,22). Targeting ER stress-induced apoptosis
and inflammation have become an important strategy in cancer
chemotherapy (23). The present study
clearly demonstrated that the typical ER stress gene GRP78
increased 30-fold and Chop10 by >3-fold in a
concentration-dependent manner, indicating that ER stress signaling
pathways have an important role in RQD-induced cell death in HepG2
cells.
In conclusion, the present study demonstrated that
RQDs are a promising in vitro anticancer agent against human
hepatocellular HepG2 cells by inducing apoptosis and necrosis,
possibly mediated through ER stress, loss of mitochondrial membrane
potential and increase of the Bax/Bcl-2 ratios leading to
hepatocellular cell death.
Acknowledgements
The present study was supported by the Science and
Technology Foundation of Guizhou (grant no. CK-856, 2013-03), the
Zunyi Medical Collage, Harbin Institute Postdoctoral Fellowship
(grant no. AUGA4110005410), the Fundamental Research Funds for the
Central Universities (grant no. HIT IBRSEM 201331) and the
Fundamental Research Funds for the Central Universities and Program
for Innovation Research of Science in Harbin Institute of
Technology (grant no. PIRS of HIT 201411).
References
|
1
|
Liu J, Lu Y, Wu Q, Goyer RA and Waalkes
MP: Mineral arsenicals in traditional medicines: Orpiment, realgar
and arsenolite. J Pharmacol Exp Ther. 326:363–368. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hu XM, Liu F and Ma R: Application and
assessment of Chinese arsenic drugs in treating malignant
hematopathy in China. Chin J Integr Med. 16:368–377. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ding W, Zhang L, Kim S, Tian W, Tong Y,
Liu J, Ma Y and Chen S: Arsenic sulfide as a potential anti cancer
drug. Mol Med Rep. 11:968–974. 2015.PubMed/NCBI
|
|
4
|
Liu X, Li X, Wang L, Lv X, Chen N, Li P,
Lu K and Wang X: Realgar induces apoptosis in the chronic
lymphocytic leukemia cell line MEC 1. Mol Med Rep. 8:1866–1870.
2013.PubMed/NCBI
|
|
5
|
Wang L, Zhou GB, Liu P, Song JH, Liang Y,
Yan XJ, Xu F, Wang BS, Mao JH, Shen ZX, et al: Dissection of
mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as
an effective treatment for promyelocytic leukemia. Proc Natl Acad
Sci USA. 105:4826–4831. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mao JH, Sun XY, Liu JX, Zhang QY, Liu P,
Huang QH, Li KK, Chen Q, Chen Z and Chen SJ: As4S4 targets
RING-type E3 ligase c-CBL to induce degradation of BCR-ABL in
chronic myelogenous leukemia. Proc Natl Acad Sci USA.
107:21683–21688. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang QY, Mao JH, Liu P, Huang QH, Lu J,
Xie YY, Weng L, Zhang Y, Chen Q, Chen SJ, et al: A systems biology
understanding of the synergistic effects of arsenic sulfide and
Imatinib in BCR/ABL-associated leukemia. Proc Natl Acad Sci USA.
106:3378–3383. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu HH, Wu DP, Jin J, Li JY, Ma J, Wang
JX, Jiang H, Chen SJ and Huang XJ: Oral tetra-arsenic tetra-sulfide
formula versus intravenous arsenic trioxide as first-line treatment
of acute promyelocytic leukemia: A multicenter randomized
controlled trial. J Clin Oncol. 31:4215–4221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu J, Shao Y, Liu J, Chen G and Ho PC: The
medicinal use of realgar (As4S4) and its
recent development as an anticancer agent. J Ethnopharmacol.
135:595–602. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baláž P and Sedlák J: Arsenic in cancer
treatment: Challenges for application of realgar nanoparticles (a
minireview). Toxins (Basel). 2:1568–1581. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu JZ and Ho PC: Evaluation of the in
vitro activity and in vivo bioavailability of realgar nanoparticles
prepared by cryo-grinding. Eur J Pharm Sci. 29:35–44. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao QH, Zhang Y, Liu Y, Wang HL, Shen YY,
Yang WJ and Wen LP: Anticancer effect of realgar nanoparticles on
mouse melanoma skin cancer in vivo via transdermal drug delivery.
Med Oncol. 27:203–212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tian Y, Wang X, Xi R, Pan W, Jiang S, Li
Z, Zhao Y, Gao G and Liu D: Enhanced antitumor activity of realgar
mediated by milling it to nanosize. Int J Nanomedicine. 9:745–757.
2014.PubMed/NCBI
|
|
14
|
Xie QJ, Cao XL, Bai L, Wu ZR, Ma YP and Li
HY: Anti-tumor effects and apoptosis induction by Realgar
bioleaching solution in Sarcoma-180 cells in vitro and transplanted
tumors in mice in vivo. Asian Pac J Cancer Prev. 15:2883–2888.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang J, Lin M, Zhang T, Yan Y, Ho PC, Xu
QH and Loh KP: Arsenic(II) sulfide quantum dots prepared by a wet
process from its bulk. J Am Chem Soc. 130:11596–11597. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Subbarayan PR and Ardalan B: In the war
against solid tumors arsenic trioxide needs partners. J
Gastrointest Cancer. 45:363–371. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qin Y, Jing F, Hai J, et al: Anti-tumor
effects of realgar quantum dots and Liushen pills in cervical
tumor-bearing mice. Chin J N Drugs Clin Remedies. 34:64–67.
2015.
|
|
18
|
Wei S, Cao H, Zhou X, Wu H and Yang J:
Prokaryotically and eukaryotically expressed interleukin-24 induces
breast cancer growth suppression via activation of apoptosis and
inhibition of tumor angiogenesis. Mol Med Rep. 11:3673–3681.
2015.PubMed/NCBI
|
|
19
|
Al-Fatlawi AA, Al-Fatlawi AA, Irshad M,
Zafaryab M, Rizvi MM and Ahmad A: Rice bran phytic acid induced
apoptosis through regulation of Bcl-2/Bax and p53 genes in HepG2
human hepatocellular carcinoma cells. Asian Pac J Cancer Prev.
15:3731–3736. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang HB, Song W, Cheng MD, Fan HF, Gu X,
Qiao Y, Lu X, Yu RH and Chen LY: Deoxycholic acid inhibits the
growth of BGC-823 gastric carcinoma cells via a p53 mediated
pathway. Mol Med Rep. 11:2749–2754. 2015.PubMed/NCBI
|
|
21
|
Il Jung H, Jo MJ, Kim HR, Choi YH and Kim
GD: Extract of Saccharina japonica induces apoptosis companied by
cell cycle arrest and endoplasmic reticulum stress in SK-Hep1 human
hepatocellular carcinoma cells. Asian Pac J Cancer Prev.
15:2993–2999. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang B, Peng X, Li G, Xu Y, Xia X and
Wang Q: Oxidative stress is involved in Patulin induced apoptosis
in HEK293 cells. Toxicon. 94:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Verfaillie T, Garg AD and Agostinis P:
Targeting ER stress induced apoptosis and inflammation in cancer.
Cancer Lett. 332:249–264. 2013. View Article : Google Scholar : PubMed/NCBI
|