|
1
|
Yu AL, Gilman AL, Ozkaynak MF, London WB,
Kreissman SG, Chen HX, Smith M, Anderson B, Villablanca JG, Matthay
KK, et al: Children's Oncology Group: Anti-GD2 antibody with
GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J
Med. 363:1324–1334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Navid F, Sondel PM, Barfield R, Shulkin
BL, Kaufman RA, Allay JA, Gan J, Hutson P, Seo S, Kim K, et al:
Phase I trial of a novel anti-GD2 monoclonal antibody,
Hu14.18K322A, designed to decrease toxicity in children with
refractory or recurrent neuroblastoma. J Clin Oncol. 32:1445–1452.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen G and Emens LA: Chemoimmunotherapy:
Reengineering tumor immunity. Cancer Immunol Immunother.
62:203–216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Emens LA: Chemoimmunotherapy. Cancer J.
16:295–303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ullrich E, Ménard C, Flament C, Terme M,
Mignot G, Bonmort M, Plumas J, Chaperot L, Chaput N and Zitvogel L:
Dendritic cells and innate defense against tumor cells. Cytokine
Growth Factor Rev. 19:79–92. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sica A: Role of tumour-associated
macrophages in cancer-related inflammation. Exp Oncol. 32:153–158.
2010.PubMed/NCBI
|
|
7
|
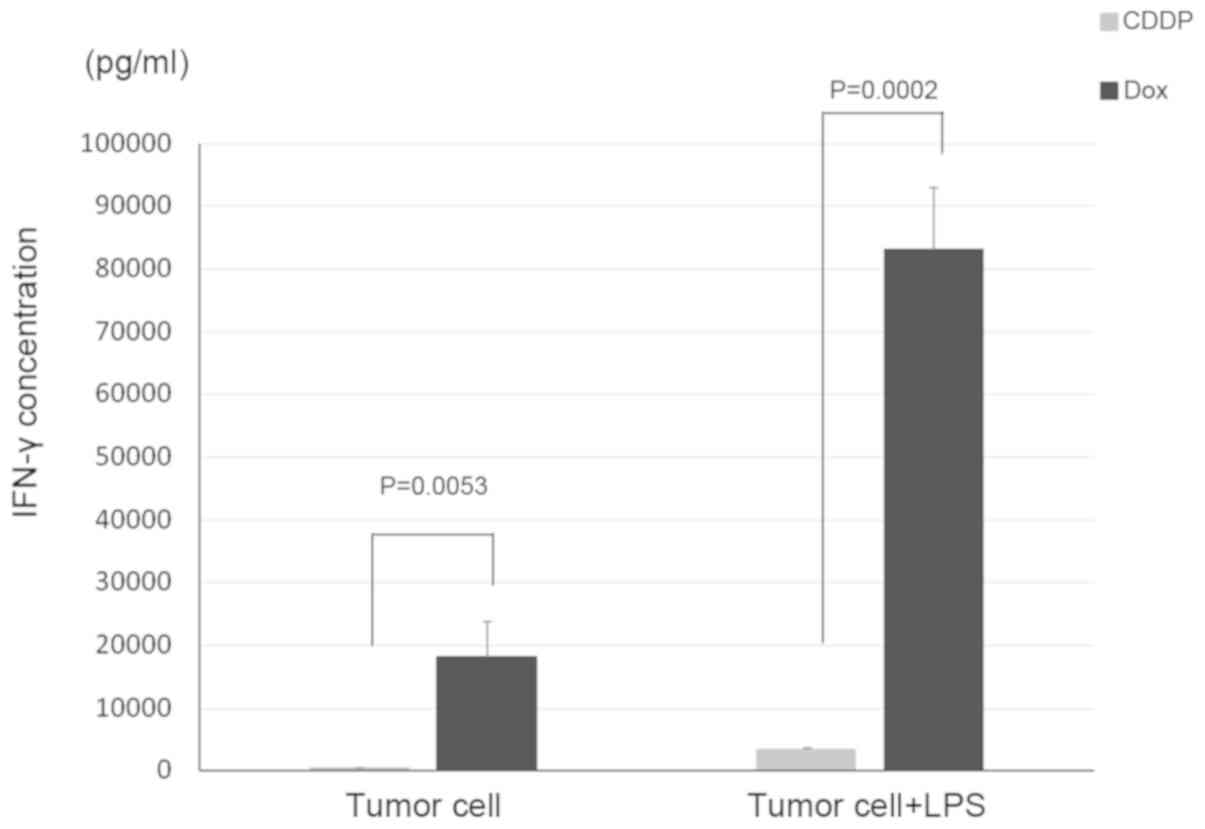
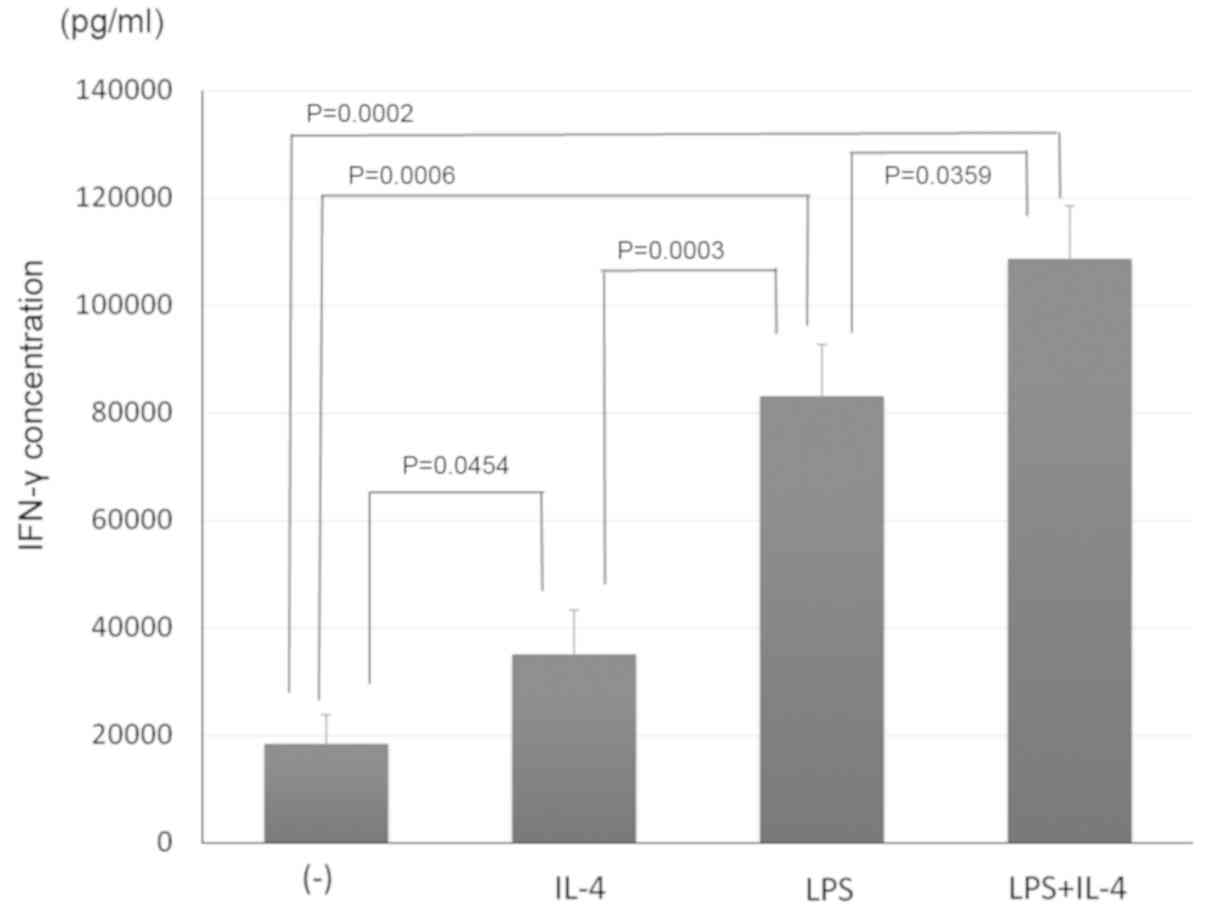
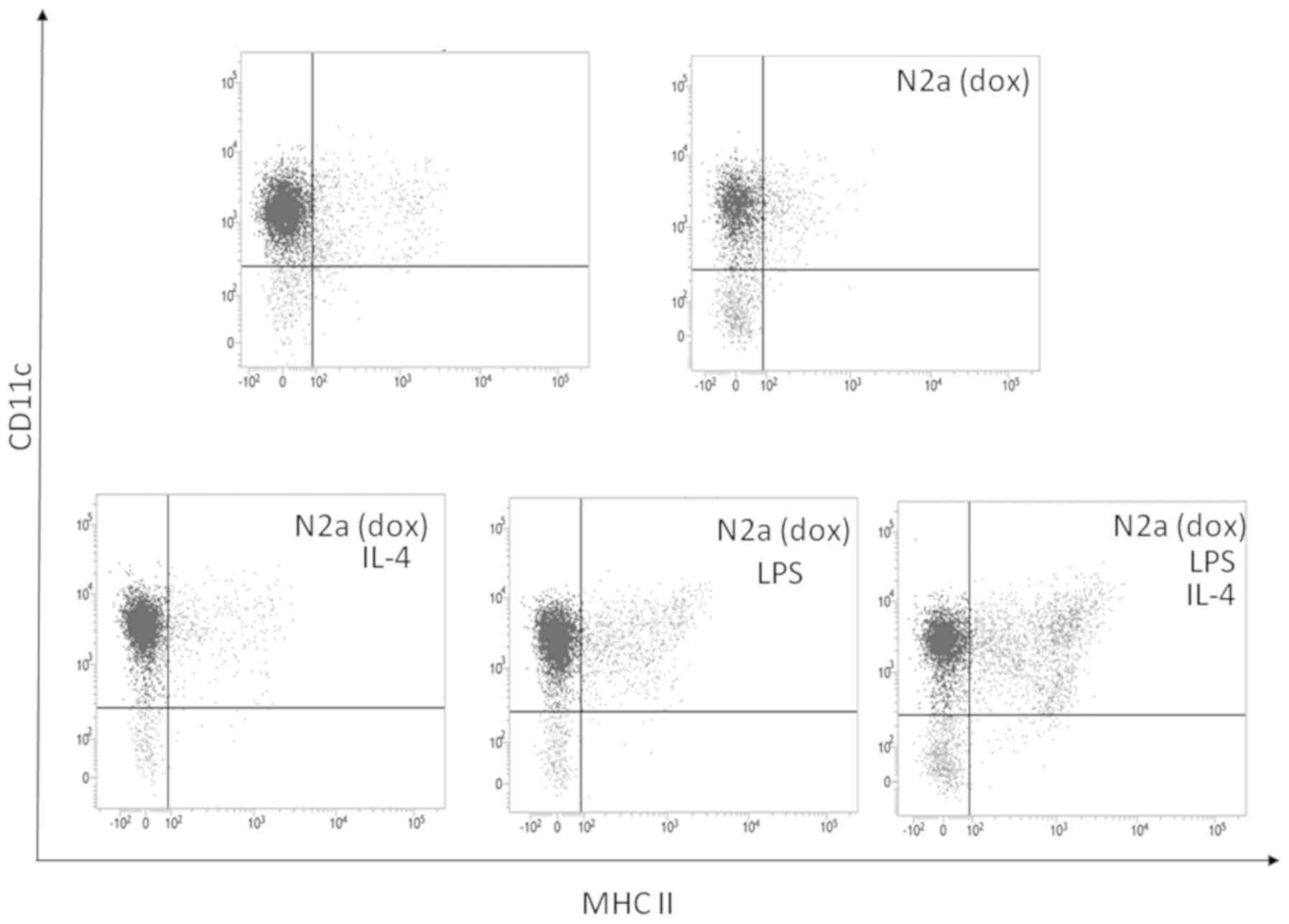
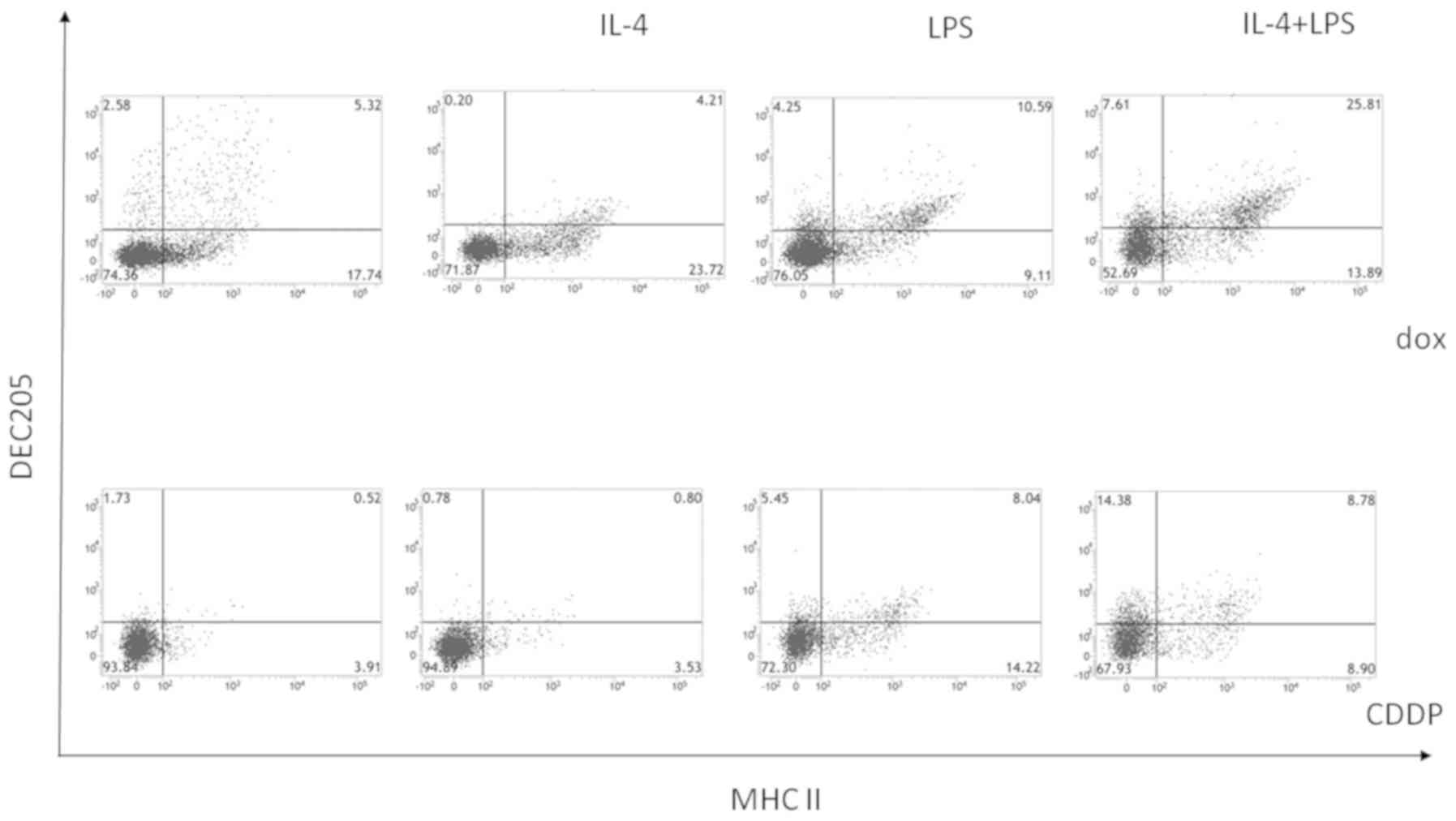
Inoue S, Setoyama Y and Odaka A:
Doxorubicin treatment induces tumor cell death followed by
immunomodulation in a murine neuroblastoma model. Exp Ther Med.
7:703–708. 2014.PubMed/NCBI
|
|
8
|
Maris JM: Recent advances in
neuroblastoma. N Engl J Med. 362:2202–2211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Smith MA, Altekruse SF, Adamson PC, Reaman
GH and Seibel NL: Declining childhood and adolescent cancer
mortality. Cancer. 120:2497–2506. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maris JM, Hogarty MD, Bagatell R and Cohn
SL: Neuroblastoma. Lancet. 369:2106–2120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hara J: Development of treatment
strategies for advanced neuroblastoma. Int J Clin Oncol.
17:196–203. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Parsons K, Bernhardt B and Strickland B:
Targeted immunotherapy for high-risk neuroblastoma - the role of
monoclonal antibodies. Ann Pharmacother. 47:210–218. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mackall CL, Merchant MS and Fry TJ:
Immune-based therapies for childhood cancer. Nat Rev Clin Oncol.
11:693–703. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Apetoh L, Ghiringhelli F, Tesniere A,
Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E,
Saulnier P, et al: Toll-like receptor 4-dependent contribution of
the immune system to anticancer chemotherapy and radiotherapy. Nat
Med. 13:1050–1059. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roth MD, Gitlitz BJ, Kiertscher SM, Park
AN, Mendenhall M, Moldawer N and Figlin RA: Granulocyte macrophage
colony-stimulating factor and interleukin 4 enhance the number and
antigen-presenting activity of circulating CD14+ and
CD83+ cells in cancer patients. Cancer Res.
60:1934–1941. 2000.PubMed/NCBI
|
|
16
|
Kiertscher SM, Gitlitz BJ, Figlin RA and
Roth MD: Granulocyte/macrophage-colony stimulating factor and
interleukin-4 expand and activate type-1 dendritic cells (DC1) when
administered in vivo to cancer patients. Int J Cancer. 107:256–261.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Menetrier-Caux C, Thomachot MC, Alberti L,
Montmain G and Blay JY: IL-4 prevents the blockade of dendritic
cell differentiation induced by tumor cells. Cancer Res.
61:3096–3104. 2001.PubMed/NCBI
|
|
18
|
Ashman LK: The immunogenicity of tumour
cells. Immunol Cell Biol. 65:271–277. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Inaba K, Swiggard WJ, Inaba M, Meltzer J,
Mirza A, Sasagawa T, Nussenzweig MC and Steinman RM: Tissue
distribution of the DEC-205 protein that is detected by the
monoclonal antibody NLDC-145. I. Expression on dendritic cells and
other subsets of mouse leukocytes. Cell Immunol. 163:148–156. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dudziak D, Kamphorst AO, Heidkamp GF,
Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW,
Park CG, et al: Differential antigen processing by dendritic cell
subsets in vivo. Science. 315:107–111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Neubert K, Lehmann CH, Heger L, Baranska
A, Staedtler AM, Buchholz VR, Yamazaki S, Heidkamp GF, Eissing N,
Zebroski H, et al: Antigen delivery to
CD11c+CD8− dendritic cells induces protective
immune responses against experimental melanoma in mice in vivo. J
Immunol. 192:5830–5838. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lahoud MH, Ahmet F, Zhang JG, Meuter S,
Policheni AN, Kitsoulis S, Lee CN, O'Keeffe M, Sullivan LC, Brooks
AG, et al: DEC-205 is a cell surface receptor for CpG
oligonucleotides. Proc Natl Acad Sci USA. 109:16270–16275. 2012.
View Article : Google Scholar : PubMed/NCBI
|