Introduction
Benzodiazepines are extensively used to treat
insomnia; however, their uses are limited by concerns regarding
long-term efficacy and the potential for abuse, dependence and
adverse effects. Thus, complementary and alternative medicine is
often a source for treating insomnia. Chinese herbal medicine
(CHM), a form of complementary and alternative medicine, is often
explored for treating insomnia, either with single herbs or with
herbal formulas. Recently, >200 studies of CHM for insomnia from
a systematic review indicated that CHM was similar to Western
medication for treating insomnia, and that the frequency of adverse
events associated with CHM were similar to that of the placebo;
however, they were less frequent than that of Western medication
(1). Therefore, identifying a single
herb or herbal formula from CHM to treat insomnia is
advantageous.
Albizia julibrissin Durazz is a small tree
with smooth, gray-brown bark and doubly pinnate leaves. Clusters of
pink flower heads are borne in summer (2) and the tree is widely distributed
throughout Korea, China, Japan and Africa. The main active
ingredients of the flowers include triterpenoids and their
glycosides, flavonoids, alkaloids, organic acids, sterols, lignans
and tannin. Quercetin
[2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one, QR] is
a major constituent of the flowers of Flos albiziae (FA) and
QR is identified in vegetables, fruits, red wine and other herbal
preparations. Pharmacological research on aqueous extracts of FA
(FAE) and QR indicate that they have broad bioactivity, such as
antithrombotic and anti-inflammatory effects, anti-infectious and
immunomodulatory activities (3),
sedative and hypnotic effects (2), the
ability to inhibit cancer cell proliferation and induce apoptosis
(4,5),
antidepressant activity (6), and
antioxidative and hypolipidemic properties (7–10). Certain
studies have indicated that the antidepressant effects of FAE may
be associated with increased plasma serotonin [5-hydroxytryptamine
(5-HT)], norepinephrine and dopamine via inhibition of uptake
(11), indicating that FAE or QR may
have functions at the level of the central nervous system and
monoamine neurotransmitters.
Serotonin, an important monoamine neurotransmitter
in the brain, is reported to have a role in the modulation of
sleep. The serotonergic system is necessary for sleep onset and
maintenance, and the administration of the 5-HT synthesis inhibitor
p-chlorophenylalanine (PCPA) induces insomnia that is
selectively antagonized/reversed by the 5-HT precursor
5-hydroxytryptophan (5-HTP) (12).
Studies indicate that certain single CHM herbs or extracts may
potentiate sleep induced by pentobarbital via the serotonergic
system (13,14). In addition, it has been reported that
anxiolytic-like effects of Albizia julibrissin may be
mediated by changes of the serotonergic nervous system (15).
Thus, the hypothesis that FAE and QR may potentiate
pentobarbital-induced sleep via the serotonergic system was
explored in the present study. Mice were administered both
compounds and changes in sleeping times and sleep latencies treated
with hypnotic or sub-hypnotic doses of pentobarbital and 5-HTP and
PCPA were measured to study the mechanism of the hypnotic effects
of FAE and QR.
Materials and methods
Animals
Male ICR mice (24±2 g) were from Zhejiang University
of Traditional Chinese Medicine (Zhejiang, China), and they were
housed under controlled environmental conditions (22±2°C, humidity
50±10%, 12 h light/dark cycle, lights on at 8:00 a.m.) with ad
libitum access to food and water. Mice were acclimated 1 week
before testing and fasted for 12 h prior to the onset of the
experiments. Experiments were carried out between 13:00 and 17:00
p.m. in a quiet room (22–24°C). All procedures were conducted in
accordance with the European Community guidelines for the use of
experimental animals and approved by the Shaoxing University
Committee on Animal Care and Use (Zhejiang, China).
Drugs and drug administration
Pentobarbital, 5-HTP, PCPA (Sigma-Aldrich, St.
Louis, MO, USA), aqueous FAE, QR (Xi'an Kailai Biological
Engineering Co., Ltd., Xi'an, China) and diazepam (DZ; 10 mg/2 ml;
manufactured by People's Pharmaceutical Manufacturer, Tianjin,
China) were used. For intragastric (ig) administration (10 ml/kg),
FAE and DZ injection were dissolved in distilled water, and QR was
suspended in 1% carboxymethyl-cellulose in physiological saline.
For intraperitoneal (ip) injection (10 ml/kg), 5-HTP and
pentobarbital were dissolved with physiological saline, and PCPA
was suspended in 0.5% gum acacia/physiological saline. The hypnotic
dose of pentobarbital (sleep onset 100%) used was 50 mg/kg, and 28
mg/kg was the sub-hypnotic dose (sleep onset 0%). FAE, QR and DZ
were administered (ig) 60 min prior to pentobarbital administration
(ip). 5-HTP was injected (ip) 15 min prior to pentobarbital
administration (ip). In a PCPA pretreatment test, mice received an
injection of PCPA (300 mg/kg between 12:00 a.m. and 13:00 p.m. for
24 h) prior to the injection of pentobarbital.
Evaluation of sleep onset and sleeping
time
The observers were blinded to the drug treatments.
Following pentobarbital administration, each mouse was observed for
sleep onset, as evidenced by rolling to their back and a loss of
righting reflex for >2 min. This was considered to be sleeping.
Mice that lost the righting reflex for <2 min were considered to
be awake. Sleep latency was recorded from pentobarbital injection
to 1 min after the loss of the righting reflex. Sleep time was
recorded from 1 min after the reflex loss to recovery of the
righting reflex.
Data and statistical analysis
For statistical analysis, data were processed with
Microsoft Excel and SPSS software (SPSS, Inc., Chicago, IL, USA).
Values are mean ± standard deviation for the indicated number of
experimental animals. For statistical comparisons, data were
analyzed by analysis of variance followed by the
Students-Newman-Keuls test for post-hoc comparisons. For the
sub-hypnotic pentobarbital dose test, a χ2 test was used
to compare the number of mice that fell asleep. P<0.05 was
considered to indicate a statistically significant difference.
Results
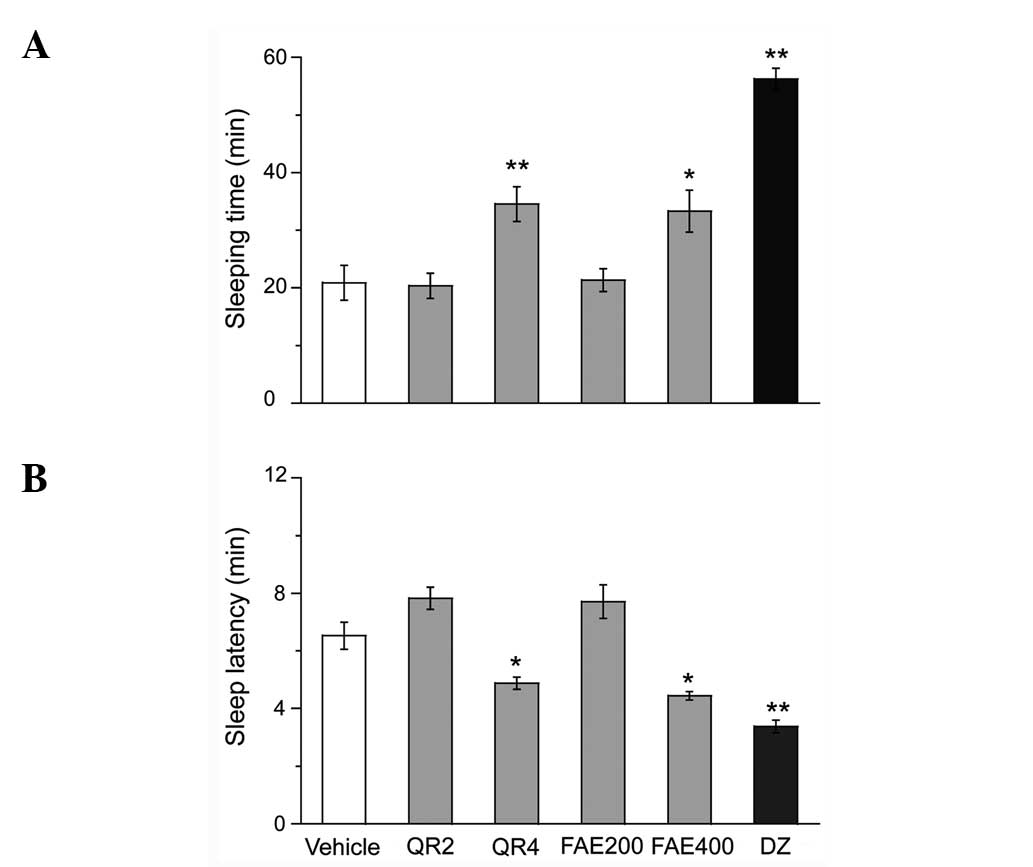
Effect of FAE and QR on the onset and
duration of sleep in pentobarbital-treated mice
FAE and QR had synergistic effects with
pentobarbital. Mice treated with hypnotic doses of pentobarbital
(50 mg/kg), FAE (400 mg/kg) or QR (4 mg/kg) had significantly
prolonged sleeping time (Fig. 1A) and
shorter sleep latency (Fig. 1B)
compared with vehicle mice. Mice treated with the sub-hypnotic
doses of pentobarbital (28 mg/kg), FAE (400 or 800 mg/kg) or QR (4
or 8 mg/kg) had significantly more rapid rate of sleep onset that
was dose dependent (Table I). As a
positive control, DZ (2 mg/kg) also potentiated the hypnotic
activity of pentobarbital in mice. FAE and QR administered alone
did not induce sleep.
 | Table I.Effect of FAE and QR on sleep onset of
mice induced by sub-hypnotic dosage of pentobarbital (28
mg/kg). |
Table I.
Effect of FAE and QR on sleep onset of
mice induced by sub-hypnotic dosage of pentobarbital (28
mg/kg).
| Groups | Total no. | No. falling
asleep | Sleep onset,% |
|---|
| Vehicle | 15 | 0 |
0.0 |
| DZ, 2 mg/kg | 10 | 10 | 100.0 |
| FAE, |
|
|
|
| 200
mg/kg | 15 | 1 |
6.7 |
| 400
mg/kg | 15 | 4 |
26.7a |
| 800
mg/kg | 15 | 7 |
46.7b |
| QR, |
|
|
|
| 2
mg/kg | 15 | 2 | 13.3 |
| 4
mg/kg | 15 | 5 |
33.3a |
| 8
mg/kg | 15 | 8 |
53.3b |
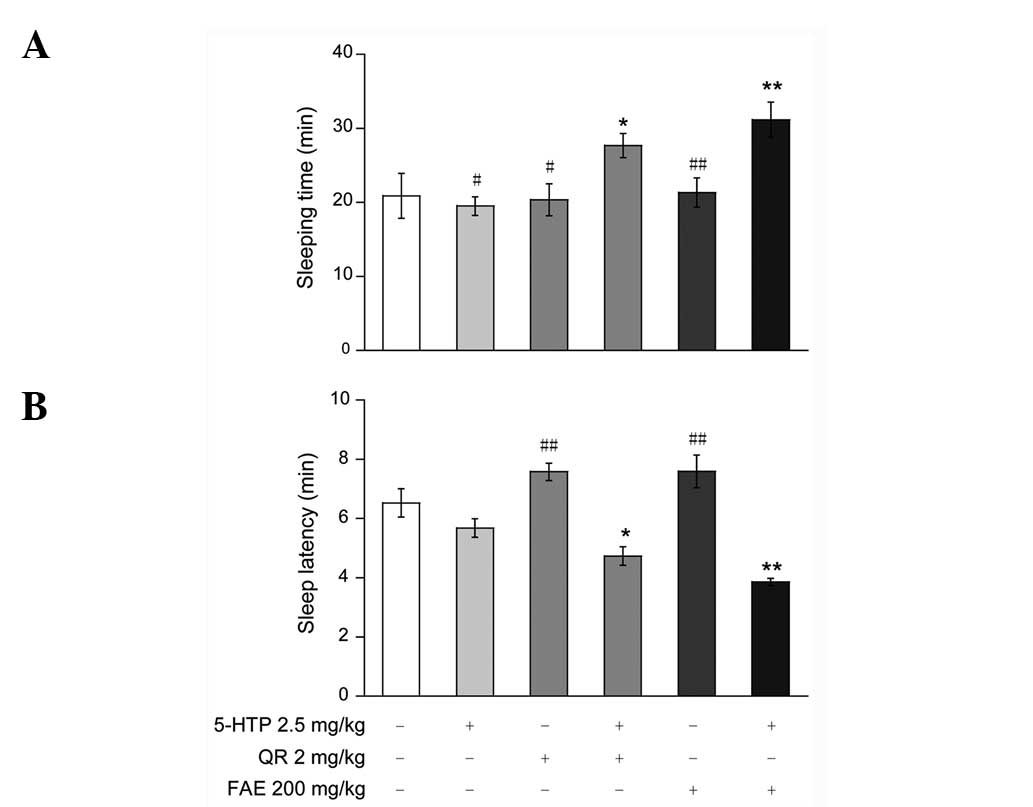
Synergic effects of FAE or QR and
5-HTP on sleep induced by pentobarbital
To investigate the association between the hypnotic
activity of FAE or QR and the serotonergic system, mice were
treated with FAE (200 mg/kg) or QR (2 mg/kg) for 60 min and with
5-HTP (2.5 mg/kg) for 15 min prior to the administration of
pentobarbital (50 mg/kg, ip). FAE (200 g/kg), QR (2 mg/kg) and
5-HTP (2.5 mg/kg) administered individually did not affect sleeping
time or sleep latency induced by hypnotic doses of pentobarbital
(Fig. 2A and B). The rate of sleep
onset induced by sub-hypnotic doses of pentobarbital was also
unaffected (Table II). However,
co-administration of FAE (200 mg/kg) or QR (2 mg/kg) and 5-HTP (2.5
mg/kg) synergistically prolonged sleeping time (Fig. 2A) and shortened sleep latency
significantly (Fig. 2B).
Co-administration also significantly increased the rate of sleep
onset (Table II) in mice treated with
sub-hypnotic doses of pentobarbital.
 | Table II.Synergic effects of FAE or QR with
5-HTP on sleep onset of mice treated with sub-hypnotic dosage of
pentobarbital (28 mg/kg). |
Table II.
Synergic effects of FAE or QR with
5-HTP on sleep onset of mice treated with sub-hypnotic dosage of
pentobarbital (28 mg/kg).
| Groups | Total no. | No. falling
asleep | Sleep onset, % |
|---|
| Vehicle | 15 | 0 | 0.0 |
| 5-HTP, 2.5 mg/kg | 15 | 2 | 13.3a |
| FAE, 200 mg/kg | 15 | 1 |
6.7a |
| QR, 2 mg/kg | 15 | 2 | 13.3a |
| 5-HTP, 2.5 mg/kg | 15 | 7 | 46.7b |
| + FAE, 200 mg/kg |
|
|
|
| 5-HTP, 2.5 mg/kg | 15 | 8 | 53.3b |
| + QR, 2 mg/kg |
|
|
|
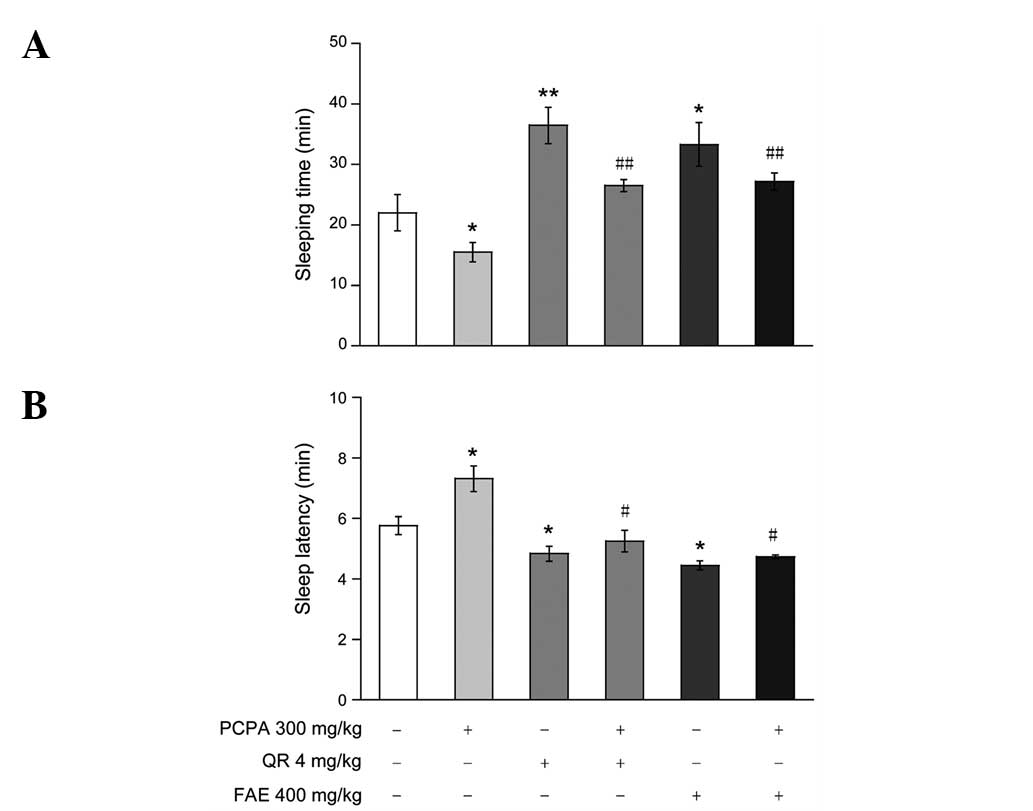
Effect of FAE or QR on PCPA-induced
insomnia in pentobarbital-treated mice
Treatment with PCPA (300 mg/kg, subcutaneous) has
been shown to induce insomnia in mice. In accordance with previous
studies (13,14), mice pretreated with PCPA for 24 h prior
to pentobarbital injection had significantly shortened sleeping
time (Fig. 3A) and prolonged sleep
latency (Fig. 3B). FAE (400 mg/kg) and
QR (4 mg/kg) significantly attenuated insomnia induced by PCPA,
which was reflected by increased sleep time (Fig. 3A) and decreased sleep latency (Fig. 3B).
Discussion
QR is a major flavonoid constituent of Albizia
julibrissin Durazz flowers with purported medicinal effects.
Data indicate that the active ingredients of FA, QR and
isoquercetin, had hypnotic effects via potentiation of
pentobarbital-induced sleep in ddY strain mice (5–6 weeks old)
(2). FAE and QR (hydrolysis product of
quercitrin) dose-dependently augmented the hypnotic activity of
pentobarbital by prolonging sleeping time (Fig. 1A) and by increasing the rate of sleep
onset (Fig. 1B; Table I) at 200–400 mg/kg FAE or 2–4 mg/kg QR.
Higher doses of each (800 or 8 mg/kg, respectively) did not change
sleeping time and sleep latency (data not shown). The differences
in the present data and those of previous studies may be explained
by different animal strains, mouse ages, and drug doses and
administration routes. By contrast, the data suggest
double-direction regulation, specifically, inhibition and sedation
at lower doses and excitation at higher doses. This effect has been
documented for several compounds: Bakuchiol (16), rhubarb (17), naringenin (18) and Panax Notoginseng Saponins
(19).
Serotonin not only has a role in inhibiting sleep
and promoting wakefulness, it is also associated with the
initiation and maintenance of sleep. Such complex effects of
serotonin on the regulation of sleep and wakefulness are attributed
to serotonin acting at different brain sites and 5-HT sub-type
receptors associated with the control of the sleep-wake cycle
(20). 5-HTP, the precursor of 5-HT,
prolongs pentobarbital-induced sleeping time in a dose-dependent
manner (14). Chronic administration
of PCPA, which inhibits tryptophan hydroxylase, can inhibit >95%
of serotonin biosynthesis, resulting in complete insomnia or
substantially reduced sleep (21).
These insomnia effects can be reversed by subsequent treatment with
5-HTP, which may restore serotonin biosynthesis, thereby restoring
sleep (22). This PCPA/5-HTP model
suggests that 5-HT and 5-HTP are important for sleep-wake
regulation. With this model, several studies suggest that CHM, such
as tetrandrine, spinosin and ferulic acid (13,14,23), may potentiate pentobarbital-induced
sleep via the serotonergic system in mice.
FAE and QR had synergistic effects with 5-HTP by
potentiating pentobarbital-induced sleep in mice, and acute
treatment of PCPA 24 h prior to pentobarbital was observed to
induce sleep suppression and this suppression can be inhibited by
FA (Fig. 3). These results suggest
that FAE and QR possess hypnotic effects, which may be mediated by
the serotonergic system. Previous studies of FAE and QR have
focused on their pharmacological effects, such as inhibition of
cancer cell proliferation and induction of apoptosis,
antidepressant effects, and antioxidant and hypolipidemic
properties (6–9). The present study suggests that FAE and QR
may also offer promise for the treatment of insomnia and diseases
that may induce sleep disorders.
In conclusion, the present study is the first to
reveal that the serotonergic system may be involved in
hypnotic-sedative activity of FAE and QR. Further studies are
required to elucidate the mechanisms behind these observations and
to describe the associations between these drugs and different 5-HT
receptor subtypes at different brain sites.
Acknowledgements
The present study was funded by the Technology
Innovation Project of University Students in Zhejiang Province and
Shaoxing City (grant nos. 2014R426026 and 2013124) and the
Scientific Research Fund of Shaoxing University (grant no.
20125025) and the Science & Technology Bureau of Shaoxing City
(grant no. 2014B70052).
References
|
1
|
Yeung WF, Chung KF, Poon MM, Ho FY, Zhang
SP, Zhang ZJ, Ziea ET and Wong VT: Chinese herbal medicine for
insomnia: A systematic review of randomized controlled trials.
Sleep Med Rev. 16:497–507. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kang TH, Jeong SJ, Kim NY, Higuchi R and
Kim YC: Sedative activity of two flavonol glycosides isolated from
the flowers of Albizzia julibrissin Durazz. J Ethnopharmacol.
71:321–323. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bischoff SC: Quercetin: Potentials in the
prevention and therapy of disease. Curr Opin Clin Nutr Metab Care.
11:733–740. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu R, Zhang Y, Ye X, Xue S, Shi J, Pan J
and Chen Q: Inhibition effects and induction of apoptosis of
flavonoids on the prostate cancer cell line PC-3 in vitro. Food
Chem. 138:48–53. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Angst E, Park JL, Moro A, Lu QY, Lu X, Li
G, King J, Chen M, Reber HA, Go VL, et al: The flavonoid quercetin
inhibits pancreatic cancer growth in vitro and in vivo. Pancreas.
42:223–229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anjaneyulu M, Chopra K and Kaur I:
Antidepressant activity of quercetin, a bioflavonoid, in
streptozotocin-induced diabetic mice. J Med Food. 6:391–395. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang Y, Gao C, Xing M, Li Y, Zhu L, Wang
D, Yang X, Liu L and Yao P: Quercetin prevents ethanol-induced
dyslipidemia and mitochondrial oxidative damage. Food Chem Toxicol.
50:1194–1200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boots AW, Haenen GR and Bast A: Health
effects of quercetin: From antioxidant to nutraceutical. Eur J
Pharmacol. 585:325–337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen X: Protective effects of quercetin on
liver injury induced by ethanol. Pharmacogn Mag. 6:135–141. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu S, Hou W, Yao P, Li N, Zhang B, Hao L,
Nüssler AK and Liu L: Heme oxygenase-1 mediates the protective role
of quercetin against ethanol-induced rat hepatocytes oxidative
damage. Toxicol In Vitro. 26:74–80. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi X, Jiang C, Zhao X, et al: Study on
the efects of Albizia Julibrissin flower on cognitive function and
plasma 5-HT, NE and DA in patients with depression: A randomized
parallel controlled multicenter clinical trial. J Pract Tradit Chin
Intern Med. 27:18–20. 2013.
|
|
12
|
Pujol JF, Buguet A, Froment JL, Jones B
and Jouvet M: The central metabolism of serotonin in the cat during
insomnia. A neurophysiological and biochemical study after
administration of P-chlorophenylalanine or destruction of the Raphé
system. Brain Res. 29:195–212. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tu Y, Cheng SX, Sun HT, Ma TZ and Zhang S:
Ferulic acid potentiates pentobarbital-induced sleep via the
serotonergic system. Neurosci Lett. 525:95–99. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao X, Cui XY, Chen BQ, Chu QP, Yao HY,
Ku BS and Zhang YH: Tetrandrine, a bisbenzylisoquinoline alkaloid
from Chinese herb Radix, augmented the hypnotic effect of
pentobarbital through serotonergic system. Eur J Pharmacol.
506:101–105. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jung JW, Cho JH, Ahn NY, Oh HR, Kim SY,
Jang CG and Ryu JH: Effect of chronic Albizzia julibrissin
treatment on 5-hydroxytryptamine1A receptors in rat brain.
Pharmacol Biochem Behav. 81:205–210. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mao H, Wang H, Ma S, Xu Y, Zhang H, Wang
Y, Niu Z, Fan G, Zhu Y and Gao XM: Bidirectional regulation of
bakuchiol, an estrogenic-like compound, on catecholamine secretion.
Toxicol Appl Pharmacol. 274:180–189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qin Y, Wang JB, Kong WJ, Zhao YL, Yang HY,
Dai CM, Fang F, Zhang L, Li BC, Jin C, et al: The diarrhoeogenic
and antidiarrhoeal bidirectional effects of rhubarb and its
potential mechanism. J Ethnopharmacol. 133:1096–1102. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li HY, Liang B, Chen ZT, et al: Two-way
effects on nasopharyngeal carcinoma CNE2 cells growth and
mechanisms of different dose naringenin. Chin Pharm J.
48:2006–2012. 2013.
|
|
19
|
Wang XY and Zhang T: Research progress of
the dual regulatory effects of Panax Notoginseng Saponins on
angiogenesis. Chin Tradit Chin Med Pharm. 28:1818–1822. 2013.
|
|
20
|
Dugovic C: Role of serotonin in sleep
mechanisms. Rev Neurol (Paris). 157:S16–S19. 2001.PubMed/NCBI
|
|
21
|
Weitzman ED, Rapport MM, McGregor P and
Jacoby J: Sleep patterns of the monkey and brain serotonin
concentration: Effect of p-chlorophenylalanine. Science.
160:1361–1363. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Touret M, Sarda N, Gharib A, Geffard M and
Jouvet M: The role of 5-hydroxytryptophan (5-HTP) in the regulation
of the sleep/wake cycle in parachlorophenylalanine (p-CPA)
pretreated rat: A multiple approach study. Exp Brain Res.
86:117–124. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang LE, Bai YJ, Shi XR, Cui XY, Cui SY,
Zhang F, Zhang QY, Zhao YY and Zhang YH: Spinosin, a C-glycoside
flavonoid from semen Zizhiphi Spinozae, potentiated
pentobarbital-induced sleep via the serotonergic system. Pharmacol
Biochem Behav. 90:399–403. 2008. View Article : Google Scholar : PubMed/NCBI
|