Introduction
A disintegrin and metalloproteinase with
thombospondin motifs (ADAMTSs) are secreted members of the
zinc-dependent metalloproteinases that contain one or more
thrombospondin type 1 repeats (TSP1) as the ancillary domains.
There are ≥26 ADAMTS proteinases identified thus far; however, not
all of them are known to possess enzymatic activity. The ADAMTS
proteinases are important regulators of cellular events, as the
enzymes have been shown to exhibit a vast array of activities,
including cleavage of pro-collagen and von Willebrand factor (VWF),
tumor suppression and proteolysis activities associated with
arthritis, morphogenesis, angiogenesis and even ovulation [as
reviewed previously (1,2)].
Also known as the VWF-cleaving protease, ADAMTS13 is
noted for its ability in cleaving and reducing the size of the
ultra-large (UL) form of the VWF. Reduction in ADAMTS13 activity
from either hereditary or acquired deficiency causes accumulation
of UL-VWF multimers, platelet aggregation and arterial thrombosis
that leads to fatal thrombotic thrombocytopenic purpura [as
reviewed previously (1,3)]. By contrast, ADAMTS15 is a potential
tumor suppressor. Only a limited number of in-depth investigations
have been carried out on the enzyme; however, expression and
profiling studies have shown that the ADAMTS15 gene is
genetically inactivated in colon cancer and breast carcinoma,
although the exact role of the enzyme remains to be delineated
(4,5).
Structure-wise, ADAMTS13 and 15 share a common
domain organization with the other members of the ADAMTS
proteinases. At the N-terminal of their polypeptides is a
pro-peptide and a metalloproteinase domain in which the catalytic
activity of the proteinases resides. Succeeding the
metalloproteinase domain is a disintegrin-like domain followed by
the first TSP1, a cysteine-rich domain, a spacer domain and 7 more
TSP1 repeats and 2 CUB domains. As with the members of the matrix
metalloproteinase (MMP) and ADAM proteinases, the metalloproteinase
domain of ADAMTS13 bears the typical hallmarks of the reprolysin or
adamalysin proteinases, namely a zinc-binding motif ‘HExxHxxGxxH’
that is essential for substrate turnover. Notably, human ADAMTS15
has a slightly modified zinc-binding motif, ‘HExxNxxGxxH,’ instead
of the usual conserved sequence (2,6,7).
Tissue inhibitor of metalloproteinases (TIMPs) are
the endogenous inhibitors of the MMPs, ADAMs and certain members of
the ADAMTS family, including ADAMTS1, 4 and 5 (8–11). TIMPs are
small proteins of 22–28 kDa in molecular mass. There are 4 human
TIMPs and each has its own distinct profile of metalloproteinase
inhibition. While the activity of TIMPs against the MMPs and ADAMs
has been studied rather extensively, the inhibitory profiles of
TIMPs against the ADAMTS proteinases remain largely unexplored.
To expand the current understanding of the role of
TIMPs in ADAMTS13 and 15 regulation, the enzymes were subjected to
direct inhibition with the full-length and the N-terminal domain
forms of TIMP (N-TIMP)-1, −2, −3 and −4. The responses of the
ADAMTS proteinases to broad spectrum hydroxamate inhibitors,
batimastat (also known as BB-94) and ilomastat (GM6001, galardin),
were also examined. The data show that ADAMTS13 and 15 are not
inhibited by the full-length or the N-TIMP-1 to −4. Notably, while
ADAMTS13 shows little or no inhibition by batimastat and ilomastat,
ADAMTS15 can be effectively inhibited by batimastat with a
Kiapp value of <300 nM.
Materials and methods
Materials
Human ADAMTS5, 13 and 15, full-length TIMP-3 and the
recombinant human aggrecan substrate G1-IGD-G2 were all products of
R&D Systems (Minneapolis, MN, USA). Full-length TIMP-1, −2 and
−4, as well as N-TIMP-1 to −4 were produced in house either from
Sf9 insect cells or Escherichia coli inclusion bodies, as
described previously (12–15). The fluorescence-quenching peptide for
the ADAMTS13 assay, FRETS-VWF73 [DRE-Dap(Nma)-APN LVY MVTG-Dpa- PAS
DEI KRL PGD IQV VPI GVG PNA NVQ ELE RIG WPN API LIQ DFE TLP REA PDL
VLQR], was purchased from AnaSpec, Inc. (Fremont, CA, USA). The
hydroxamate inhibitors batimastat (BB-94) and ilomastat (GM6001,
galardin) were purchased from Selleck Chemicals Inc. (Houston, TX,
USA). All the chemicals and reagents were products of Thermo
Scientific (Rockford, IL, USA) unless stated otherwise.
Inhibition studies of ADAMTS5 and 15
with the full-length form and N-TIMPs using recombinant human
aggrecan G1-IGD-G2 domains
The procedure for the assay was modified from the
one designed for ADAMTS4 by Hashimoto et al (16). In brief, ADAMTS5 and 15 (≤20 nM) were
pre-incubated with TIMPs (various concentrations, ≤1 µM for certain
N-TIMPs) or hydroxamate inhibitors in FAB buffer [10 mmol/l
CaCl2, 50 mmol/l Tris-HCl (pH 7.5), 0.05% Brij-35, 1%
dimethyl sulfoxide and 0.02% NaN3] in a total volume of
20 µl for 1 h at room temperature before 2 µg of recombinant human
aggrecan domains G1-IGD-G2 was added to initiate digestion.
Digestion occurred for 4 h at 37°C before the samples were analyzed
on an 8% reducing SDS-PAGE followed by staining in 0.2% Coomassie
Brilliant Blue. The Kiapp value of the
inhibitors was calculated based on densitometric analysis of the
130 kDa undigested G1-IGD-G2 bands using ImageJ software developed
at the National Institutes of Health (Bethesda, MD, USA).
Inhibition studies of ADAMTS13 with
the full-length form and N-TIMPs using VWF73 fluorescence-quenching
peptide
The assay for ADAMTS13 was carried out in 2 ml
cuvettes in Na acetate buffer [25 mmol/l Na acetate, 50 mmol/l
NaCl, 20 mmol/l CaCl2 and 0.05% Brij (pH 6.0)] in a
manner similar to the protocol developed for evaluating the
Kiapp of ADAM17 and MMPs (12,17). To
measure the inhibitory activity of TIMPs on ADAMTS13, ≤2 nM of
ADAMTS was pre-incubated with increasing concentrations of TIMPs
(≤300 nM) in a total volume of 2 ml for 1 h at room temperature.
Reactions were initiated by adding 2.5 µM of VWF73
fluorescence-quenching peptide and the activity of the enzyme was
followed for ≥45 min using a LS55 spectrofluorometer (Perkin-Elmer,
Waltham, MA, USA) pre-warmed to 30°C.
The Kiapp values of
TIMPs were calculated by plotting the steady-state rates against
TIMP concentrations with GraphPad Prism software using the Morrison
equation: Vs =
(V0/2Et) ×
[(Et - It -
Kiapp) +
{(Kiapp + It -
Et)2 +
4EtKiapp]1/2}, where
V0 denotes the rate of reaction in the absence of
inhibitor, Et is the total enzyme concentration
and It is the total inhibitor concentration.
Results
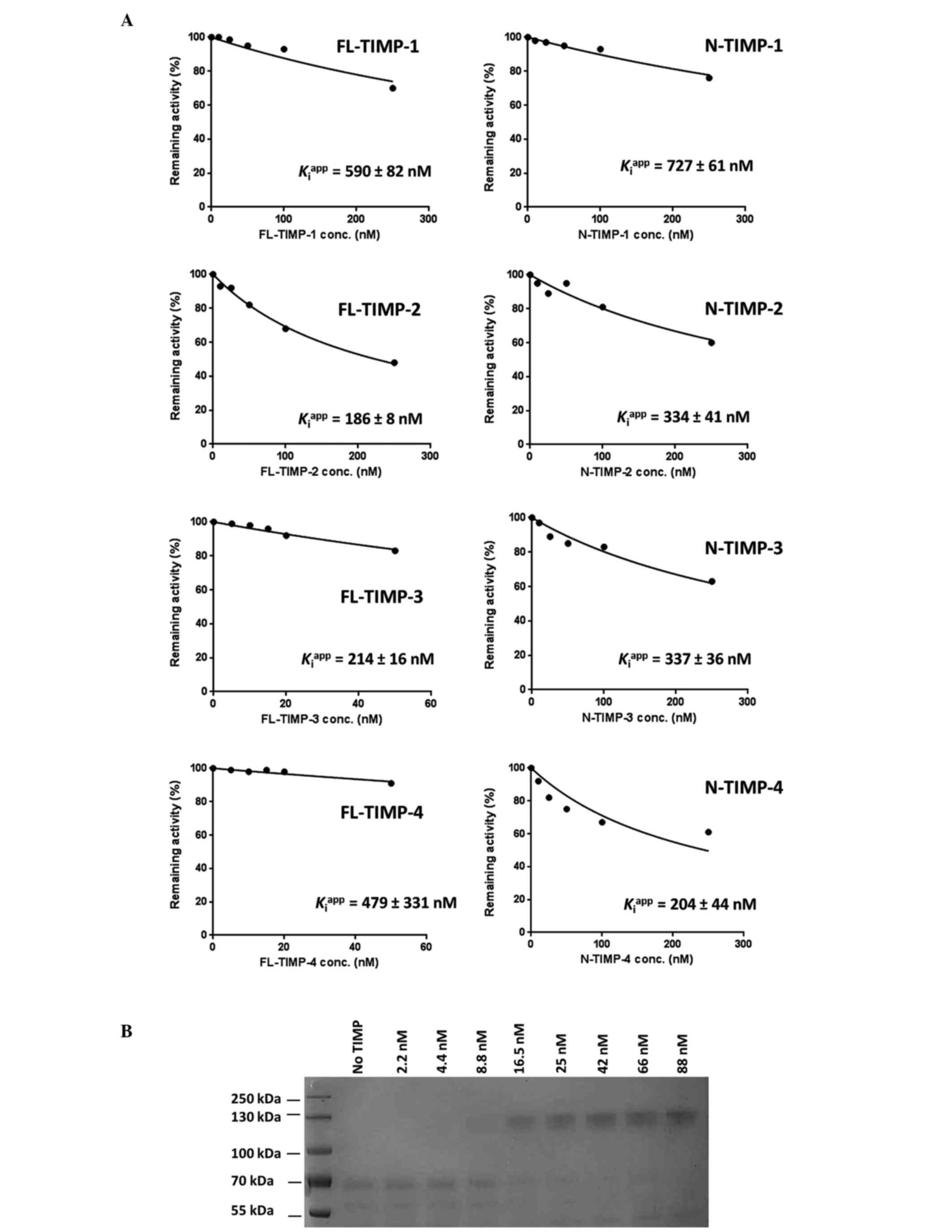
ADAMTS13 is not inhibited by the
full-length form or N-TIMP-1, −2, −3 or −4
Fig. 1A summarizes the
inhibition patterns of full-length and N-TIMP-1, −2, −3 and −4
against ADAMTS13. As shown, only full-length TIMP-2, full-length
TIMP-3 and N-TIMP-4 exhibited a notable degree of inhibitory
activity against the enzyme. The effects of other TIMPs were either
minimal or negligible. Overall, the
Kiapp values of the majority of the
TIMPs were >200 nM. Fig. 1A also
shows that the Kiapp value of the most
potent TIMP for ADAMTS13, namely full-length TIMP-2, was 186 ± 8
nM. The value is well above the normal range of affinity for a
tight-binding inhibitor, which is typically in the low nM to pM
range, as in the cases of TIMP-3 and ADAMTS4 and 5 (9,16).
To illustrate the contrasting effect of the TIMPs,
in particular TIMP-3, on other ADAMTS proteinases, another set of
inhibition studies was performed using ADAMTS5 (also known as
aggrecanase-2) as the subject. As shown in Fig. 1B, full-length TIMP-3 was a potent
inhibitor against the proteinase. At 25 nM [enzyme:inhibitor (E:I)
ratio, 1:1], full-length TIMP-3 almost completely inhibited the
ability of ADAMTS5 to digest the aggrecan substrate G1-IGD-G2
(Kiapp <2 nM), similar to the
results of a previous study (9).
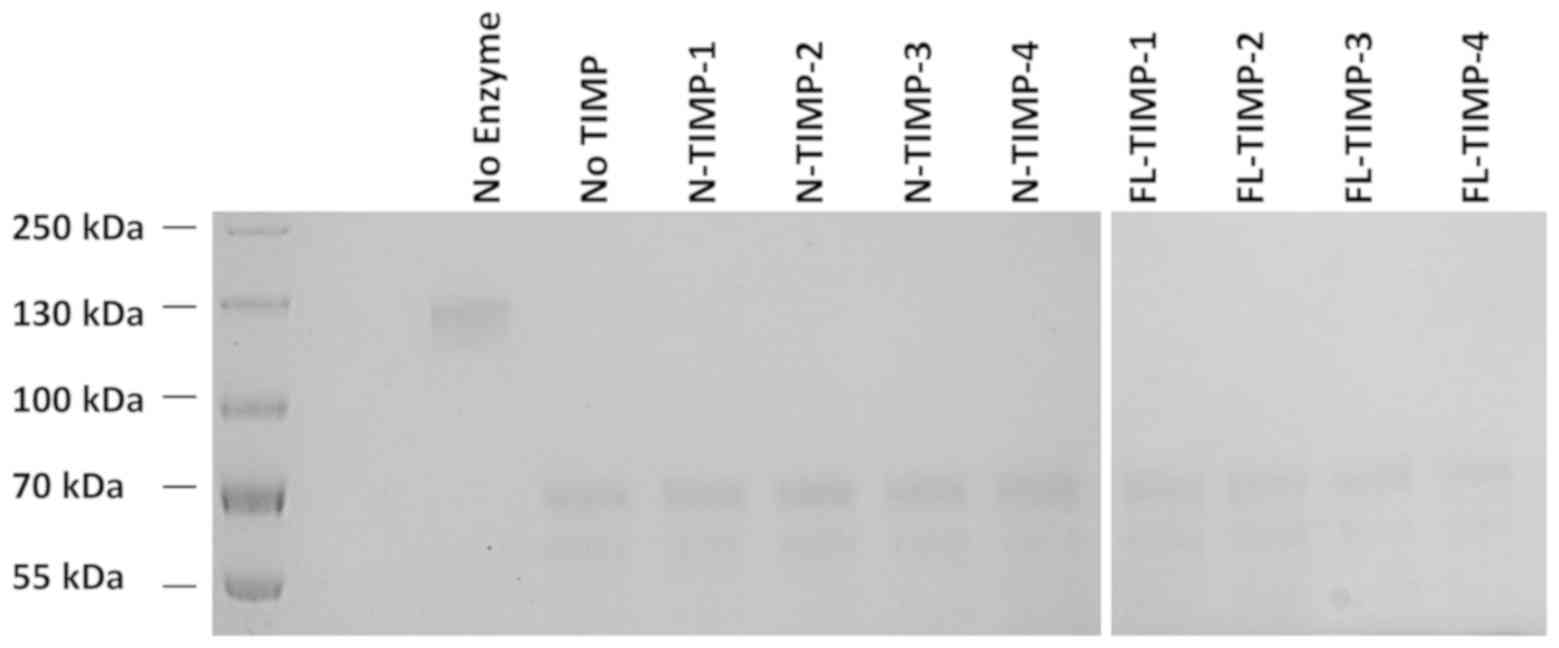
ADAMTS15 is not inhibited by the
full-length form or N-TIMP-1, −2, −3 or −4
Due to the lack of a suitable fluorescence-quenching
peptide substrate, the catalytic activity of ADAMTS15 was evaluated
by its ability to cleave the 130 kDa recombinant human aggrecan
G1-IGD-G2 domains into 70- and 55-kDa fragments. Fig. 2 shows that despite pre-incubation with
an excessive dose of the full-length or N-TIMPs (E:I, ~1:50), none
of the TIMPs exhibited any sign of inhibition against the enzyme.
Switching the incubation condition to a neutral one (pH 7.4) with
FAB buffer yielded the same outcome. Therefore, the enzymatic
activity of ADAMTS15 was not inhibited by any of the TIMPs.
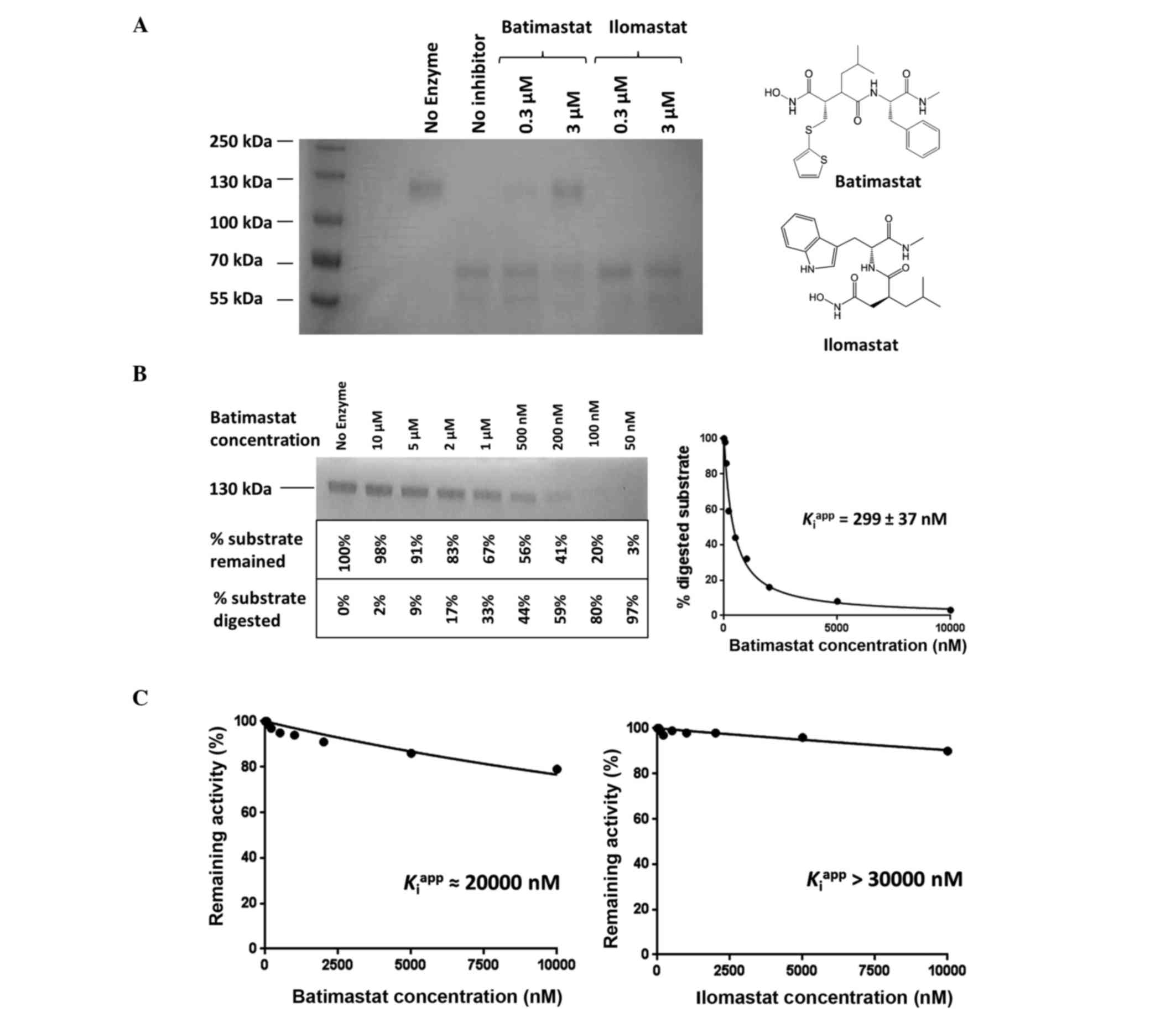
Inhibition of ADAMTS13 and 15 by broad
spectrum MMP inhibitors batimastat and ilomastat
To understand whether ADAMTS13 and 15 were sensitive
to hydroxamate-based small molecule inhibitors, the proteinases
were subjected to two doses (0.3 and 3 µM) of batimastat and
ilomastat. The results are summarized in Fig. 3A. As shown, batimastat was able to
partially inhibit ADAMTS15 at 300 nM. As the concentration of
batimastat was increased to 3 µM, the effect of the inhibitor
became even more pronounced. By contrast, ilomastat has no
noticeable activity even at the high dose of 3 µM. To quantify the
affinity of batimastat for ADAMTS15, further investigation was
carried out to examine the concentration effect. Fig. 3B illustrates how the
Kiapp value of batimastat was derived
by densitometric analysis of the undigested G1-IGD-G2 bands. Under
the assay conditions described in the present study, the
Kiapp of batimastat was determined to
be 299±37 nM, which is the indication of a good inhibitor for a
small hydroxamate molecule. Despite the potency of batimastat on
ADAMTS15, neither batimastat nor ilomastat showed any signs of
activity on ADAMTS13. The inhibition curves of batimastat and
ilomastat for ADAMTS13 are shown in Fig.
3C.
Discussion
The present findings are significant for two
reasons. First and foremost, the data suggest that the topology of
the catalytic clefts of ADAMTS13 and 15 is likely to be markedly
different from those of the MMPs and ADAMs. Among the ADAMTS
proteinases isolated to date, ADAMTS1, 4 (aggrecanase-1) and 5
(aggrecanase-2) are known to be responsive to the inhibitory
activity of the TIMPs, in particular TIMP-3 (8,9). The fact
that TIMP-3 is able to inhibit ADAMTS1, 4 and 5, as well as the
majority of the MMPs and ADAMs, hinted a structural similarity
among the active sites of these enzymes. Subsequent delineation of
the metalloproteinase domains of ADAMTS1, 4 and 5 by X-ray
crystallography revealed an overall structural similarity to those
of the MMP and ADAM proteinases (PDB 3Q2G; 2RJP; and 2RJQ)
(18,19). The inability of the TIMPs to form a
tight binary complex with ADAMTS13 and 15 indicates that the 2
ADAMTS proteinases may have a significantly different active site
configuration from those of the MMPs, ADAMs, and ADAMTS1, 4 and
5.
Furthermore, the present findings also confirm the
notion that TIMPs are not part of the regulatory mechanisms that
modulate ADAMTS13 and 15 activities. Thus far, ADAMTS13 has been
shown to be susceptible to proteolysis by thrombin and furin, as
well as inhibitory autoantibodies (20–24). These
findings, taken together with the results of the present study,
suggest that TIMPs have no role to play in its regulation. In
comparison to ADAMTS13, much less is known with regards to how
ADAMTS15 is regulated at present, as there have been extremely few
biochemical studies on the enzyme. As shown in the present study,
none of the four native TIMPs had the true ability to inhibit the
proteinase. Therefore, future studies should uncover the
physiological regulator of ADAMTS15.
Another aspect that warrants further investigation
is the discrepancy in potency between batimastat and ilomastat in
ADAMTS15 inhibition. The two are small molecules that inhibit a
wide range of MMPs and ADAM proteinases; however, only batimastat
is able to bind to ADAMTS15. Without a crystal structure of the
ADAMTS15 metalloproteinase domain, it is difficult to speculate the
reason for the discrepancy. The inhibitor could, however, be of use
in protein stabilization if the metalloproteinase domain of
ADAMTS15 is crystalized in the future. In conclusion, the present
data confirm that ADAMTS13 and 15 are not the subjects of a typical
metalloproteinase regulatory mechanism in which TIMPs have a
central and indispensable role.
Acknowledgements
The authors thank the National Natural Science
Foundation of China (grant no. 31270850) and the Research
Development Fund, XJTLU, for their generous funding of the present
study.
Glossary
Abbreviations
Abbreviations:
|
ADAM
|
a disintegrin and
metalloproteinase
|
|
ADAMTS
|
ADAM with thombospondin type 1
motifs
|
|
E:I
|
enzyme:inhibitor
|
|
MMP
|
matrix metalloproteinase
|
|
TIMP
|
tissue inhibitors of
metalloproteinase
|
|
N-TIMP
|
N-terminal domain of TIMP
|
References
|
1
|
Dubail J and Apte SS: Insights on ADAMTS
proteases and ADAMTS-like proteins from mammalian genetics. Matrix
Biol. 44(46C): 24–37. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cal S, Obaya AJ, Llamazares M, Garabaya C,
Quesada V and López-Otín C: Cloning, expression analysis, and
structural characterization of seven novel human ADAMTSs, a family
of metalloproteinases with disintegrin and thrombospondin-1
domains. Gene. 283:49–62. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lancellotti S and De Cristofaro R:
Structure and proteolytic properties of ADAMTS13, a metalloprotease
involved in the pathogenesis of thrombotic microangiopathies. Prog
Mol Biol Transl Sci. 99:105–144. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Viloria CG, Obaya AJ, Moncada-Pazos A,
Llamazares M, Astudillo A, Capellá G, Cal S and López-Otín C:
Genetic inactivation of ADAMTS15 metalloprotease in human
colorectal cancer. Cancer Res. 69:4926–4934. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Porter S, Span PN, Sweep FC, Tjan-Heijnen
VC, Pennington CJ, Pedersen TX, Johnsen M, Lund LR, Rømer J and
Edwards DR: ADAMTS8 and ADAMTS15 expression predicts survival in
human breast carcinoma. Int J Cancer. 118:1241–1247. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng XL: Structure-function and
regulation of ADAMTS-13 protease. J Thromb Haemost. 11(Suppl 1):
11–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dancevic CM, Fraser FW, Smith AD, Stupka
N, Ward AC and McCulloch DR: Biosynthesis and expression of a
disintegrin-like and metalloproteinase domain with thrombospondin-1
repeats-15: A novel versican-cleaving proteoglycanase. J Biol Chem.
288:37267–37276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rodríguez-Manzaneque JC, Westling J, Thai
SN, Luque A, Knauper V, Murphy G, Sandy JD and Iruela-Arispe ML:
ADAMTS1 cleaves aggrecan at multiple sites and is differentially
inhibited by metalloproteinase inhibitors. Biochem Biophys Res
Commun. 293:501–508. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kashiwagi M, Tortorella M, Nagase H and
Brew K: TIMP-3 is a potent inhibitor of aggrecanase 1 (ADAM-TS4)
and aggrecanase 2 (ADAM-TS5). J Biol Chem. 276:12501–12504. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Amour A, Slocombe PM, Webster A, Butler M,
Knight CG, Smith BJ, Stephens PE, Shelley C, Hutton M, Knäuper V,
et al: TNF-alpha converting enzyme (TACE) is inhibited by TIMP-3.
FEBS Lett. 435:39–44. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Amour A, Knight CG, Webster A, Slocombe
PM, Stephens PE, Knäuper V, Docherty AJ and Murphy G: The in vitro
activity of ADAM-10 is inhibited by TIMP-1 and TIMP-3. FEBS Lett.
473:275–279. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee MH, Verma V, Maskos K, Nath D, Knäuper
V, Dodds P, Amour A and Murphy G: Engineering N-terminal domain of
tissue inhibitor of metalloproteinase (TIMP)-3 to be a better
inhibitor against tumour necrosis factor-alpha-converting enzyme.
Biochem J. 364:227–234. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee MH, Verma V, Maskos K, Becherer JD,
Knäuper V, Dodds P, Amour A and Murphy G: The C-terminal domains of
TACE weaken the inhibitory action of N-TIMP-3. FEBS Lett.
520:102–106. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee MH, Rapti M and Murphy G: Delineating
the molecular basis of the inactivity of tissue inhibitor of
metalloproteinase-2 against tumor necrosis factor-alpha-converting
enzyme. J Biol Chem. 279:45121–45129. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee MH, Rapti M and Murphy G: Total
conversion of tissue inhibitor of metalloproteinase (TIMP) for
specific metalloproteinase targeting: Fine-tuning TIMP-4 for
optimal inhibition of tumor necrosis factor-α-converting enzyme. J
Biol Chem. 280:15967–15975. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hashimoto G, Aoki T, Nakamura H, Tanzawa K
and Okada Y: Inhibition of ADAMTS4 (aggrecanase-1) by tissue
inhibitors of metalloproteinases (TIMP-1, 2, 3 and 4). FEBS Lett.
494:192–195. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee MH, Rapti M and Murphy G: Unveiling
the surface epitopes that render tissue inhibitor of
metalloproteinase-1 inactive against membrane type 1-matrix
metalloproteinase. J Biol Chem. 278:40224–40230. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gerhardt S, Hassall G, Hawtin P, et al:
Crystal structures of human ADAMTS-1 reveal a conserved catalytic
domain and a disintegrin-like domain with a fold homologous to
cysteine-rich domains. J Mol Biol. 373:891–902. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mosyak L, Georgiadis K, Shane T, Svenson
K, Hebert T, McDonagh T, Mackie S, Olland S, Lin L, Zhong X, et al:
Crystal structures of the two major aggrecan degrading enzymes,
ADAMTS4 and ADAMTS5. Protein Sci. 17:16–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grillberger R, Gruber B, Skalicky S,
Schrenk G, Knöbl P, Plaimauer B, Turecek PL, Scheiflinger F and
Rottensteiner H: A novel flow-based assay reveals discrepancies in
ADAMTS-13 inhibitor assessment as compared with a conventional
clinical static assay. J Thromb Haemost. 12:1523–1532. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feys HB, Vandeputte N, Palla R, Peyvandi
F, Peerlinck K, Deckmyn H, Lijnen HR and Vanhoorelbeke K:
Inactivation of ADAMTS13 by plasmin as a potential cause of
thrombotic thrombocytopenic purpura. J Thromb Haemost. 8:2053–2062.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Doldan-Silvero A, Acevedo-Gadea C, Habib
C, Freeman J and Johari V: ADAMTS13 activity and inhibitor. Am J
Hematol. 83:811–814. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lam JK, Chion CK, Zanardelli S, Lane DA
and Crawley JT: Further characterization of ADAMTS-13 inactivation
by thrombin. J Thromb Haemost. 5:1010–1018. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Crawley JT, Lam JK, Rance JB, Mollica LR,
O'Donnell JS and Lane DA: Proteolytic inactivation of ADAMTS13 by
thrombin and plasmin. Blood. 105:1085–1093. 2005. View Article : Google Scholar : PubMed/NCBI
|