Introduction
Graves' Disease (GD), also known as toxic diffuse
goiter, can increase the level of thyroid hormone, is one of the
organ-specific autoimmune diseases and it accounts for 85% of all
clinical hyperthyroidism (1,2). The disease often presents in patients
aged from 20–40 years old, with a male to female ratio of ~1:8 and
a significant familial tendency (3).
The clinical performance of GD is not limited to the thyroid, but
is a multi-system syndrome, including the high metabolic syndrome
group, diffuse goiter, eye symptoms, lesions and thyroid extremity
diseases (4–6). Immunologically, GD is characterized by
increased circulating antibodies against thyroid-stimulating
hormone receptor (TSHR), thyroglobulin (TG) and thyroid peroxidase
(TPO). Although the precise pathogenesis involved in the process of
GD is not completely understood, certain findings indicate that
complex interactions between environmental, genetic, endogenous and
local factors are involved in its pathogenesis (7–9). A study on
the genetic susceptibility genes of GD has become of interest
(10–13).
Previous studies have demonstrated that a number of
susceptibility genes are associated with the autoimmune thyroid
diseases, including interleukin-21, TSHR, human leukocyte
antigen class I and II, cluster of differentiation 40 (CD40)
and cytotoxic T lymphocyte associated 4 (CTLA4) (10–15). Among
them, CTLA4 as a key negative regulator of the T lymphocyte
immune response has attracted an increasing focus on the
susceptibility to autoimmune disease.
CTLA4, which is expressed by activate T cells, is a
type of transmembrane protein. The CTLA4 gene is located on
human chromosome 2q33 (16). The most
significant function of the CTLA4 gene is negative
regulation of the human immune response. Several polymorphic sites
in the CTLA4 gene are associated with thyroid diseases, such
as −318C/T promoter and 49A/G exon 1, −224(AT)n dinucleotide repeat
sequence single-nucleotide polymorphism (SNP), −1722C/T, −1661A/G,
CT60 (rs3087243), CT61 (rs51157131), J031 (rsl1571302) and J027-1
(rsl1571297) (16–22). Several studies also show that the SNPs
at the 3′ untranslated region (UTR) are more associated with GD
(23–25). CT60 is located in the 3′-UTR of the
CTLA4 gene. Increasing attention has previously been paid to
the CT60 SNP of CTLA4 in patients with GD (26–28).
However, the correlation between CT60 A/G and the development of GD
varied among patients from different geographic populations
(29). Thus, there are limited reports
regarding an association between the CT60 SNP and GD in the Han
population of Southern China.
The aim of the present report was to use a
case-control study to establish a database of CT60 polymorphisms in
the Han population from Southern China and to evaluate CT60
polymorphisms as an indicator of GD susceptibility. The results
indicated that the CT60 SNP of the CTLA4 gene was associated
with susceptibility to GD in the Han population of Southern China.
In addition, the G allele of the CT60 SNP was strongly associated
with GD patients.
Materials and methods
GD patients
For the case-control study, a total of 288 unrelated
individuals with GD (66 males and 222 females) from Southern China
were recruited from the Department of Endocrinology at Sun Yat-Sen
Memorial Hospital (Guangzhou, China). The age (mean ± standard
deviation) of the GD patients was 37.7±14.2 years (range, 19–80
years). The GD patients were diagnosed from the clinical symptoms
and biochemical criteria of thyrotoxicosis, including suppressed
serum TSH levels, elevated free thyroxine (fT4) and/or free
triiodothyronine (fT3) levels, as well as the presence of TSHR
antibodies or antibodies against TG or TPO. All the patients had no
evident co-morbidities and provided informed consent.
Data from the patients were compared with those
obtained from 290 control subjects without a family history of
thyroid diseases or other autoimmune diseases from the Health Care
Center at Sun Yat-Sen Memorial Hospital. All the healthy controls
were age- and gender-matched with the GD patients and also provided
informed consent. The experimental protocol was approved by the
Ethics Committee of Sun Yat-Sen Memorial Hospital.
Thyroid function tests
Serum concentrations of fT3, fT4, TSH, anti-TG and
anti-TPO were detected with the chemiluminescence immunoassay by
the ADVIA Centaur Automated Chemiluminescent Immunoassay Analyzer
(Siemens Healthcare, Erlangen, Germany); the normal ranges were
0.82–1.79 ng/ml, 4.5–12.5 µg/dl and 0.40–4.00 µIU/ml,
respectively.
CTLA4 3′-UTR CT60 genotyping
Genomic DNA was isolated from whole blood with the
Whole Blood Genomic DNA Extraction kit (Omega Bio-Tek, Inc.,
Norcross, GA, USA) according to the manufacturer's instructions.
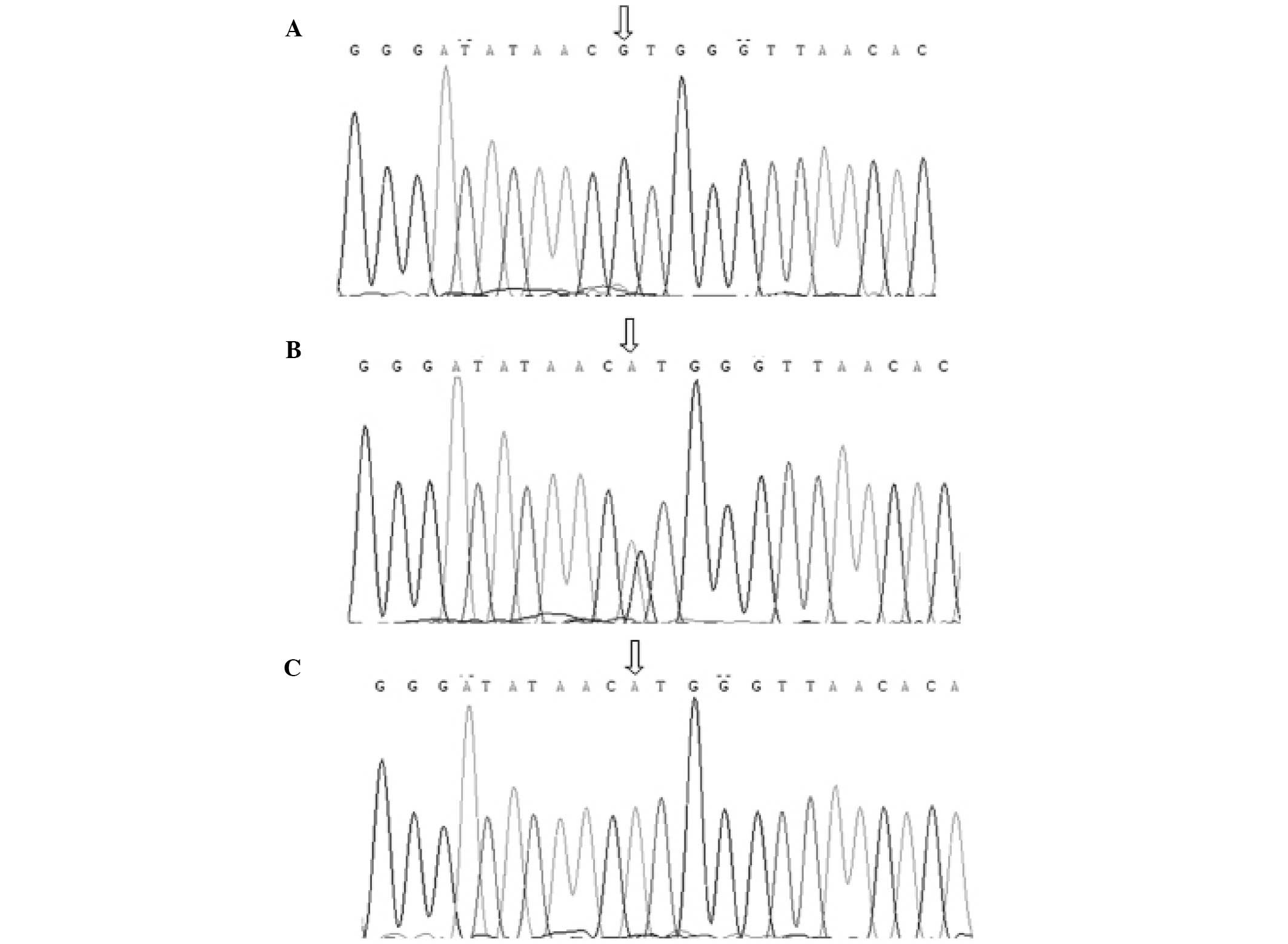
The CT60 SNP was determined by direct DNA sequencing (BigDye DNA
Sequencing kit; Applied Biosystems Inc., Norwalk, CT, USA)
following polymerase chain reaction (PCR). PCR was performed using
the following primers: Forward, 5′-ATAATGCTTCATGAGTCAGCTT-3′ and
reverse, 5′-GAG GTGAAGAACCTGTGTTAAA-3′. Amplification of target DNA
was performed on the DNA template in a total volume of 50 µl
comprising 5.0 µl of 10X PCR buffer, 4.0 µl of deoxyribonucleotide
mixture, 0.25 µl of Ex Taq polymerase, 3.0 µl of DNA template (50
ng/µl), 35.75 µl of PCR-Grade water and 1.0 µl of each 20 µmol/l
primer. PCR conditions were as follows: An initial denaturation at
94°C for 4 min; 35 cycles of 94°C for 45 sec, 58°C for 45 sec and
72°C for 45 sec; and a final extension for 4 min at 72°C.
Following amplification, the PCR products were
identified by electrophoresis (Power Pac 200; Bio-Rad, Hercules,
CA, USA) (Fig. 1) and submitted for
DNA sequencing. The sequences are shown in Fig. 2.
Statistical analysis
The Hardy-Weinberg equilibrium test for the
genotyping results in the control group was carried out by Excel
(Microsoft Office Excel, Microsoft Corp., Redmond, WA, USA). All
the statistical analyses were performed with the SPSS 20.0 software
(IBM Corp., Armonk, NY, USA). A χ2 test or the Fisher's
exact test was performed to compare the frequencies of genotypes
and alleles between the groups. The disease susceptibility of
specific genotypes and alleles were estimated with the calculated
odds ratio (OR) and 95% confidence interval (CI). P<0.05 was
considered to indicate a statistically significant difference.
Results
Hardy-Weinberg equilibrium test
A Hardy-Weinberg equilibrium of the CT60 (rs3087243)
genotype distribution was carried out in the control group. As was
listed in Table I, χ2 test
revealed that the CT60 (rs3087243) loci was in the balance of
Hardy-Weinberg (χ2 test=4.216, P=0.122). The CT60 SNP
loci can be used as a genetic marker to study the association
between the CTLA4 gene and GD.
 | Table I.Hardy-Weinberg equilibrium of the
genotyping results in the controls. |
Table I.
Hardy-Weinberg equilibrium of the
genotyping results in the controls.
| Genotype | Observed
frequency | Expected
frequency | χ2
test | P-value |
|---|
| GG | 149 | 157.9 | 4.216 | 0.122 |
| AG | 130 | 112.2 |
|
|
| AA | 11 |
19.9 |
|
|
Genotype distributions of the general
population
The population frequencies of genotype and allele of
the CT60 SNP in GD patients and controls are listed in Table II. The frequencies of genotype in the
cases (AA, 2.4; AG, 27.4; and GG, 70.1%) were significantly
different from those in the controls (AA, 3.8; AG, 44.8; and GG,
51.4%) (χ2 test=21.330, P=2.3×10−5). As is
shown in Table II, there was an
evidently higher frequency of the GG genotype in the cases compared
to the controls (P=4.0×10−6; OR, 2.223; 95% CI,
1.579–3.128). Inversely, the frequencies of the AG genotype were
significantly lower in the GD group in contrast to the control
group (P=1.3×10−5; OR, 0.465; 95% CI, 0.329–0.658).
Table II also showed that the
frequency of the G allele in the GD group was significantly higher
than that in the control group (83.9 vs. 73.8%; χ2
test=17.515, P=2.9×10−5).
 | Table II.Genotype and allele frequencies of
CT60 in GD patients and controls. |
Table II.
Genotype and allele frequencies of
CT60 in GD patients and controls.
|
Genotype/allele | GD, n (%) | Controls, n
(%) | χ2
test | P-value | OR | 95% CI |
|---|
| Genotype |
|
|
|
|
|
|
| GG | 202 (70.1) | 149 (51.4) | 21.322a |
4.0×10−6 | 2.223 | 1.579–3.128 |
| AG | 79 (27.4) | 130 (44.8) | 18.945b |
1.3×10−5 | 0.465 | 0.329–0.658 |
| AA | 7 (2.4) | 11 (3.8) | 0.889c | 0.346 | 0.632 | 0.241–1.653 |
| Allele |
|
|
|
|
|
|
| G | 483 (83.9) | 428 (73.8) | 17.515 |
2.9×10−5 | 1.844 | 1.382–2.462 |
| A | 93 (16.1) | 152 (26.2) |
|
|
|
|
Genotype distributions for different
gender
All the 578 individuals were divided into two groups
according to gender to study the association of the polymorphisms
of the CT60 SNP with GD. As is shown in Table III, following subdivision by gender,
the frequency distributions of genotype and allele of males and
females were similar to those of the general population. Compared
to the controls, the frequencies of the GG genotype and the G
allele were markedly higher and the frequencies of the AG genotype
were evidently lower in the cases. The genotype and allele
distributions of the CT60 SNP were also compared between the male
and female patients (Table IV).
Conversely, no significant differences were found in genotype and
allele frequencies between male and female patients.
 | Table III.Genotype and allele frequencies of
CT60 in male and female GD patients and controls. |
Table III.
Genotype and allele frequencies of
CT60 in male and female GD patients and controls.
| Genotype and
allele | GD, n (%) | Controls, n
(%) | χ2
test | P-value | OR | 95% CI |
|---|
| Male |
|
|
|
|
|
|
|
Genotype |
|
|
|
|
|
|
|
GG | 51 (77.3) | 49 (52.7) | 9.998 |
1.6×10−3 | 3.053 | 1.508–6.179 |
|
AG | 14 (21.2) | 42 (45.2) | 9.705 |
1.8×10−3 | 0.327 | 0.160–0.670 |
|
AA | 1 (1.5) | 2 (2.1) | 0.084 | 0.772 | 0.700 | 0.062–7.884 |
|
Allele |
|
|
|
|
|
|
|
G | 116 (87.9) | 140 (75.3) | 7.822 |
5.2×10−3 | 2.382 | 1.282–4.427 |
|
A | 16 (12.1) | 46 (24.7) |
|
|
|
|
| Female |
|
|
|
|
|
|
|
Genotype |
|
|
|
|
|
|
|
GG | 151 (68.0) | 100 (50.8) | 12.941 |
3.2×10−4 | 2.063 | 1.387–3.068 |
|
AG | 65 (29.3) | 88 (44.7) | 10.665 |
1.1×10−3 | 0.513 | 0.343–0.767 |
|
AA | 6 (2.7) | 9 (4.5) | 1.053 | 0.305 | 0.580 | 0.203–1.660 |
|
Allele |
|
|
|
|
|
|
|
G | 367 (82.7) | 288 (73.1) | 11.180 |
8.3×10−4 | 1.754 | 1.259–2.444 |
|
A | 77 (17.3) | 106 (26.9) |
|
|
|
|
 | Table IV.Genotype and allele frequencies of
CT60 in male and female GD patients. |
Table IV.
Genotype and allele frequencies of
CT60 in male and female GD patients.
| Genotypes and
alleles | Males, n (%) | Females, n (%) | χ2
test | P-value |
|---|
| Genotype |
|
|
|
|
| GG | 51 (77.3) | 151 (68.0) | 2.123 | 0.346 |
| AG | 14 (21.2) | 65 (29.3) |
|
|
| AA | 1 (1.5) | 6 (2.7) |
|
|
| Allele |
|
|
|
|
| G | 116 (87.9) | 367 (82.7) | 1.681 | 0.195 |
| A | 16 (12.1) | 77 (17.3) |
|
|
Genotype distributions of the GD
patients
A total of 288 GD patients were grouped according to
the family history, ophthalmopathy and relapse history. As is shown
in Table V, there was no significant
difference in GD patients with and without family history,
ophthalmopathy and relapse history.
 | Table V.Correlation analyses between the
genotypes and alleles of CT60 and clinical features of GD. |
Table V.
Correlation analyses between the
genotypes and alleles of CT60 and clinical features of GD.
|
| Family history, n
(%) | Ophthalmopathy, n
(%) | Relapse history, n
(%) |
|---|
|
|
|
|
|
|---|
|
Characteristics | (+) | (−) | (+) | (−) | (+) | (−) |
|---|
| Genotype |
|
|
|
|
|
|
| GG | 59 (69.4) | 143 (70.4) | 14 (70.0) | 184 (68.6) | 21 (75.0) | 177 (68.1) |
| AG | 25 (29.4) | 54 (26.6) | 5 (25.0) | 75 (28.0) | 6 (21.4) | 74 (28.4) |
| AA | 1 (1.2) | 6 (3.0) | 1 (5.0) | 9 (3.4) | 1 (3.6) | 9 (3.5) |
|
χ2
test |
| 0.962 |
| 0.209 |
| 0.627 |
|
|
P-value |
| 0.618 |
| 0.901 |
| 0.731 |
|
| Allele |
|
|
|
|
|
|
| G | 143 (84.1) | 340 (83.7) | 33 (82.5) | 443 (82.6) | 48 (85.7) | 428 (82.3) |
| A | 27 (15.9) | 66 (16.3) | 7 (17.5) | 93 (17.4) | 8 (14.3) | 92 (17.7) |
|
χ2
test |
| 0.012 |
| 0.037 |
| 0.206 |
|
|
P-value |
| 0.911 |
| 0.847 |
| 0.650 |
|
Discussion
CTLA4 (CD152), as an important immune-regulatory
molecule expressed on activated T cells, is able to inhibit a
T-cell response while triggered by costimulatory molecules. The
CTLA4 gene is located on human chromosome 2q33 and is
composed of four exons. It spans ~8.44 kb of genomic DNA and
encodes 233 amino acids (30).
CTLA4 belongs to the immunoglobulin gene superfamily and is
associated with immune tolerance. CTLA4 expresses on the activated
CD4+ and CD8+ T cells. It has sequence
homology with costimulatory molecules, including CD28, but has an
opposing role (31). The main function
of the costimulatory molecule CD28 is to stimulate and activate T
cells, however, CTLA4 is a negative regulator of T cell response.
CTLA4 produces inhibitory signals combining with the relevant
molecules in the B7 family, which has an important role in the
termination of T-cell activation. The abnormal expression of CTLA4
induces the activation of T cells, breaks down the immune tolerance
and results in autoimmune diseases.
The association of CTLA4 gene polymorphisms
with the autoimmune diseases, including type 1 diabetes (19,24), GD
(20–22,32),
abdominal disease (33), systemic
lupus erythematous disease (34),
rheumatoid arthritis (35), autoimmune
pancreatitis (36) and Addison's
disease (37), have been investigated.
The CTLA4 gene has multiple SNP loci and repeat sequences.
In recent years, increasing attention has been paid to the 3′-UTR
of the CTLA4 gene. The 3′-UTR is a complex region containing
multiple binding sites for microRNA. Therefore, the presence of SNP
or base mutation in the 3′-UTR would change the signaling pathway,
influence the protein expression and result in diseases.
The CT60 SNP, which has been demonstrated to be most
significantly correlated with GD and Hashimoto's thyroiditis (HT)
in the Caucasian population (25), is
at the 3′-UTR of the CTLA4 gene. The correlation between the
CT60 SNP and the autoimmune diseases, which has been confirmed,
varied among patients from different ethnicities or regions. Torres
et al (34) reported that CT60
(rs3087243) in the CTLA4 gene was associated with systemic
lupus erythematosus in the Spanish population. Ban et al
(38) suggested that the CT60 SNP in
the CTLA4 gene 3′-UTR may have a role in the autoimmune
thyroid disease in the Japanese population. Weng et al
(28) also found that the G/G genotype
and G allele in CT60 were associated with the onset of GD in the
Taiwanese population. However, the study of Pastuszak-Lewandoska
et al (39) demonstrated that
CT60 A/G has no association with HT or GD patients in the Polish
population. In the present study, the distributions of the CT60
polymorphisms were also analyzed in GD patients and healthy
controls. The GG genotype frequencies of the CT60 SNP in GD
patients and healthy controls were 70.2 and 51.4%, respectively
(P=4.0×10−6). It is clear that the GD group had a
significantly higher GG genotype compared with the control group.
Conversely, the AG genotype frequencies were evidently lower in the
cases compared with the controls (27.4 vs. 44.8%;
P=1.3×10−5). Furthermore, the study demonstrated that
the GG genotype was more prevalent in male and female GD patients
and fewer male and female patients had the AG genotype, compared
with the control subjects. The data provided suggest that the GG
genotype of CT60 may be associated with the onset of GD in the Han
population of Southern China.
The G allele of CT60 SNP was also revealed to
increase the susceptibility to GD. The frequency of the G allele of
the control group in the Han population of Southern China was
73.8%, which was higher compared to the Caucasian (25) (53.2%), Swedish (40) (61.3%), Dutch (41) (53.3%) and Italian populations (26) (48%) but similar to the Taiwanese
(28) (75.7%) and Japanese populations
(38) (72.6%). This may be attributed
to the differences of ethnicity, region or sample size. In
addition, the frequency of the G allele was associated with GD
(83.9 vs. 73.8%, P=0.035) in the Southern Chinese Han population,
which was consistent with the research of other ethnic groups
(26,28,39,40). These studies, including the present
report of the association between G allele and GD, may have
increasingly demonstrated our hypothesis that CT60 of CTLA4
appears to be susceptible to GD in the Han population of Southern
China.
Additionally, in order to discuss the associations
between clinical manifestations with GD, 288 GD patients were
subdivided according to the family history, ophthalmopathy and
relapse history. However, there was no significant difference
identified between GD patients with and without family history,
ophthalmopathy and relapse history. Therefore, the CT60 gene
polymorphism of CTLA4 may not be associated with the
progress of GD. However, more detailed investigations are required
to support this hypothesis.
In conclusion, the present findings demonstrated
that the CT60 SNP of the CTLA4 gene was significantly
associated with GD in the Chinese Han population from Southern
China. The G allele of CT60 indicated a positive role in the
susceptibility to GD.
Acknowledgements
The authors would like to thank all the patients who
consented to take part in the present study, as well as the doctors
and nurses for recruitment. The study was supported by Guangdong
Medical Science and Technology Research Foundation (grant no.
A2010166), Guangdong Province Science and Technology Project (grant
no. 2011B031800162) and the Key Project of Guangzhou Science and
Technology Project (grant no. 2011J4100114).
References
|
1
|
Weetman AP: Graves' disease. N Engl J Med.
343:1236–1248. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jacobson DL, Gange SJ, Rose NR and Graham
NM: Epidemiology and estimated population burden of selected
autoimmune diseases in the United States. Clin Immunol
Immunopathol. 84:223–243. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Falgarone G, Heshmati HM, Cohen R and
Reach G: Mechanisms in endocrinology. Role of emotional stress in
the pathophysiology of Graves' disease. Eur J Endocrinol.
168:R13–R18. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chaar BT, Kudva GC, Olsen TJ, Silverberg
AB and Grossman BJ: Thrombotic thrombocytopenic purpura and Graves
disease. Am J Med Sci. 334:133–135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aydin Y, Berker D, Ustün I, Balcik O,
Balli DN, Delibasi T, Peksoy I and Guler S: A very rare cause of
aplastic anemia: Graves disease. South Med J. 101:666–667. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soultati AS, Dourakis SP, Asvesti K, Nezi
V, Alexopoulou A and Archimandritis AJ: Autoimmune blistering
disease in a patient with Graves disease and vitiligo. Am J Med
Sci. 333:309–312. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brix TH, Christensen K, Holm NV, Harvald B
and Hegedüs L: A population-based study of Graves' disease in
Danish twins. Clin Endocrinol (Oxf). 48:397–400. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tomer Y, Barbesino G, Greenberg DA,
Concepcion E and Davies TF: Mapping the major susceptibility loci
for familial Graves' and Hashimoto's diseases: Evidence for genetic
heterogeneity and gene interactions. J Clin Endocrinol Metab.
84:4656–4664. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brix TH, Kyvik KO, Christensen K and
Hegedüs L: Evidence for a major role of heredity in Graves'
disease: A population-based study of two Danish twin cohorts. J
Clin Endocrinol Metab. 86:930–934. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ban Y, Davies TF, Greenberg DA, et al:
Analysis of the CTLA-4, CD28, and inducible costimulator (ICOS)
genes in autoimmune thyroid disease. Genes Immun. 4:586–593. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brand OJ, Barrett JC, Simmonds MJ, et al:
Association of the thyroid stimulating hormone receptor gene (TSHR)
with Graves' disease. Hum Mol Genet. 18:1704–1713. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Heward JM, Allahabadia A, Armitage M, et
al: The development of Graves' disease and the CTLA-4 gene on
chromosome 2q33. J Clin Endocrinol Metab. 84:2398–2401. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jacobson EM, Huber A and Tomer Y: The HLA
gene complex in thyroid autoimmunity: From epidemiology to
etiology. J Autoimmun. 30:58–62. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li M, Sun H, Liu S, et al: CD40 C/T-1
polymorphism plays different roles in Graves' disease and
Hashimoto's thyroiditis: A meta-analysis. Endocr J. 59:1041–1050.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zeng H, Yan H, Zhang Z, Fang W, Ding R,
Huang L, Chen M and Zhang J: Association between IL-21 gene
rs907715 polymorphisms and Graves' disease in a Southern Chinese
population. Exp Ther Med. 8:213–218. 2014.PubMed/NCBI
|
|
16
|
Brunet JF, Denizot F, Luciani MF,
RouxDosseto M, Suzan M, Mattei MG and Golstein P: A new member of
the immunoglobulin superfamily - CTLA-4. Nature. 328:267–270. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yanagawa T, Hidaka Y, Guimaraes V, Soliman
M and DeGroot LJ: CTLA-4 gene polymorphism associated with Graves'
disease in a Caucasian population. J Clin Endocrinol Metab.
80:41–45. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Frydecka I, Daroszewski J, Suwalska K,
Zołedziowska M, Tutak A, Słowik M, Potoczek S and Dobosz T: CTLA-4
(CD152) gene polymorphism at position 49 in exon 1 in Graves'
disease in a Polish population of the Lower Silesian region. Arch
Immunol Ther Exp (Warsz). 52:369–374. 2004.PubMed/NCBI
|
|
19
|
Mochizuki M, Amemiya S, Kobayashi K,
Kobayashi K, Shimura Y, Ishihara T, Nakagomi Y, Onigata K, Tamai S,
Kasuga A, et al: Association of the CTLA-4 gene 49 A/G polymorphism
with type 1 diabetes and autoimmune thyroid disease in Japanese
children. Diabetes Care. 26:843–847. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Han SZ, Zhang SH, Li R, Zhang WY and Li Y:
The common −318C/T polymorphism in the promoter region of CTLA4
gene is associated with reduced risk of ophthalmopathy in Chinese
Graves' patients. Int J Immunogenet. 33:281–287. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Q, Yang YM and Lv XY: Association of
Graves' disease and Graves' ophthalmopathy with the polymorphisms
in promoter and exon 1 of cytotoxic T lymphocyte associated
antigen-4 gene. J Zhejiang Univ Sci B. 7:887–891. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ban Y and Tomer Y: Susceptibility genes in
thyroid autoimmunity. Clin Dev Immunol. 12:47–58. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Furugaki K, Shirasawa S, Ishikawa N, Ito
K, Ito K, Kubota S, Kuma K, Tamai H, Akamizu T, Hiratani H, et al:
Association of the T-cell regulatory gene CTLA4 with Graves'
disease and autoimmune thyroid disease in the Japanese. J Hum
Genet. 49:166–168. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Benmansour J, Stayoussef M, AlJenaidi FA,
Rajab MH, Rayana CB, Said HB, Mahjoub T and Almawi WY: Association
of single nucleotide polymorphisms in cytotoxic T-lymphocyte
antigen 4 and susceptibility to autoimmune type 1 diabetes in
Tunisians. Clin Vaccine Immunol. 17:1473–1477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ueda H, Howson JM, Esposito L, Heward J,
Snook H, Chamberlain G, Rainbow DB, Hunter KM, Smith AN, Di Genova
G, et al: Association of the T-cell regulatory gene CTLA4 with
susceptibility to autoimmune disease. Nature. 423:506–511. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Petrone A, Giorgi G, Galgani A, Alemanno
I, Corsello SM, Signore A, Di Mario U, Nisticò L, Cascino I and
Buzzetti R: CT60 single nucleotide polymorphisms of the cytotoxic
T-lymphocyte-associated antigen-4 gene region is associated with
Graves' disease in an Italian population. Thyroid. 15:232–238.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Daroszewski J, Pawlak E, Karabon L,
Frydecka I, Jonkisz A, Slowik M and Bolanowski M: Soluble CTLA-4
receptor an immunological marker of Graves' disease and severity of
ophthalmopathy is associated with CTLA-4 Jo31 and CT60 gene
polymorphisms. Eur J Endocrinol. 161:787–793. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Weng YC, Wu MJ and Lin WS: CT60 single
nucleotide polymorphism of the CTLA-4 gene is associated with
susceptibility to Graves' disease in the Taiwanese population. Ann
Clin Lab Sci. 35:259–264. 2005.PubMed/NCBI
|
|
29
|
Song HD, Liang J, Shi JY, et al:
Functional SNPs in the SCGB3A2 promoter are associated with
susceptibility to Graves' disease. Hum Mol Genet. 18:1156–1170.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Teft WA, Kirchhof MG and Madrenas J: A
molecular perspective of CTLA-4 function. Annu Rev Immunol.
24:65–97. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kimkong I, Nakkuntod J, SaeNgow S,
Snabboon T, Avihingsanon Y and Hirankarn N: Association between
CTLA-4 polymorphisms and the susceptibility to systemic lupus
erythematosus and Graves' disease in Thai population. Asian Pac J
Allergy Immunol. 29:229–235. 2011.PubMed/NCBI
|
|
32
|
Takahashi M and Kimura A: HLA and CTLA4
polymorphisms may confer a synergistic risk in the susceptibility
to Graves' disease. J Hum Genet. 55:323–326. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hunt KA, McGovern DP, Kumar PJ, et al: A
common CTLA4 haplotype associated with coeliac disease. Eur J Hum
Genet. 13:440–444. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Torres B, Aguilar F, Franco E, Sánchez E,
Sánchez-Román J, Jiménez Alonso J, Núñez-Roldán A, Martín J and
González-Escribano MF: Association of the CT60 marker of the CTLA4
gene with systemic lupus erythematosus. Arthritis Rheum.
50:2211–2215. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lei C, Dongqing Z, Yeqing S, Oaks MK,
Lishan C, Jianzhong J, Jie Q, Fang D, Ningli L, Xinghai H, et al:
Association of the CTLA-4 gene with rheumatoid arthritis in Chinese
Han population. Eur J Hum Genet. 13:823–828. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chang MC, Chang YT, Tien YW, Liang PC, Jan
IS, Wei SC and Wong JM: T-cell regulatory gene CTLA-4
polymorphism/haplotype association with autoimmune pancreatitis.
Clin Chem. 53:1700–1705. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Blomhoff A, Lie BA, Myhre AG, Kemp EH,
Weetman AP, Akselsen HE, Huseby ES and Undlien DE: Polymorphisms in
the cytotoxic T lymphocyte antigen-4 gene region confer
susceptibility to Addison's disease. J Clin Endocrinol Metab.
89:3474–3476. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ban Y, Tozaki T, Taniyama M, Tomita M and
Ban Y: Association of a CTLA-4 3′ untranslated region (CT60) single
nucleotide polymorphism with autoimmune thyroid disease in the
Japanese population. Autoimmunity. 38:151–153. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
PastuszakLewandoska D, Sewerynek E,
Domańska D, Gładyś A, Skrzypczak R and Brzeziańska E: CTLA-4 gene
polymorphisms and their influence on predisposition to autoimmune
thyroid diseases (Graves' disease and Hashimoto's thyroiditis).
Arch Med Sci. 8:415–421. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mayans S, Lackovic K, Nyholm C, Lindgren
P, Ruikka K, Eliasson M, Cilio CM and Holmberg D: CT60 genotype
does not affect CTLA-4 isoform expression despite association to
T1D and AITD in northern Sweden. BMC Med Genet. 8:32007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhernakova A, Eerligh P, Barrera P, et al:
CTLA4 is differentially associated with autoimmune diseases in the
Dutch population. Hum Genet. 118:58–66. 2005. View Article : Google Scholar : PubMed/NCBI
|