Introduction
Liver transplantation (LT) has been performed since
1963 and is now a worldwide procedure (1). LT has a 30-day and 5-year mortality rate
of 5 and 30%, respectively (2).
Abnormalities of the pulmonary vasculature found in association
with coexisting chronic liver disease were first reported in the
1950s (3,4). The pulmonary complications, such as
hepatopulmonary syndrome (HPS) and portopulmonary hypertension
(PoPH), make LT a procedure with more risks. Additionally, these
liver disease complications reduce the chances of LT success,
worsen the overall prognosis of patients and represent a challenge
to the anesthesiologist (5,6). There is limited information with regards
to the epidemiology, pathophysiology and treatment of HPS and PoPH
(1).
HPS and PoPH are associated with pulmonary arterial
hypertension (PAH) and/or liver disease (7). HPS is the most common condition,
identified in 5–30% of cirrhosis patients, manifested by abnormal
oxygenation due to excessive pulmonary microvascular dilation
(8). HPS is a well-recognized cause of
a poorer outcome in the liver disease patient. PoPH is
characterized by the development of PAH in the setting of portal
hypertension with or without hepatic disease (8). PoPH is observed in cirrhotic and
non-cirrhotic portal hypertension and is not associated with the
etiology of liver disease or the severity of portal hypertension.
It is now clear that patients with PoPH or HPS should be properly
diagnosed preoperatively and the severity of the disease should be
graded to initiate the right treatment promptly (1). The proposed algorithm for diagnosing and
grading HPS is based on the hemodynamic characteristics of
pulmonary artery (1,9). However, the mechanical characteristics of
pulmonary artery with HPS and PoPH remain to be elucidated.
Therefore, the aim of the present study was to
investigate the mechanical characteristics of HPS and PoPH in
beagle dog.
Materials and methods
Animals
A total of 30 healthy beagle dogs (12 spayed females
and 18 neutered males; median age, 3.2 years; range, 2.4–6.0;
weight, ~15 kg), were used in the experiment. All the animals were
kept in a clean level condition, with the room temperature
maintained at 20±2°C, relative humidity at 65±10%, diurnal cycle
and free access to standard food and tap water (10). Surgical procedures were performed under
general anesthesia with medetomidine hydrochloride (0.1 ml/kg) and
butorphanol tartrate (0.1 ml/kg), and under local anesthesia with
lidocaine hydrochloride (2% with 1:80,000 epinephrine). Blood
pressure, heart rate, respiratory rate and body temperature were
closely monitored. Penicillin (400 U/g) was administered
intramuscularly 3 consecutive days after the surgery to prevent
infection.
The dogs were assigned to three groups randomly: HPS
model, PoPH model and control. They had daily interaction with
their caregivers and periodic access to outside playgrounds.
Experimental protocols were approved by the Institutional Animal
Care of Baotou Medical College (Inner Mongolia Autonomous Region,
China).
Surgical and experimental
procedures
Chronic common bile duct ligation (CBDL) in the
animal is an established experimental model for human HPS (11). CBDL was performed for HPS model dogs as
described previously (12). Normal
control animals underwent mobilization of the common bile duct
without ligation. Animals were evaluated 2 weeks after CBDL. All
the animals had hepatic histological analysis and measurements of
portal venous pressure and spleen weight (13). Lung tissues were obtained from each
animal.
Sephadex microspheres (G50, 50–150 mm, medium;
Pharmacia Biotech, Freiburg, Germany) were used to establish the
experimental model for human PoPH, as previously described
(10). In brief, 25 g microspheres
were placed in 500 ml of 0.9% saline (number of beads:
1.7×104/ml), and subsequently the mixture was placed
into an autoclave (120°C for 30 min) for disinfection. Following
disinfection, the size of the swollen beads should be 105–310 µm.
The emboli were kept in a 4°C refrigerator. Under an aseptic
condition and through a midline abdominal incision, a catheter
filled with heparin was inserted into the portal vein through the
pancreaticoduodenal vein, with only its tip located in the main
portal vein truck. The outer end was covered with a heparin lock
and it was subsequently placed subcutaneously in the right
hypogastrium (10). The dogs received
intra-portal injection of Sephadex microspheres at a 5-day interval
(10 mg/kg body weight, mixed in 5 ml saline, administered in 30
sec) 6 times in total; while the controls were administered the
same volume of saline in an identical manner. In order to evenly
distribute the microspheres in the liver, the body of the dog was
gently turned whilst injecting the emboli (10). Following every injection, the catheter
was washed with saline and subsequently filled with heparin to
avoid occlusion (10).
Histological examination
The lung specimen of the main pulmonary artery was
collected by thoracotomy subsequent to sacrificing the by
intravenous injection of potassium chloride under anaesthetic. The
specimens were fixed in formalin (10%) and embedded in paraffin,
sectioned (5-mm), stained with hematoxylin and eosin and examined
by light microscopy (10,14).
Hemodynamic characteristics of the
main pulmonary artery
The pulmonary artery pressure (PAP) was measured
using MedLab-U/4cs (Nanjing Medease Co., Ltd., Nanjing, China)
every month after the initial CBDL or injection of the
microspheres. The PAP prior to the CBDL or injection was regarded
as the baseline. The level of the midaxillary line was set as the
zero point (15) and subsequently the
transducer was set to the same level to calibrate the machine prior
to connecting to the catheter. The unit of measurement was mmHg (1
mmHg=0.133 kPa). The diameter and wall thickness of the pulmonary
artery was measured by laparotomy. The velocity of blood flow in
the pulmonary artery (PAV) was measured by the ALC-Ultrasonic blood
flow system (Shanghai Alcott Biotech Co., Ltd., Shanghai, China).
Pulmonary vascular resistance was calculated as PAP/PAV
(mmHg/l/min). The ratio of the wall area and vascular
cross-sectional area (Rwa-csa) of the main pulmonary artery was
measured at the one-third position of the pulmonary valve side
between the pulmonary valve and the bifurcation of pulmonary artery
(16).
Statistical analysis
The measurements are expressed as mean ± standard
deviation. Statistical analysis was performed by SPSS 13.0 (SPSS,
Inc., Chicago, IL, USA) for windows. Data were analyzed with the
two-tailed Student's t-test or analysis of variance with Bonferroni
correction for multiple comparisons between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Pulmonary artery changes subsequent to
CBDL and Sephadex microspheres injection
To assess whether the structure and hemodynamic
characteristics of the pulmonary artery with HPS and PoPH changed,
CBDL was used to establish the HPS model, and Sephadex microspheres
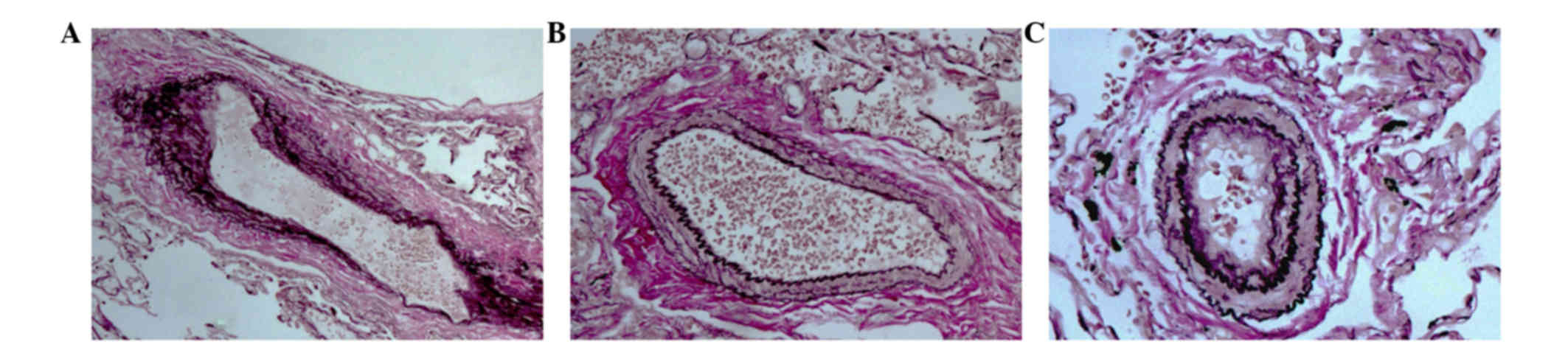
injection was used to establish the PoPH model (Fig. 1). In the HPS model dog, the main
pulmonary artery was enlarged and its wall became thin, the
vascular diameter was larger, and the cross-sectional area was
increased compared to the control (Fig. 1A
and C). By contrast, in the PoPH model dog, the main pulmonary
artery was smaller and its wall became thick, the vascular diameter
was smaller, and the cross-sectional area was decreased compared to
the control (Fig. 1B and C). Thus, HPS
and PoPH had opposing changes in the morphology of the main
pulmonary artery.
Hemodynamic characteristics of the
main pulmonary artery
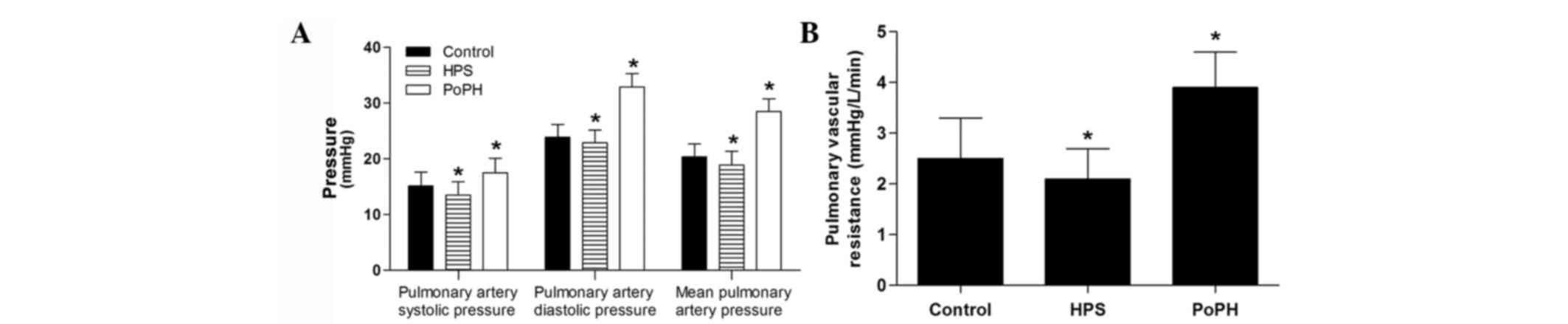
PAP of the control dogs was maintained on the
baseline through the experiment (data not shown). In the HPS group,
the systolic pressure and mean PAP were decreased (Fig. 2A). In the PoPH group, all the systolic
pressure, diastolic pressure, and mean PAP increased (Fig. 2A). The pulmonary vascular resistance in
the HPS group was also decreased, but was enhanced in the PoPH
group (Fig. 2B).
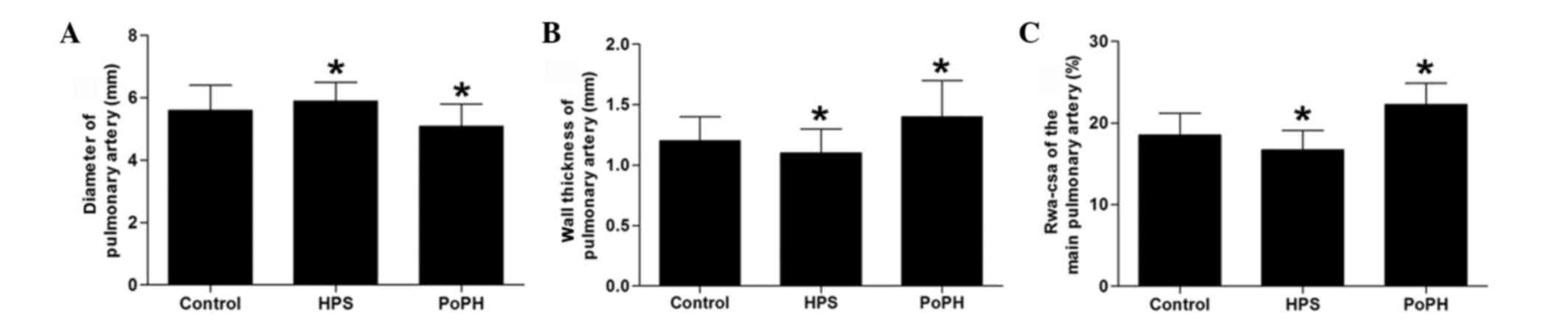
Similar to the aforementioned observations, the
diameter of the main pulmonary artery was enlarged in the HPS model
dog, and was narrowed in the PoPH model dog (Fig. 3A). The wall thickness of the main
pulmonary artery became thin in the HPS group, and thick in the
PoPH group (Fig. 3B). Corresponding to
those changes, the ratio of the wall area and vascular
cross-sectional area (Rwa-csa) was significantly decreased in the
HPS group, and significantly increased in the PoPH group (Fig. 3C).
Discussion
PoPH and HPS are frequent complications of portal
hypertension and cirrhosis (1). In the
present study, the PoPH and HPS models were established using
beagle dogs with methods of CBDL and Sephadex microspheres
injection. Arterial blood was drawn from the femoral artery as
previously described (17). The
alveolar-arterial oxygen gradient was calculated as 150 -
(PaCO2/0.8) - PaO2 (18). When the detection of PaCO2
was <70 mmHg, the HPS model was effective. When there is portal
hypertension, PAP is >25 mmHg, pulmonary vascular resistance
>240 dyn.sec.cm−5 and pulmonary artery occlusion
pressure <15 mmHg, the PoPH model is successful. Subsequently,
the hemodynamic characteristics of PoPH and HPS were
investigated.
PAH is a set of diseases or clinical syndrome with a
high incidence rate and mortality, which is characterized by an
increase in PAP and pulmonary vascular resistance caused by
etiology of vasoconstriction, vascular remodeling and thrombosis
(19). The diagnostic criteria of PAH
presented by the World Health Organization is mean PAP >25 mmHg
in the resting state and >25 mmHg during exercise. When the PAH
is not the correct treatment, it can cause right ventricular
failure with increased volume load, and eventually can lead to
fatality (20). Although the etiology
of PAH has been investigated for >100 years and the quality of
life has improved for certain patients, the pathogenesis of PAH
remains elusive (21,22). Current studies are focusing on the
mechanism of PAH from a novel perspective to identify a promising
target for treatment (23,24). Currently, HPS and PoPH have become
areas of interest worldwide (1,18,25). HPS is a well-recognized cause of
worsened outcome in the liver disease patient, which is defined by
the combination of intrapulmonary vascular dilatation and hypoxemia
in patients with chronic liver disease or portal hypertension
(26). PoPH is the association between
pulmonary hypertension and portal hypertension with or without
hepatic disease (1). The hemodynamic
monitor is particularly useful for the diagnosis of HPS and PoPH,
and for the anesthesiologist in correct decision making (1).
In the present study, HPS and PoPH were further
validated as different complications. In the HPS model dog, the
main pulmonary artery was enlarged and its wall became thin, the
vascular diameter was larger, and the cross-sectional area was
increased when compared to the control (Fig. 1A and C). By contrast, in the PoPH model
dog, the main pulmonary artery was smaller and its wall became
thick, the vascular diameter was smaller, and the cross-sectional
area was decreased when compared to the control (Fig. 1B and C). Therefore, the pulmonary
morphology and hemodynamic properties of HPS and PoPH are changed
in the opposing direction. As HPS and PoPH are observed in the same
patients (27), we could hypothesize
that there is a balance of hemodynamic properties and changes in
the pulmonary artery morphology between HPS and PoPH, which may
decide the development of lung complications. The present findings
renew the traditional view that pulmonary hypertension is due to
the enhanced peripheral resistance.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81160188).
References
|
1
|
Aldenkortt F, Aldenkortt M, Caviezel L,
Waeber JL, Weber A and Schiffer E: Portopulmonary hypertension and
hepatopulmonary syndrome. World J Gastroenterol. 20:8072–8081.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dienstag JL and Cosimi AB: Liver
transplantation - a vision realized. N Engl J Med. 367:1483–1485.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mantz FA Jr and Craige E: Portal axis
thrombosis with spontaneous portacaval shunt and resultant cor
pulmonale. AMA Arch Pathol. 52:91–97. 1951.PubMed/NCBI
|
|
4
|
Hoffbauer FW and Rydell R: Multiple
pulmonary arteriovenous fistulas in juvenile cirrhosis. Am J Med.
21:450–460. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fauconnet P, Klopfenstein CE and Schiffer
E: Hepatopulmonary syndrome: The anaesthetic considerations. Eur J
Anaesthesiol. 30:721–730. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krowka MJ: Management of pulmonary
complications in pretransplant patients. Clin Liver Dis.
15:765–777. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fritz JS, Fallon MB and Kawut SM:
Pulmonary vascular complications of liver disease. Am J Respir Crit
Care Med. 187:133–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Machicao VI, Balakrishnan M and Fallon MB:
Pulmonary complications in chronic liver disease. Hepatology.
59:1627–1637. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Møller S, Henriksen JH and Bendtsen F:
Extrahepatic complications to cirrhosis and portal hypertension:
Haemodynamic and homeostatic aspects. World J Gastroenterol.
20:15499–15517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin W, Deng L, Zhang Q, Lin D, Zhu J, Chen
Y, Chen B and Li J: A canine portal hypertension model induced by
intra-portal administration of Sephadex microsphere. J
Gastroenterol Hepatol. 25:778–785. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang Y, Chen B, Chen Y, Zu B, Yi B and Lu
K: A comparison of two common bile duct ligation methods to
establish hepatopulmonary syndrome animal models. Lab Anim.
49:71–79. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Easter DW, Wade JB and Boyer JL:
Structural integrity of hepatocyte tight junctions. J Cell Biol.
96:745–749. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fallon MB, Abrams GA, Luo B, Hou Z, Dai J
and Ku DD: The role of endothelial nitric oxide synthase in the
pathogenesis of a rat model of hepatopulmonary syndrome.
Gastroenterology. 113:606–614. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang W, Hu B, Wu W, Batra S, Blackburn MR,
Alcorn JL, Fallon MB and Zhang J: Alveolar type II epithelial cell
dysfunction in rat experimental hepatopulmonary syndrome (HPS).
PLoS One. 9:e1134512014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Groszmann RJ, Glickman M, Blei AT, Storer
E and Conn HO: Wedged and free hepatic venous pressure measured
with a balloon catheter. Gastroenterology. 76:253–258.
1979.PubMed/NCBI
|
|
16
|
Kasai H, Sugiura T, Tanabe N, Sakurai Y,
Yahaba M, Matsuura Y, Shigeta A, Kawata N, Sakao S, Kasahara Y, et
al: Electrocardiogram-gated 320-slice multidetector computed
tomography for the measurement of pulmonary arterial distensibility
in chronic thromboembolic pulmonary hypertension. PLoS One.
9:e1115632014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fallon MB, Abrams GA, McGrath JW, Hou Z
and Luo B: Common bile duct ligation in the rat: A model of
intrapulmonary vasodilatation and hepatopulmonary syndrome. Am J
Physiol. 272:G779–G784. 1997.PubMed/NCBI
|
|
18
|
Zhang J, Luo B, Tang L, Wang Y, Stockard
CR, Kadish I, Van Groen T, Grizzle WE, Ponnazhagan S and Fallon MB:
Pulmonary angiogenesis in a rat model of hepatopulmonary syndrome.
Gastroenterology. 136:1070–1080. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chan SY and Loscalzo J: Pathogenic
mechanisms of pulmonary arterial hypertension. J Mol Cell Cardiol.
44:14–30. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stenmark KR and McMurtry IF: Vascular
remodeling versus vasoconstriction in chronic hypoxic pulmonary
hypertension: A time for reappraisal? Circ Res. 97:95–98. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zaiman A, Fijalkowska I, Hassoun PM and
Tuder RM: One hundred years of research in the pathogenesis of
pulmonary hypertension. Am J Respir Cell Mol Biol. 33:425–431.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tabima DM and Chesler NC: The effects of
vasoactivity and hypoxic pulmonary hypertension on extralobar
pulmonary artery biomechanics. J Biomech. 43:1864–1869. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dyer K, Lanning C, Das B, Lee PF, Ivy DD,
Valdes-Cruz L and Shandas R: Noninvasive Doppler tissue measurement
of pulmonary artery compliance in children with pulmonary
hypertension. J Am Soc Echocardiogr. 19:403–412. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rossmann JS: Elastomechanical properties
of bovine veins. J Mech Behav Biomed Mater. 3:210–215. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Singh C and Sager JS: Pulmonary
complications of cirrhosis. Medical Clin North Am. 93871–883.
(viii)2009. View Article : Google Scholar
|
|
26
|
Rodríquez-Roisin R, Krowka MJ, Hervé P and
Fallon MB: ERS (European Respiratory Society) Task Force-PHD
Scientific Committee: Highlights of the ERS Task Force on
pulmonary-hepatic vascular disorders (PHD). J Hepatol. 42:924–927.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chung S, Lee K, Chang SA and Kim DK:
Aggravation of hepatopulmonary syndrome after sildenafil treatment
in a patient with coexisting portopulmonary hypertension. Korean
Circ J. 45:77–80. 2015. View Article : Google Scholar : PubMed/NCBI
|