Introduction
Treatment with γδ T cells is one of the commonly
used cellular adoptive immunotherapies. The γδ T cells were
recognized as one special type of cell that is between innate and
adaptive immune cells (1). The cells
mainly locate at the mucosae of the respiratory tracts, intestine
and urogenital tract and subcutaneous tissues. The majority of the
γδ T cells do not express cluster of differentiation 4 (CD4) or
CD8. They can specifically recognize antigens in a major
histocompatibility complex-nonrestricted manner, playing an
important role in the defense of the body against infection,
autoimmune disease and antitumor processes (2). γδ T cells are only 0.15–5% of the
peripheral blood T lymph cells, and therefore, the γδ T cells that
are used in clinical cellular adoptive immunotherapies have to be
large-scale cultured in vitro. However, the biological
features of in vitro cultured γδ T cells are rarely
reported. Currently, numerous studies of the features of clinically
used cytokine-induced killer (CIK) cells have been reported.
Rettinger et al (3) found that
the maximum specific growth rate of CIK was achieved on day 10 and
the cell number began to decrease on day 30. The proliferation rate
and activity of the cells were closely associated with the cell
number and cell density, and the proliferation of CIK cells is time
restricted. The study of Yang et al (4) showed that extended culture time can
increase the cytotoxic activity of CIK cells; ~28-day culture time
is the optimum time for CIK cell culture, increasing the effective
cell number and significantly increasing the killing effect against
tumor cells. However, the growth features of the cells reported in
these studies are not consistent. Additionally, there are
significant differences between types of immune cells. Therefore,
the present study examined the biological features of in
vitro cultured γδ T cells, comparing the growth rates,
phenotype, secreted cytokines and killing activity of the γδ T
cells cultured for different times. The optimal status of the in
vitro cultured γδ T cells was investigated, which provides
information for clinical treatment to determine the optimal culture
time and the number of reinfused cells.
Materials and methods
Materials
Fetal bovine serum (FBS), trypsin, RPMI-1640 medium
and OpTmizer medium were purchased from Gibco (Grand Island, NY,
USA). Gastric cancer SGC-901 cells were purchased from SIBCB (CAS,
Shanghai, China). Zoledronate was purchased from Novartis Pharma
Schweiz (AG, Rotkreuz, Switzerland) and interleukin (IL)-2 was
purchased from Beijing SL Pharmaceutical Co., Ltd. (Beijing,
China). Lymphocyte Separation Medium was purchased from PAA
Laboratories (Linz, Austria), phorbol 12-myristate 13-acetate (PMA)
and ionomycin were purchased from Sigma Chemical Co. (St. Louis,
MO, USA), and brefeldin A (BFA) was purchased from eBioscience Inc.
(San Diego, CA, USA). PE-conjugated anti-T-cell receptor (TCR)-γδ
antibody (347907), APC-conjugated anti-human CD3 (555342),
FITC-conjugated interferon (IFN)-γ (554551), APC-conjugated tumor
necrosis factor (TNF)-α (554514) and the Cytofix/Cytoperm™
Fixation/Permeabilization kit were purchased from BD Biosciences
(Ann Arbor, MI, USA). Cell counting kit-8 (CCK-8) was purchased
from Beyotime Intitute of Biotechnology (Shanghai, China). The
automated cell counter Countess® was purchased from Invitrogen/Life
Technologies (Carlsbad, CA, USA). The Thermo MK3 microplate reader
was purchased from Thermo Scientific (Rockford, IL, USA) and the BD
Accuri C6 cytometer was purchased from BD Biosciences.
Culture of human γδ T cells
Peripheral blood (100 ml) was taken from 4 healthy
volunteers who provided written informed consent. Ethical approval
was provided from the Ethics Committee from the Department of
Oncology, Tianjin Union Medical Center (Tianjin, China).
Mononuclear cells were separated and washed with normal saline 3
times. Subsequently, the cells were suspended with OpTmizer medium
supplied with 1.33 µl zoledronic acid and 1,000 U/ml IL-2. The
culture of cells was maintained in a humidified incubator at 37°C
and 5% CO2. Cell numbers were counted every 3–4 days by
an automated cell counter and adjusted to 1×106
cells/ml. The multiplication factor and growth rate were
calculated.
Detection of the phenotypes and
cytokines secreted by the cultured γδ T cells
The cells were harvested on days 7, 10, 14 and 17.
The cells were washed with PBS and adjusted to 1×106
cells/ml and incubated with the PE-conjugated anti-TCR-γδ antibody
and APC-conjugated anti-human CD3 antibody. Phenotypes were
analyzed by flow cytometry. The γδ T cells were supplied with 20
ng/ml PMA, 0.5 µg/ml ionomycin and 3 µg/ml BFA, and were
subsequently cultured in a humidified incubator at 37°C and 5%
CO2 for 6 h. Cells were harvested and incubated with
fluorescein-conjugated antibody at 4°C in the dark for 20 min, and
were washed with PBS twice. The cells were resuspended by
permeabilization buffer and incubated at 4°C for 1 h. The cells
were washed with wash buffer twice and incubated with
fluorescein-conjugated antibody at 4°C in the dark for 20 min.
Cells were subsequently resuspended with 1% paraformaldehyde
dissolved in PBS following washing with wash buffer twice.
Cytokines secreted by γδ T cells were analyzed by flow
cytometry.
Detection of killing activity of γδ T
cells against SGC-7901 cells by the CCK-8 kit
SGC-7901 cells in the exponential phase were
harvested and washed with RPMI-1640 medium supplied with 5% FBS and
adjusted to 5×104 cells/ml, serving as the target cells.
γδ T cells were harvested on days 7, 10, 14 and 17. The cells were
washed with RPMI-1640 supplied with 5% FBS and were diluted to
1×106, 5×105, 2.5×105,
5×104 and 2.5×104 cells/ml, serving as the
effector cells. Subsequently, the effector cells and the target
cells were mixed with ratios (E:T) of 20:1, 10:1, 5:1, 1:1 or 1:2
in triplicate. The cell mixtures were cultured in a humidified
incubator at 37°C and 5% CO2 for 24 h. The killing
activities were detected by the CCK-8 kit and Thermo MK3 microplate
reader at 450 nm.
Statistical analysis
Data were analyzed by SPSS 19.0 (IBM, Corp., Armonk,
NY, USA) using a pair-matching t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
In vitro growth of γδ T cells at
different time-points
The γδ T cells cultured in OpTmizer medium were
counted on days 0, 4, 7, 10, 14 and 17 by an automated cell
counter. On days 7–14, the cells entered the exponential phase. On
day 14, the maximum multiplication factor (10.146±0.252) was
observed. The proliferation rate decreased following day 14.
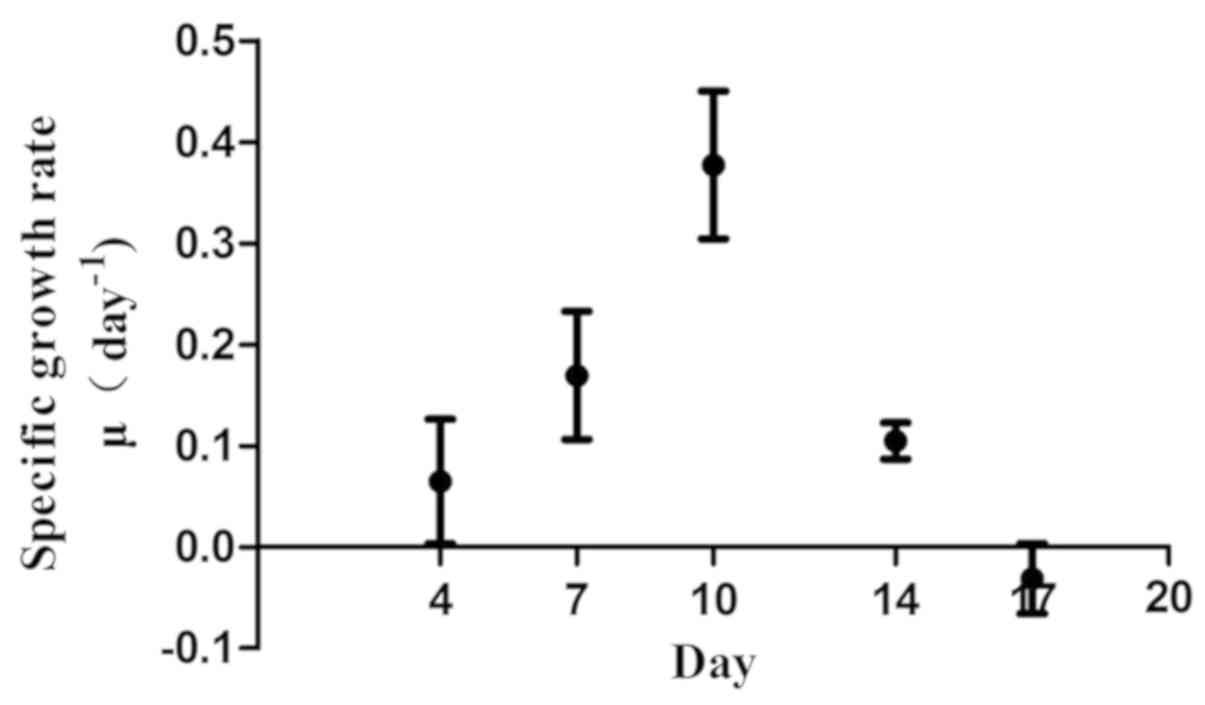
Specific growth rate µ reached a maximum (0.377±0.073) on day 10
and subsequently decreased. On day 17, a negative specific growth
rate was observed (Figs. 1 and
2).
Phenotypes of γδ T cells on different
days
The γδ T cells were harvested on days 7, 10, 14 and
17. Phenotypes of these cells were analyzed by the C6 cytometer.
High percentages (61.2±0.052 to 67.4±0.055%) of γδ T cells were
acquired at these points in time, without a significant difference
(Figs. 3 and 4).
Cytokines secreted by γδ T cells at
different points in time
The γδ T cells were harvested on days 7, 10, 14 and
17. The secretion of IFN-γ and TNF-α by the cells was analyzed by
the C6 cytometer. The amount of IFN-γ and TNF-α increased gradually
from day 7 to 14 and reached the peak on day 14. Following this,
IFN-γ and TNF-α decreased gradually. Compared with day 7, the
percentages of IFN-γ and TNF-α secreted by the γδ T cells were
significantly higher (P<0.05) (Figs.
5 and 6).
Killing activities of γδ T cells at
different points in time
The γδ T cells were harvested on days 7, 10, 14 and
17. Subsequently, they were mixed with SGC-7901 with the ratios of
20:1, 10:1, 5:1, 1:1, or 1:2, and the killing activities of the γδ
T cells were analyzed. The results showed that the killing
activities of γδ T cells harvested on days 7, 10 and 14 were
efficient; however, there was no significant difference
(P>0.05). On day 17, the killing activities of the γδ T cells
against SGC-7901 with the ratios of 10:1 and 5:1 decreased. On days
7, 10 and 14, the killing activities of γδ T cells with the ratios
of 20:1, 10:1, 5:1 and 1:1 were higher than that of the cells with
the ratios of 1:1 and 1:2. On day 17, the killing activities of γδ
T cells with the ratios of 20:1 and 10:1 were higher than that of
the cells with the ratios of 5:1, 1:1 and 1:2 (Fig. 7; Tables I
and II).
 | Table I.Killing activity of γδ T cells
cultured with different E:T ratios to SGC-7901 cells on different
days. |
Table I.
Killing activity of γδ T cells
cultured with different E:T ratios to SGC-7901 cells on different
days.
| E:T ratio | n | Day 7 | Day 10 | Day 14 | Day 17 |
|---|
| 20:1 | 4 | 91.7±4.9a,b | 94.6±1.1a,b | 97.0±1.5a–c | 90.7±1.6a–c |
| 10:1 | 4 | 93.8±1.9a,b | 96.9±1.6a,b | 90.8±3.4a,b | 80.9±2.5a–f |
| 5:1 | 4 | 92.7±2.9a,b | 91.9±3.6a,b | 83.1±4.2a,b | 73.1±2.4d,e |
| 1:1 | 4 | 73.8±2.7 | 70.1±2.0 | 68.4±2.0 | 67.9±1.7 |
| 1:2 | 4 | 66.3±2.3 | 63.3±1.6 | 65.5±2.2 | 60.9±2.7 |
Discussion
Since the discovery of the γ receptor and δ receptor
of the T cells in the 1980s, the roles of γδ T cells in
immunoregulation and immunosurveillance have been identified
(5,6).
Viey et al (7) found that the
cultured γδ T cells were able to kill tumor cells. Currently,
research of γδ T cells in the immunotherapy against tumors has
increased (8–10). However, the biological features of
in vitro cultured γδ T cells are rarely reported. In
addition, understanding the state, phenotypes, secreted cytokines
and killing activities will provide information for further study
and clinical treatment.
Specific growth rate (µ) is one important index that
indicates the dynamics of cell growth (11). In the present study, cell number
reached the peak on day 14. However, the maximum specific growth
rate (µmax) was achieved on day 10. This indicated that although
the total number of the cells increased, the growth rate decreased
after day 10. On day 17, the specific growth rate (µ) became
negative and the total number of γδ T cells began to decrease.
The main effector cells of γδ T cells are the cells
with the phenotype of CD3+TCRγδ+. These cells
can express multiple cytokines following antigen stimulation,
particularly Th1 cytokines (12), such
as IFN-γ and TNF-α. In the present study, cytokines were analyzed
by the C6 cytometer on different days. High percentages of γδ T
cells were acquired on days 7, 10, 14 and 17 without a significant
difference. Cytokines were also analyzed by the C6 cytometer, and
the quantities of the cytokines secreted by the γδ T cells
gradually increased from days 7 to 14 and reached the peak on day
14. Following this the quantities of the cytokines decreased.
To study the killing activities of the cells in
vitro, γδ T cells that were cultured for different days were
mixed with SGC-7901 cells in different ratios. Compared with the
cells cultured for 17 days, the γδ T cells that were in the
exponential phase (days 7 to 14) showed higher killing activities.
A more efficient effect occurred when the ratio was >5:1, which
indicated that reinfused γδ T cells should be increased if
possible.
In conclusion, it is suitable to reinfuse the γδ T
cells in their exponential phase (days 7 to 14). The optimum time
for reinfusion is ~day 10 and the ratio should be >5:1.
References
|
1
|
Holtmeier W and Kabelitz D: Gammadelta T
cells link innate and adaptive immune responses. Chem Immunol
Allergy. 86:151–183. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carding SR and Egan PJ: Gammadelta T
cells: Functional plasticity and heterogeneity. Nat Rev Immunol.
2:336–345. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rettinger E, Meyer V, Kreyenberg H, et al:
Cytotoxic capacity of IL-15-stimulated cytokine-induced killer
cells against human acute myeloid leukemia and rhabdomyosarcoma in
humanized preclinical mouse model. Front Oncol. 2:322012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang K, Zhao Y, Gao X, et al: Generation
of CIK cells under different expansion time and their biological
properties. China Medical Herald. 10:12–14. 2013.
|
|
5
|
Beetz S, Marischen L, Kabelitz D and Wesch
D: Human gamma delta T cells: Candidates for the development of
immunotherapeutic strategies. Immunol Res. 37:97–111. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cao Y: Antitumor actions of γδT
lymphocytes. Int J Immunol. 29:357–360. 2006.
|
|
7
|
Viey E, Fromont G, Escudier B, Morel Y, Da
Rocha S, Chouaib S and Caignard A: Phosphostim-activated gamma
delta T cells kill autologous metastatic renal cell carcinoma. J
Immunol. 174:1338–1347. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Caccamo N, Meraviglia S, Scarpa F, La
Mendola C, Santini D, Bonanno CT, Misiano G, Dieli F and Salerno A:
Aminobisphosphonate-activated gammadelta T cells in immunotherapy
of cancer: Doubts no more. Expert Opin Biol Ther. 8:875–883. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kabelitz D, Wesch D and He W: Perspectives
of gammadelta T cells in tumor immunology. Cancer Res. 67:5–8.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoshida Y, Nakajima J, Wada H and Kakimi
K: γδ T-cell immunotherapy for lung cancer. Surg Today. 41:606–611.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li D, Shuxiang Z, Zhu M, et al: Effects of
glucose and glutamine concentration on the growth and metabolism of
hybridoma cells. J East China Univ Sci Technol. 29:359–362.
2003.
|
|
12
|
Gao Y, Yang W, Pan M, Scully E, Girardi M,
Augenlicht LH, Craft J and Yin Z: Gamma delta T cells provide an
early source of interferon gamma in tumor immunity. J Exp Med.
198:433–442. 2003. View Article : Google Scholar : PubMed/NCBI
|