Introduction
Hepatic ischemia/reperfusion (I/R) injury has been
indicated in the pathogenesis of a variety of clinical conditions,
including trauma, reconstructive vascular surgery, liver
transplantation and liver resection surgery (1–4).
Accumulating evidence has shown that cysteinyl leukotrienes (LTs)
were associated with hepatic I/R injury. LTC4 synthesis enzymes,
including leukotriene C4 synthase (LTC4S), microsomal glutathione
S-transferase (mGST) 2 and mGST3, can conjugate LTA4 and reduce
glutathione to form LTC4, which is the first synthesis step of the
cysteinyl LTs, LTC4, LTD4 and LTE4. A pivotal inflammatory
transcription factor, nuclear factor-κB (NF-κB), appears to have a
central role in the cascade of inflammatory mediators induced
during I/R injury (5). NF-κB
activation has been shown to occur in models of warm and cold I/R
injury. LPS downregulates cysteinyl LT release and LTC4 synthase
gene expression in mononuclear phagocytes by an NF-κB-mediated
mechanism (6). Nitric oxide (NO) is
enzymatically synthesized from L-arginine by three known NO
synthase (NOS) isoforms: Constitutively expressed endothelial NOS,
neuronal NOS and the inducible NOS (iNOS) (7,8). The
association between cysteinyl LTs and NO has been shown in previous
studies (9–11). When cells were stimulated with a
combination of cytokines or with interleukin-1, LTB4 decreased
hepatocyte NO synthesis in a concentration-dependent manner
(9). Reduced synthesis of
NO2− was associated with reduced iNOS mRNA
levels suggesting that the induction of iNOS was inhibited. These
findings demonstrate that eicosanoids can regulate hepatocyte NO
synthesis in vitro. Numerous studies have suggested that NO
is associated with NF-κB in hepatic I/R injury (12–17). Our
previous study has suggested that the NO donor sodium nitroprusside
(SNP) downregulated the mRNA expression of LTC4S by
inhibiting NF-κB activation in an IκBα-independent manner (12). Recently, we reported that a selective
liver NO donor,
O2-vinyl1-(pyrrolidin-1-yl)diazen-1-ium-1,2-diolate
(V-PYRRO/NO), downregulated the mRNA expression of LTC4S
(18). However, whether the underlying
influence on LTC4S mRNA expression levels is involved in
NF-κB activation remains to be elucidated.
Materials and methods
Materials
In total, 18 male Sprague-Dawley rats, weighing
230–250 g, were obtained from the Experimental Animal Center,
Nanchang University (Nanchang, China). V-PYRRO/NO was purchased
from Cayman Chemical Company, Inc. (Ann Arbor, MI, USA). TRIzol
reagent and MmuLV reverse transcription (RT) were from Gibco-BRL
(Gaithersburg, MD, USA), and reduced glutathione and Taq DNA
polymerase were from Sangon Biotech Co., Ltd. (Shanghai, China).
cDNA probes for rat LTC4S were synthesized by Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). NF-κB p50, IκBα and β-actin
rabbit polyclonal antibodies together with NF-κB p65 mouse
monoclonal antibody were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). The enhanced chemiluminescence detection
kit for horseradish peroxidase (HRP) was from Biological Industries
(Biological Industries, Kibbutz Beit-Haemek, Israel).
Polyvinylidene difluoride (PVDF) membranes were from Millipore
(Billerica, MA, USA). The Polymer Detection system for
immunohistological staining, DAB kit, HRP-linked goat anti-rabbit
(#ZB-2301) and goat anti-mouse antibody (#ZB-2305) were from
Zhongshan Biological Co. (Beijing, China). All other chemicals were
of the highest purity commercially available.
Animal model of hepatic I/R
injury
The rats were housed and treated in accordance with
the Guidelines for the Care and Use of the Experimental Animals
Center of Nanchang University (Nanchang, China). The study was
approved by the Local Animal Ethics Committee. Animals were fasted
for 12 h, but allowed to drink water prior to the surgery, and were
randomized into 3 groups consisting of 6 animals. In the I/R group,
animals were anesthetized with 50 mg/kg pentobarbital
intraperitoneally, the external jugular vein catheter was created
using a polyethylene tube of 0.9 mm inner diameter (BD Biosciences
Medical Devices Co. Ltd., Suzhou, China) and was subjected to
midline laparotomy, the liver was exposed, and the left lateral and
median lobes were rendered ischemic by clamping the hepatic
arterial and portal venous blood supply using a microaneurysm
clamp. Following 60 min of hepatic ischemia (or sham), livers were
reperfused for 5 h by removing the clamp and the peritoneal cavity
was sutured closed for 5 h. Saline solution (3 ml/kg/min) was
intravenously injected by external jugular vein at 15 min before
the start of ischemia through 5 h reperfusion. In the sham group
(control), surgeries were performed on anesthetized rats in which
hepatic blood flow was not occluded. In the V-PYRRO/NO (1.06
µmol/kg/h) + I/R group, surgeries were performed on anesthetized
rat as for the I/R group, and V-PYRRO/NO (1.06 µmol/kg/h) was
intravenously injected through the external jugular vein catheter
using a micro-injector (19) at 15 min
before the start of ischemia through 5 h reperfusion, respectively.
Following 5 h of reperfusion, the livers were removed, medium lobe
fixed in 10% formalin for immunohistochemistry, and the left lobule
was snap frozen in liquid nitrogen and subsequently stored at −80°C
for RNA determination and western blot analysis.
RT-polymerase chain reaction
(PCR)
The mRNA expression levels of LTC4S were detected as
described in our previous studies (2,10,11). Briefly, total RNA was isolated from
whole liver tissue using TRIzol reagent, according to the
manufacturer's protocol, and quantified by measurement of
ultraviolet absorption at 260 nm. A total of 1 µg of total RNA from
each sample was RT to synthesize the single-stranded cDNA using an
antisense specific primer and 200 units of MmuLV RT (Gibco-BRL).
Sequences of the PCR primers for rat β-actin and LTC4S were derived
from published sequences (10,11) (Table I).
Aliquots of the synthesized cDNA (1.5 µl) were amplified with a
proper cycle using each primer and 1.5 units of Taq DNA polymerase
in a Mastercycler gradient (Eppendorf, Hamburg, Germany). The
reactants were cycled at 95°C for 45 sec, 55.8/58°C for 45 sec and
72°C for 45 sec. The PCR products were separated by electrophoresis
using a 1.5% ethidium bromide-stained agarose gel and visualized by
ultraviolet transillumination. The intensity of each band was
measured by a Bio-Imaging Analyzer (Bio-Rad, Berkeley, CA, USA) and
quantified using Quantity One version 4.2.2 software (Bio-Rad).
Using amplification of β-actin as a control, the degree of
expression of the mRNA of these products was compared.
 | Table I.Oligonucleotide primer used for the
analysis of LTC4S and β-actin genes by RT-PCR. |
Table I.
Oligonucleotide primer used for the
analysis of LTC4S and β-actin genes by RT-PCR.
| Genes | Sense and
antisense | PCR product, bp | PCR cycles | Annealing
temperature°C |
|---|
| LTC4S |
5′-CGAGTACTTTCCGCTGTTC-3′ | 237 | 35 | 55.8 |
|
|
5′-TAGTGTGCCAGGGAGGAAG-3′ |
|
|
|
| β-actin |
5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′ | 660 | 25 | 58.0 |
|
|
5′-CTAGAAGCATTTGCGGTGGACGATGGAGGG-3′ |
|
|
|
Western blot analysis
The protein expression levels of NF-κB p50, p65 or
IκBα were performed as described in our previous study (12). Deep-frozen liver samples were lysed in
150 mmol/l NaCl, 50 mmol/l Tris-HCl (pH 7.5), 1% NP-40, 0.25
deoxycholate, 0.1% SDS supplemented with the protease inhibitor
phenylmethanesulfonyl fluoride, pepstatin, leupeptin and aprotinin.
The protein concentration was determined as described by Lowry
et al (20). Nuclear extracts
were prepared from liver tissue as described by Deryckere and
Gannon (21). Equal amounts of liver
lysates (100 µg) or nuclear extracts (50 µg) were loaded on an
SDS-PAGE gel (12%), and electroblotted onto PVDF membranes. The
transfer efficiency was visualized using prestained molecular
weight protein standards (Fermentas, Sangon). Membranes were
subsequently soaked for 1 h at 25°C in 5% (w/v) non-fat dried milk.
The PVDF membranes were subsequently incubated overnight at 4°C
with specific rat polyclonal or monoclonal antibodies raised
against a peptide of human NF-κB p50 (#SC-114), p65 (#SC-8008) or
IκBα (#SC-371) and β-actin (#SC-1616), used at dilutions of 1:500
or 1:1,000. After washing, the blot was incubated for 1 h at 25°C
with a HRP-linked goat anti-rabbit or goat anti-mouse antibody
(1:5,000 dilution) in 0.1% phosphate-buffer solution with Tween-20
and 5% (w/v) non-fat dried milk. The washing steps were repeated
and subsequently enhanced chemiluminescence detection was performed
according to the manufacturer's protocols (Biological
Industries).
Immunohistochemistry
The indirect immunoperoxidase method was used to
localize NF-κB p65 in paraffin-embedded sections from the control,
I/R and V-PYRRO/NO + I/R group rats and was performed using the
Polymer Detection System for immunohistological staining and DAB
kit (Zhongshan Biological Co.), according to the manufacturer's
protocols. When the sections were deparaffinized and rehydrated,
endogenous peroxidase was quenched by incubation of the sections in
3% H2O2 in methanol for 20 min. Following
antigen retrieval, the sections were blocked for nonspecific
binding of the antibody with phosphate-buffered saline (PBS)
containing 10% normal calf serum for 30 min and subsequently
incubated overnight at 4°C with mouse NF-κB p65 monoclonal antibody
(Santa Cruz Biotechnology, Inc.) at a dilution of 1:100 in 0.5%
bovine serum albumin in PBS. After three washes with PBS, the
sections were incubated for 1 h in a solution containing goat
anti-mouse immunoglobulin G-HRP polymer. The sections were washed,
stained with diaminobenzidine and counterstained with
hematoxylin.
Statistical analysis
Data are expressed as mean ± standard deviation.
Kruskal-Wallis test was used to compare the 3 groups. The Student's
t-test was used for the comparison of two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
RT-PCR analysis of hepatic mRNA
expression levels of LTC4S in the control, I/R and V-PYRRO/NO + I/R
group rats
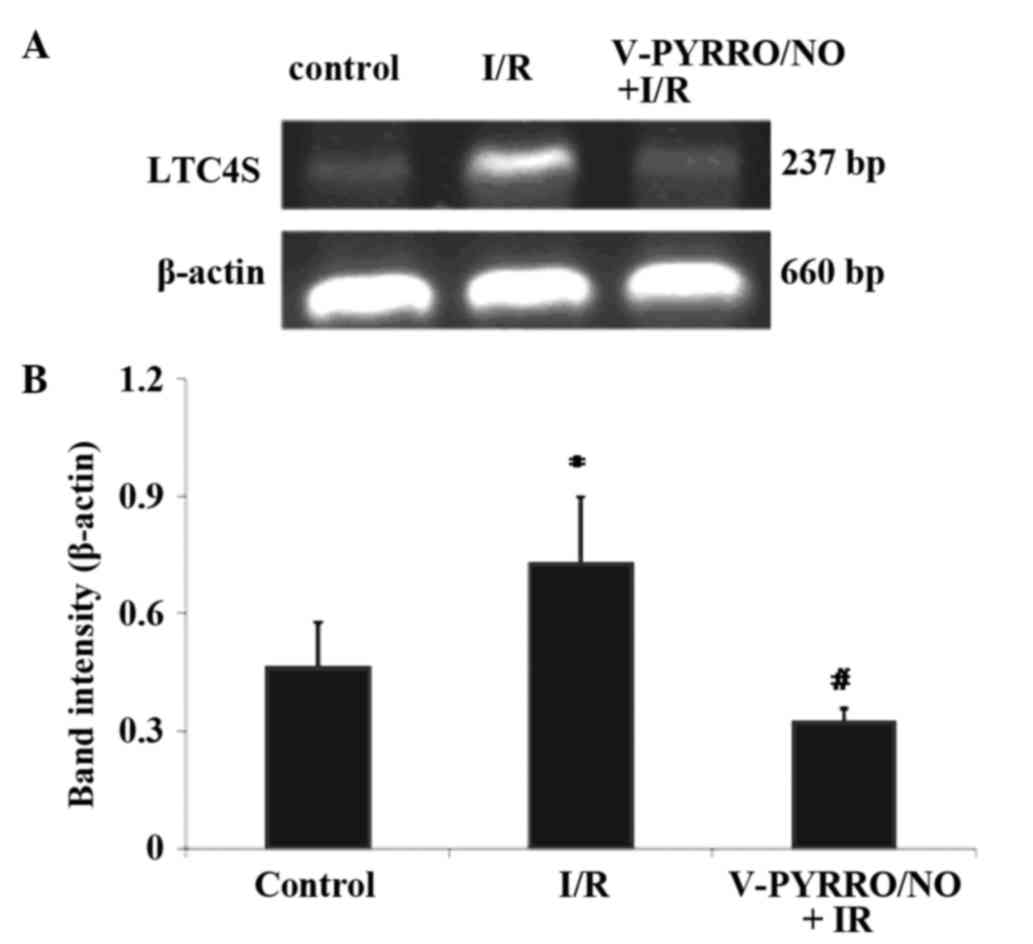
A representation of the hepatic mRNA expression
levels of LTC4S is shown in Fig. 1A and
B, exhibited as densitometric analysis of the LTC4S PCR
products in the control, I/R and V-PYRRO/NO (1.06 µmol/kg/h) + I/R
group rats. The mRNA expression of LTC4S in the I/R group
was significantly higher compared with the control groups
(P<0.05). Compared with the I/R group, the mRNA expression of
LTC4S in the liver tissue was significantly decreased after 5 h
reperfusion in the V-PYRRO/NO (1.06 µmol/kg/h) + I/R group
(P<0.05).
Immunoblot analysis of hepatic protein
expression of NF-κB p-50, p-65 and IκB in control, I/R and
V-PYRRO/NO + I/R group rats
NO was demonstrated to be associated with NF-κB in
hepatic I/R injury (12–17). The present study examined the protein
expression levels of NF-κB p-50, p-65 and IκBα in nuclear extracts
and whole liver lysates with western blot analysis. As indicated in
Fig. 2, the nuclear NF-κB p65 and p50
protein expression levels in the I/R group were significantly
increased compared to the control group, whereas the protein levels
of cytoplasmic NF-κB p65 and p50 in the I/R group were markedly
lower compared to in the control group; V-PYRRO/NO (1.06 µmol/kg/h)
decreased the protein levels of NF-κB p65 and p50 in the nuclear
extracts while it increased the protein levels of NF-κB p65 and p50
in the liver lysates during hepatic I/R in rats. However, there was
no difference in the IκBα protein level in all the groups.
Immunohistochemical staining for NF-κB
p65 in the liver sections in the control, I/R and V-PYRRO/NO + I/R
group rats
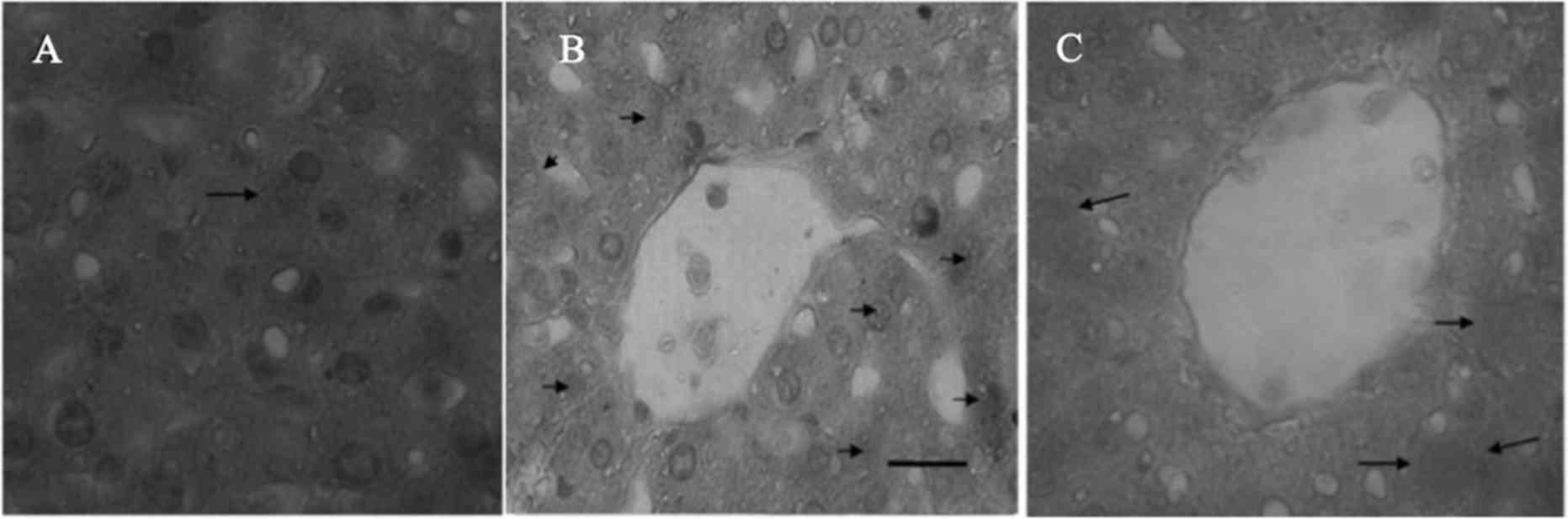
To further examine NF-κB translocation in rat liver
tissue, immunohistochemical staining was performed for NF-κB p65 to
detect the cytoplasmic and nuclear staining in paraffin-embedded
liver sections from the control, I/R and V-PYRRO/NO (1.06
µmol/kg/h) + I/R group rats. The cytoplasmic and nuclei staining
for NF-κB p65 was slight in the normal (Fig. 3A) and V-PYRRO/NO (1.06 µmol/kg/h) + I/R
(Fig. 3C) group liver tissues, and was
strong in the I/R liver tissues (Fig.
3B).
Discussion
Numerous studies have indicated LTs in the
pathogenesis of the hepatic I/R injury (2,22,23). The biosynthesis of cysteinyl LTs (LTC4,
LTD4 and LTE4) is catalyzed by LTC4S, mGST2 and mGST3 (24,25). A
previous study demonstrated that LTC4S mRNA was detected in
whole liver, hepatocytes and sinusoidal endothelial cells, but not
in Kupffer cells (26). Endogenous NO
has also been identified as a key messenger molecule in the
cardiovascular, nervous and immune systems (27). Our previous study and others studies
have reported the association that exists between cysteinyl LTs and
NO (8,10,28,29). The present study further elucidates
whether a selective liver NO donor, V-PYRRO/NO, could regulate the
gene expression of LTC4S in rats. The results revealed that
V-PYRRO/NO completely reveresd the upregulation of LTC4S
gene expression in hepatic I/R rats.
Whether NO can activate the NF-κB signaling pathway
remains to be elucidated (7). LPS has
been reported to downregulate cysteinyl LT release and LTC4S
gene expression in mononuclear phagocytes by an NF-κB-mediated
mechanism (6). The major pathway for
NF-κB activation is well known to depend on the activation of the
IκK complex, which leads to the phosphorylation of serine residues
of IκB and the degradation of IκB via the ubiquitin-proteasome
system (30). Our previous study
suggested that SNP downregulated the mRNA expression of
LTC4S by inhibiting NF-κB activation in an IκBα-independent
manner (11). In order to investigate
whether a selective liver NO donor, V-PYRRO/NO, can regulate the
gene expression of LTC4S via NF-κB signaling pathway in
rats, the protein levels of NF-κB p-50, p-65 and IκBα were examined
in nuclear extracts and whole liver lysates with western blotting
analysis. V-PYRRO/NO clearly decreased the protein levels of NF-κB
p65 and p50 in the nuclear extracts but increased the protein
levels of NF-κB p65 and p50 in the liver lysates during hepatic I/R
in rats (Fig. 2); but the IκBα protein
expression presented no differences in all the groups. To further
evaluate the alterations of NF-κB translocation in the liver
tissue, immunohistochemical staining was performed for NF-κB p65 to
detect the cytoplasmic and nuclear staining in paraffin-embedded
liver sections. The data showed slight cytoplasmic and nuclei
positive staining for NF-κB p65 in the normal and V-PYRRO/NO + I/R
group liver tissues, and the I/R liver tissue exhibited strong
cytoplasmic and nuclei positive staining. These results suggest
that an exogenous NO donor, V-PYRRO/NO, inhibited the NF-κB
activation in a manner independent of IκBα degradation during
hepatic I/R injury in rats. Considering the above result of
LTC4S gene expression levels, V-PYRRO/NO evidently
downregulated the mRNA expression of LTC4S by inhibiting
NF-κB activation independent of IκBα degradation. This result was
in accordance with a previous study, which suggested that NF-κB is
activated by c-Src dependent tyrosine phosphorylation of IκBα but
not IκBβ during I/R injury, and this process occurs in the absence
of IκBα ubiquitin-dependent degradation (11,31).
However, whether V-PYRRO/NO can regulate LTC4S gene
expression via NF-κB signaling pathway by c-Src dependent tyrosine
phosphorylation of IκBα remains to be elucidated.
In conclusion, the present findings demonstrated
that a selective liver NO donor, V-PYRRO/NO, may downregulate the
mRNA expression of LTC4S by inhibiting NF-κB activation in
an IκBα-independent manner.
Acknowledgements
The present study was supported by National Natural
Science Foundation of China (grant no. 81260504) and Educational
Commission of Jiangxi Province of China (grant no. GJJ12073).
References
|
1
|
Montalvo-Jave EE, Piña E, Montalvo-Arenas
C, Urrutia R, Benavente-Chenhalls L, Peña-Sanchez J and Geller DA:
Role of ischemic preconditioning in liver surgery and hepatic
transplantation. J Gastrointest Surg. 13:2074–2083. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang SL, Huang X, Chen HF, Xu D, Chen LJ,
Kong Y and Lou YJ: Increased leukotriene c4 synthesis accompanied
enhanced leukotriene c4 synthase expression and activities of
ischemia-reperfusion-injured liver in rats. J Surg Res. 140:36–44.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hong F and Yang S: Ischemic
preconditioning decreased leukotriene C4 formation by depressing
leukotriene C4 synthase expression and activity during hepatic I/R
injury in rats. J Surg Res. 178:1015–1021. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qiao YL, Qian JM, Wang FR, Ma ZY and Wang
QW: Butyrate protects liver against ischemia reperfusion injury by
inhibiting nuclear factor kappa B activation in Kupffer cells. J
Surg Res. 187:653–659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsoulfas G and Geller DA: NF-κB in
transplantation: Friend or foe? Transpl Infect Dis. 3:212–219.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Serio KJ, Johns SC, Luo L, Hodulik CR and
Bigby TD: Lipopolysaccharide down-regulates the leukotriene C4
synthase gene in the monocyte-like cell line, THP-1. J Immunol.
170:2121–2128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Demirbilek S, Karaman A, Gürünlüoğlu K,
Taş E, Akın M, Aksoy RT, Türkmen E, Edalı MN and Baykarabulut A:
Polyenylphosphatidylcholine pretreatment protects rat liver from
ischemia/reperfusion injury. Hepatol Res. 34:84–91. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Laroux FS, Pavlick KP, Hines IN, Kawachi
S, Harada H, Bharwani S, Hoffman JM and Grisham MB: Role of nitric
oxide in inflammation. Acta Physiol Scand. 173:113–118. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harbrecht BG, Kim YM, Wirant EM, Shapiro
RA and Billiar TR: PGE2 and LTB4 inhibit cytokine-stimulated nitric
oxide synthase type 2 expression in isolated rat hepatocytes.
Prostaglandins. 52:103–116. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang SL and Lou YJ: Sodium nitroprusside
decreased leukotriene C4 generation by inhibiting leukotriene C4
synthase expression and activity in hepatic ischemia-reperfusion
injured rats. Biochem Pharmacol. 73:724–735. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang SL, Chen LJ, Kong Y, Xu D and Lou YJ:
Sodium nitroprusside regulates mRNA expressions of LTC4 synthesis
enzymes in hepatic ischemia/reperfusion injury rats via NF-kappaB
signaling pathway. Pharmacology. 80:11–20. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hur GM, Ryu YS, Yun HY, Jeon BH, Kim YM,
Seok JH and Lee JH: Hepatic ischemia/reperfusion in rats induces
iNOS gene transcription by activation of NF-κB. Biochem Biophys Res
Commun. 261:917–922. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Inoue T, Kwon AH, Oda M, Kaibori M,
Kamiyama Y, Nishizawa M, Ito S and Okumura T: Hypoxia and heat
inhibit inducible nitric oxide synthase gene expression by
different mechanisms in rat hepatocytes. Hepatology. 32:1037–1044.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Isobe M, Katsuramaki T, Hirata K, Kimura
H, Nagayama M and Matsuno T: Beneficial effects of inducible nitric
oxide synthase inhibitor on reperfusion injury in the pig liver.
Transplantation. 68:803–813. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jessup JM, Samara R, Battle P and Laguinge
LM: Carcinoembryonic antigen promotes tumor cell survival in liver
through an IL-10-dependent pathway. Clin Exp Metastasis.
21:709–717. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kiemer AK, Vollmar AM, Bilzer M, Gerwig T
and Gerbes AL: Atrial natriuretic peptide reduces expression of
TNF-α mRNA during reperfusion of the rat liver upon decreased
activation of NF-κB and AP-1. J Hepatol. 33:236–246. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luedde T, Assmus U, Wüstefeld T, Meyer zu
Vilsendorf A, Roskams T, Schmidt-Supprian M, Rajewsky K, Brenner
DA, Manns MP, Pasparakis M, et al: Deletion of IKK2 in hepatocytes
does not sensitize these cells to TNF-induced apoptosis but
protects from ischemia/reperfusion injury. J Clin Invest.
115:849–859. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hong FF, He CS, Tu GL, Guo FX, Chen XB and
Yang SL: Nitric oxide donor regulated mRNA expressions of LTC4
synthesis enzymes in hepatic ischemia reperfusion injury rats.
Frontier and Future Development of Information Technology in
Medicine and Education. (Lecture Notes in Electrical Engineering).
Li S, Jin Q, Jiang X and Park JJ: 269:Springer Science Business
Media Dordrecht. 3359–3366. 2014. View Article : Google Scholar
|
|
19
|
Saavedra JE, Billiar TR, Williams DL, Kim
YM, Watkins SC and Keefer LK: Targeting nitric oxide (NO) delivery
in vivo. Design of a liver-selective NO donor prodrug that blocks
tumor necrosis factor-alpha-induced apoptosis and toxicity in the
liver. J Med Chem. 40:1947–1954. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
21
|
Deryckere F and Gannon F: A one-hour
minipreparation technique for extraction of DNA-binding proteins
from animal tissues. Biotechniques. 16:4051994.PubMed/NCBI
|
|
22
|
Daglar G, Karaca T, Yuksek YN, Gozalan U,
Akbiyik F, Sokmensuer C, Gurel B and Kama NA: Effect of montelukast
and MK-886 on hepatic ischemia-reperfusion injury in rats. J Surg
Res. 153:31–38. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takamatsu Y, Shimada K, Chijiiwa K, Kuroki
S, Yamaguchi K and Tanaka M: Role of leukotrienes on hepatic
ischemia/reperfusion injury in rats. J Surg Res. 119:14–20. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jakobsson PJ, Mancini JA and
Ford-Hutchinson AW: Identification and characterization of a novel
human microsomal glutathione S-transferase with leukotriene C4
synthase activity and significant sequence identity to
5-lipoxygenase-activating protein and leukotriene C4 synthase. J
Biol Chem. 271:22203–22210. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jakobsson PJ, Mancini JA, Riendeau D and
Ford-Hutchinson AW: Identification and characterization of a novel
microsomal enzyme with glutathione-dependent transferase and
peroxidase activities. J Biol Chem. 272:22934–22939. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shimada K, Navarro J, Goeger DE, Mustafa
SB, Weigel PH and Weinman SA: Expression and regulation of
leukotriene-synthesis enzymes in rat liver cells. Hepatology.
28:1275–1281. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Megson LL: Nitric oxide donor drugs. Drugs
fulure. 25:701–715. 2000. View Article : Google Scholar
|
|
28
|
Lärfars G, Lantoine F, Devynck MA,
Palmblad J and Gyllenhammar H: Activation of nitric oxide release
and oxidative metabolism by leukotrienes B4, C4 and D4 in human
polymorphonuclear leukocytes. Blood. 93:1399–1405. 1999.PubMed/NCBI
|
|
29
|
Beckh K, Lange AB, Adler G and Weidenbach
H: Effects of nitric oxide on leukotriene D4 decreased bile
secretion in the perfused rat liver. Life Sci. 61:1947–1952. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mercurio F, Zhu H, Murray BW, Shevchenko
A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A, et al:
IKK-1 and IKK-2: Cytokine-activated IkappaB kinases essential for
NF-κB activation. Science. 278:860–866. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fan C, Li Q, Ross D and Engelhardt JF:
Tyrosine phosphorylation of I kappa B alpha activates NF kappa B
through a redox-regulated and c-Src-dependent mechanism following
hypoxia/reoxygenation. J Biol Chem. 278:2072–2080. 2003. View Article : Google Scholar : PubMed/NCBI
|