Introduction
Diabetes mellitus is a metabolic disease in which
blood sugar concentrations are high over a prolonged period.
Without proper treatment, diabetes mellitus can trigger a number of
complications, including chronic kidney failure (1). Diabetic nephropathy is a critical event
in end-stage renal failure worldwide. Its morphological features
include glomerular hypertrophy, mesangial expansion, basement
membrane thickening, interstitial fibrosis, tubular atrophy and
arteriolar thickening (2). Numerous
studies indicate that oxidative stress is a typical factor
associated with the main pathways involved in the development and
progression of diabetic microvascular and macrovascular
complications of diabetes. There are a number of pathways that have
been associated with increased production of intracellular reactive
oxygen species (ROS), such as nicotinamide adenine dinucleotide
phosphate (NADPH) oxidase, mitochondrial respiratory chain via
oxidative phosphorylation, advanced glycation end products, defects
in the polyol pathway and uncoupled nitric oxide synthase (2). Excess amounts of ROS directly damage
cellular micromolecules and macromolecules, which eventually cause
end-stage renal disease. Diminishing the production of ROS may be a
favorable therapeutic treatment to improve renal damage from
diabetic nephropathy.
Resveratrol (Re) is found in various plants,
including grapes, berries and peanuts. It is also present in wine,
particularly red wine. Re has been the focus of numerous studies
investigating its biological attributes, which include mainly
antioxidant activities (3). Re is
believed to have antioxidant properties, protecting the body
against the type of damage associated with an increased risk for
conditions, such as cancer and heart disease. As Re is believed to
have numerous health benefits, a number of manufacturers have tried
to capitalize by the production of Re supplements.
Isoflavones are able to neutralize free radicals.
Among the isoflavones, genistein (Ge) has the highest antioxidant
activity. Ge may act as direct antioxidant, similar to a number of
other isoflavones, and thus may alleviate the damaging effects of
free radicals in tissues (4,5). Ge is a strong antioxidant that eliminates
damaging free radicals and reduces lipid peroxidation, and
increases the activity of other antioxidant enzymes, such as
glutathione peroxidase, superoxide dismutase and glutathione
reductase (6).
These previous studies emphasize the importance of
Re and Ge in antioxidant activity and cellular protection from ROS
damage. However, to the best of our knowledge, there are no
previous studies evaluating the synergistic effects of Re with Ge
on the high-glucose (HG) treatment in kidney cells and its possible
protective mechanisms. The present study established a HG treatment
in an MDCK cell line and evaluated the possible synergistic
antioxidant efficacy of Re/Ge combination treatment. Specifically,
the sources of ROS generation from the HG incubation of MDCK cells
were examined and the molecular mechanisms underlying synergistic
antioxidant activity were determined.
Materials and methods
Cell line and reagents
The MDCK cell line was obtained from the Bioresource
Collection and Research Center (Hsinchu, Taiwan).
Dichlorofluorescein diacetate (DCFH-DA) was acquired from
Invitrogen Co. (Carlsbad, CA, USA). The primary antibodies against
p47phox, p22, γ-glutamylcysteine synthetase (γ-GCS), catalase,
superoxide dismutase-1 (SOD-1), glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) and secondary antibodies were obtained from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The primary
antibodies against β-actin, hydroxyphenyl fluorescein (HPF) and
other chemicals were purchased from Sigma-Aldrich (St. Louis, MO,
USA).
Cell culture and treatment
MDCK (1×106) cells were cultured in a
minimum essential medium (MEM) (90%) supplemented with 2 mM
L-glutamine and Earle's BBS adjusted to contain 1.5 g/l sodium
bicarbonate, 0.1 mM non-essential amino acids, 1.0 mM sodium
pyruvate, 10% fetal bovine serum, 100 U/ml penicillin and 100 µg/ml
streptomycin in 100-mm cultured dishes at 37°C in a humidified
atmosphere of 5% CO2. When cells reached 80% confluence
in the 100-mm cultured dishes, they were washed with
phosphate-buffered saline (PBS) and trypsinized for use in various
experiments. The MDCK cells were plated in 60- or 100-mm culture
dishes for 24 h. The culture medium was replaced with five types of
media: i) Normal glucose MEM medium (NG) containing 5.5 mM glucose,
ii) HG MEM medium (HG) containing 30 mM glucose, iii) 3 µM Re alone
in HG medium, iv) 1 µM Ge alone in HG medium and v) Re and Ge in HG
medium for 48 h. The selective inhibitors and the particular
concentrations used to inhibit the intracellular ROS generation
from various enzymes were referenced and modified from our previous
study (7). The particular
concentrations of the selective inhibitors did not affect the cell
viability of MDCK cells. The selective inhibitor of mitochondrial
complex I [20 nM rotenone (RO)], mitochondrial complex II [5 µM
carboxin (Car) or 5 µM 2-ethenoyltrifluoroacetone (TTFA)],
mitochondrial complex III [0.1 nM antimycin A (AA)], NADPH oxidase
[2 µM 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (AEB)
or 30 µM apocynin (Apo)], xanthine oxidase [10 µM allopurinol
(Allo)] and liopoxygenase [5 µM nordydihydroguaiaretic acid
(Nordy)] were pretreated for 1 h, followed by HG treatment for 48
h. These inhibitors were purchased from Sigma-Aldrich.
Measurement of intracellular ROS by
flow cytometry
Production of intracellular ROS was detected by flow
cytometry using the DCFH-DA probe (Sigma-Aldrich). The MDCK
(2×105) cells were plated in 60-mm culture dishes for 24
h. The culture medium was replaced with five types of media, as
aforementioned. Cells were treated with 10 µM DCFH-DA for 30 min in
the dark, washed once with PBS, collected by centrifugation (200 ×
g) for 5 min at room temperature, and were subsequently suspended
in PBS. Intracellular ROS levels indicated by the fluorescence of
dichlorofluorescein (DCF) were evaluated by excitation at 488 nm
and measured through a 530/22-nm barrier filter using a
Becton-Dickinson FACScan flow cytometer (BD Biosciences, San Diego,
CA, USA) (7). The data were acquired,
analyzed and plotted by the CellQuest Pro software (BD Biosciences)
and the SigmaPlot 10.0 software (Systate Software, Inc., San Jose,
CA, USA).
Western blot analysis
The MDCK (4×105) cells were plated in
100-mm culture dishes for 24 h. The culture medium was replaced
with five types of media, as aforementioned. Following treatment,
the cells were washed with PBS, resuspended in a protein extraction
buffer for 10 min, and centrifuged at 12,000 × g for 10 min at 4°C
to obtain the total extracted proteins (in the supernatant).
Protein concentrations were measured with a Bio-Rad protein assay
reagent (Bio-Rad, Richmond, CA, USA). The extracted cellular
proteins were boiled in loading buffer, and an aliquot
corresponding to 60–100 µg of protein was separated on a 12% sodium
dodecyl sulfate (SDS)-polyacrylamide gel. Following
electrophoresis, proteins were electrotransferred onto a
polyvinylidene fluoride transfer membrane. Following blotting, the
membranes were incubated with various primary antibodies overnight
[p47phox polyclonal antibody (rabbit anti-human; 1:1,000 dilution;
cat. no. sc-14015; Santa Cruz Biotechnology, Inc.), p22 polyclonal
antibody (rabbit anti-human; 1:500 dilution; cat. no. sc-20781;
Santa Cruz Biotechnology, Inc.), γ-GCS polyclonal antibody (rabbit
anti-human; 1:750 dilution; cat. no. sc-22755; Santa Cruz
Biotechnology, Inc.), SOD-1 monoclonal antibody (mouse anti-human;
1:750 dilution; cat. no. sc-17767; Santa Cruz Biotechnology, Inc.),
GAPDH polyclonal antibody (rabbit anti-human; 1:5,000 dilution;
cat. no. sc-25778; Santa Cruz Biotechnology, Inc.), catalase
polyclonal antibody (rabbit anti-human; 1:5,000 dilution; cat. no.
219010; Merck Millipore Corporation) or β-actin polyclonal antibody
(rabbit anti-human; 1:1,000 dilution; cat. no. A5060; Sigma-Aldrich
Co.)] and were washed with PBST solution 0.05% Tween-20 in PBS
(PBST). Subsequent to washing, the secondary antibodies (goat
anti-rabbit; 1:10,000 dilution; cat. no. sc-2004; or goat
anti-mouse; 1:5,000 dilution; cat. no. sc-2005; Santa Cruz
Biotechnology, Inc.) labeled with horseradish-peroxidase was added
to the membrane for 1 h and the sample was washed with PBST
solution. The antigen-antibody complexes were detected by enhanced
chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ, USA)
with a chemiluminescence analyzer.
Statistical analysis
Data are presented as the mean ± standard deviation
of at least 3 independent experiments and were analyzed using
Student's t-test by the Sigma Plot 10.0 software. P<0.05 was
considered to indicate a statistically significant difference.
Results
Antioxidant activity of Re and Ge in
HG treatment
The antioxidant activity of Re and Ge in HG
treatment was first evaluated in MDCK cells by DCFH-DA staining and
flow cytometry. The DCFH-DA is a probe for intracellular ROS. In
Fig. 1, 30 mM glucose (HG) incubation
induced a 1.7-fold intracellular ROS increase, as compared with 5.5
mM glucose (NG) treatment. Re (3, 5 and 10 µM) treatment led to a
significant inhibition of intracellular ROS as compared with the HG
treatment. The significant inhibition of intracellular ROS appeared
following 1, 20, 40 and 50 µM Ge treatment, but not in 5 and 10 µM
of Ge treatment. To evaluate the synergistic antioxidant activity
in Re and Ge, 3 µM of Re and 1 µM of Ge were selected for the
further experiments.
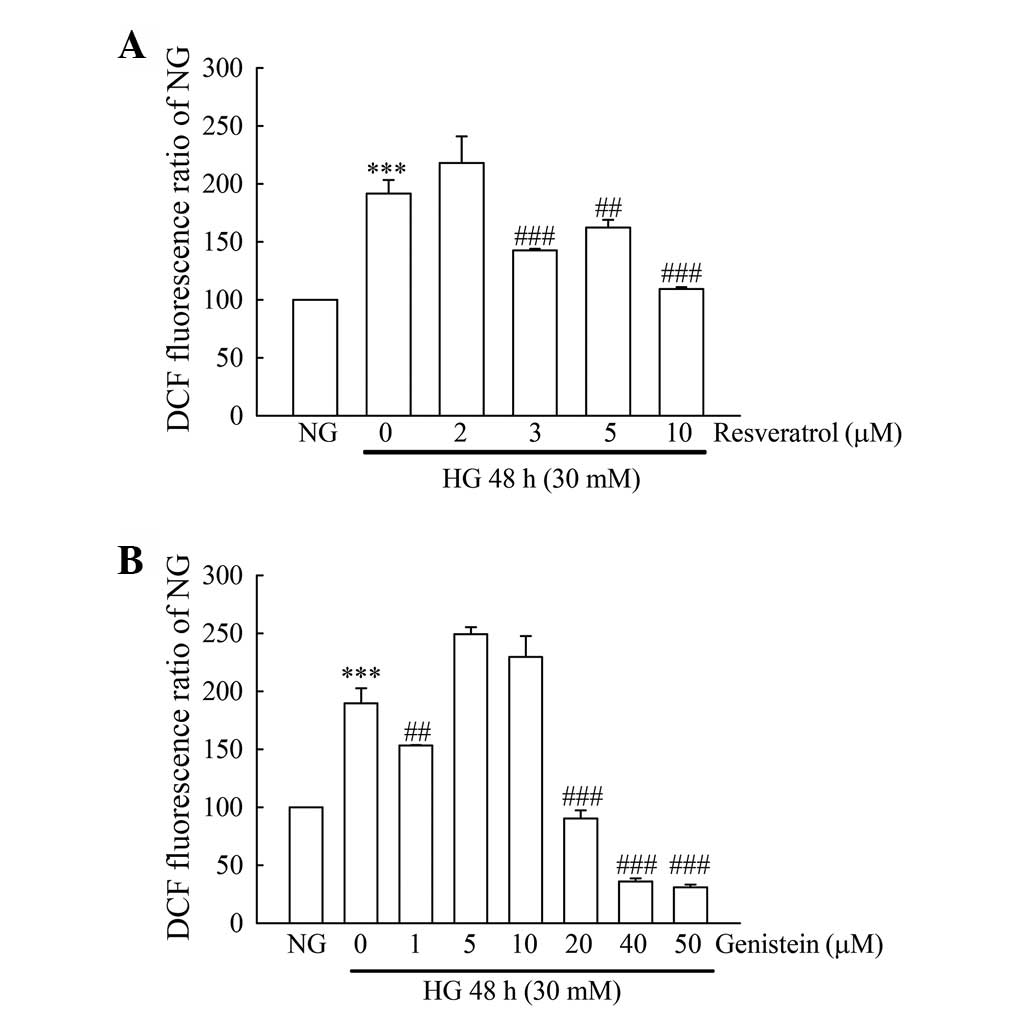 | Figure 1.Evaluation of intracellular ROS in
resveratrol- or genistein-treated HG-incubated MDCK cells. MDCK
cells (2×105) were plated in 60-mm cultured dishes for
24 h. MDCK cells were separately grown under NG and HG media for 48
h. Except for the NG and HG groups, cells in the HG incubation were
treated with or without (A) resveratrol (2, 3, 5 and 10 µM) or (B)
genistein (1, 5, 10, 20, 40 and 50 µM). Following treatment, the
intracellular ROS production was detected by flow cytometry using
DCFH-DA staining. The values shown are mean ± standard deviation
(n=5–8 samples/experiment). Significant differences from the NG
group are ***P<0.001 and the HG group are ##P<0.01
and ###P<0.001, respectively. ROS, reactive oxygen
species; HG, high glucose; MDCK, Madin-Darby canine kidney; NG,
normal glucose; DCFH-DA, dichlorofluorescein diacetate. |
Re with Ge synergistically inhibits
intracellular ROS in HG treatment
The antioxidant activity of Re alone, Ge alone and
the Re/Ge combination in HG-treated MDCK cells was evaluated by
DCFH-DA staining and flow cytometry. Glucose (HG) of 30 mM of
treatment induced a 1.7-fold intracellular ROS as compared with 5.5
mM glucose (NG) treatment (Fig. 2). Re
alone or Ge alone led to significant inhibition of intracellular
ROS as compared with HG treatment. Of note, the intracellular ROS
was significantly inhibited in the combined administration of Re
and Ge, as compared with a single compound treatment.
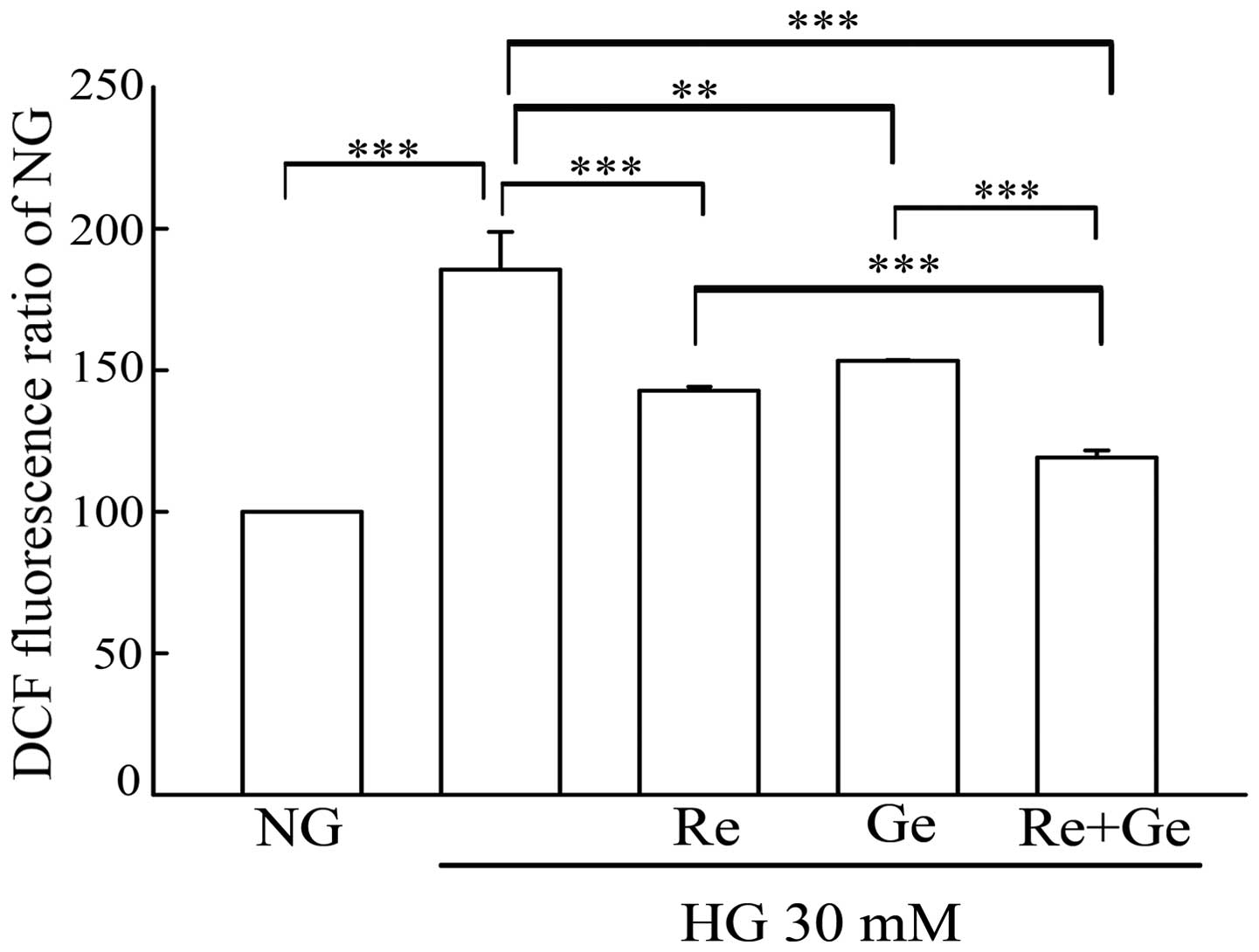 | Figure 2.Evaluation of intracellular ROS in the
Re/Ge combination-treated HG-incubated MDCK cells. MDCK cells
(2×105) were plated in 60-mm cultured dishes for 24 h.
MDCK cells were separately grown under NG and HG media for 48 h.
Except for the NG and HG groups, cells in the HG incubation were
treated with or without 3 µM Re alone, 1 µM Ge alone, and the Re/Ge
combination. Following treatment, intracellular ROS production was
detected by flow cytometry using DCFH-DA staining. The values shown
are mean ± standard deviation (n=5–8 samples/experiment).
**P<0.01 and ***P<0.001. ROS, reactive oxygen species; Re,
resveratrol; Ge, genistein; HG, high glucose; MDCK, Madin-Darby
canine kidney; NG, normal glucose; DCFH-DA, dichlorofluorescein
diacetate. |
Multiple sources of ROS are induced by
HG treatment in MDCK cells
To evaluate the ROS sources of HG-treated MDCK
cells, cells were pre-incubated with 20 nM RO (a complex I
inhibitor), 5 µM TTFA (a complex II inhibitor), 5 µM Car (a complex
II inhibitor), 0.1 nM AA (a complex III inhibitor), 2 µM AEB (a
NADPH oxidase inhibitor), 30 µM Apo (an NADPH oxidase inhibitor),
10 µM Allo (a xanthine oxidase inhibitor) or 5 µM Nordy (a
lipoxygenase inhibitor) for 1 h, followed by co-incubation with 30
mM glucose (HG) for 48 h. Following treatment, the intracellular
ROS production was measured using the DCFH-DA probe. A significant
increase in intracellular ROS following HG treatment was observed
in the MDCK cells (Fig. 3). The
HG-induced intracellular ROS produced, measured using the DCFH-DA
probe and flow cytometry, was significantly scavenged when cells
were pre-incubated for 1 h with RO, AEB, Apo, Allo and Nordy in the
MDCK cells (Fig. 3). However, TTFA,
Car and AA did not scavenge the HG-induced intracellular ROS. These
results indicated that there were 4 main sources of ROS, including
mitochondrial complex I, NADPH oxidase, xanthine oxidase and
lipoxygenase, induced by HG incubation in MDCK cells.
 | Figure 3.Identification of the originating
sites of HG-induced ROS overproduction using a group of specific
inhibitors. MDCK cells (2×105) were plated in 60-mm
cultured dishes for 24 h. MDCK cells were separately grown under NG
and HG media for 48 h. Except for the NG and HG groups, cells in
the HG group were pretreated with or without various concentrations
of designated inhibitors including 20 nM rotenone (RO;
mitochondrial complex I), 5 µM of 2-thenoyltrifluoroacetone (TTFA;
mitochondrial complex II), 5 µM carboxin (Car; mitochondrial
complex II), 0.01 nM antimycin A (AA; mitochondrial complex III), 1
µM 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (AEB;
NADPH oxidase), 30 µM apocynin (Apo; NADPH oxidase), 20 µM
allopurinol (Allo; xanthine oxidase) and 5 µM
nordydihydroguaiaretic acid (Nordy; lipoxygenase) for 1 h.
Intracellular ROS production was detected by flow cytometry using
DCFH-DA staining. The values shown are mean ± standard deviation
(n=5–8 samples/experiment). Significant differences from the NG
group are *P<0.05 and the HG group are #P<0.05,
##P<0.01 and ###P<0.001, respectively.
ROS, reactive oxygen species; HG, high glucose; MDCK, Madin-Darby
canine kidney; NG, normal glucose; DCFH-DA, dichlorofluorescein
diacetate. |
Re/Ge synergistically inhibits NADPH
oxidase expression in HG treatment
NADPH oxidase is a main source of intracellular ROS
production. The intracellular ROS produced by HG treatment also
involved NADPH oxidase. The expression of two subunits of NADPH
oxidase, p47phox and p22, were further evaluated in HG incubation.
In Fig. 4, the expression of p22 was
increased to 1.78-fold in HG-treated cells. Of note, Re or Ge alone
treatment led to a 2.25- and 2.89-fold increase of p22 expression.
There was no increased expression following treatment with the
Re/Ge combination in the HG-treated cells (only 0.99-fold p22).
Treatment with Re or Ge alone decreased the p47phox expression to
0.54- and 0.59-fold of the NG treatment. The Re/Ge combination
caused more inhibition of p47phox expression, which was 0.28-fold
of the NG incubation.
 | Figure 4.Expression of NADPH oxidase subunits
in Re/Ge combination-treated HG-incubated MDCK cells. MDCK cells
(4×105) were plated in 100-mm cultured dishes for 24 h.
MDCK cells were separately grown under NG and HG media for 48 h.
Except for the NG and HG groups, cells in the HG incubation were
treated with or without 3 µM Re alone, 1 µM Ge alone, and Re/Ge
combination. Following treatment, the expression levels of the
NADPH oxidase subunits, p47phox and p22, were detected using
western blotting. NADPH, nicotinamide adenine dinucleotide
phosphate; Re, resveratrol; Ge, genistein; HG, high glucose; NG,
normal glucose; MDCK, Madin-Darby canine kidney. |
Re with Ge synergistically effects the
expression of antioxidant enzymes in HG treatment
γ-GCS, catalase and SOD-1 are three critical
antioxidant enzymes, which act against cellular ROS during
oxidative stress. To evaluate the expression of these antioxidant
enzymes in HG incubation, western blotting was used. The expression
of γ-GCS decreased to 0.75-fold in the HG treatment as compared
with NG incubation (Fig. 5). The
expression level of γ-GCS following treatment with Re alone in HG
incubation was decreased to 0.63-fold. However, an increase to
1.13-fold was observed in the expression of γ-GCS when MDCK cells
were treated with Ge alone in HG for 48 h. Treatment with the Re/Ge
combination in HG-treated cells for 48 h further increased the
γ-GCS expression to 1.23-fold as compared with NG incubation. The
data appear to indicate that the increased expression of γ-GCS for
the Re/Ge combination treatment in HG-treated cells is an important
event for ROS elimination. The catalase expression was markedly
decreased to 0.76- and 0.68-fold in HG incubation and Re alone
treatment, respectively. Notably, catalase expression was 0.95-fold
in the Re/Ge combination treatment in the HG incubation. The
expression of SOD-1 in MDCK cells was increased to 1.9-fold in the
HG treatment at 48 h; however, the Re/Ge combination in the HG
incubation resulted in 1.03-fold of SOD-1 expression as compared
with NG incubation.
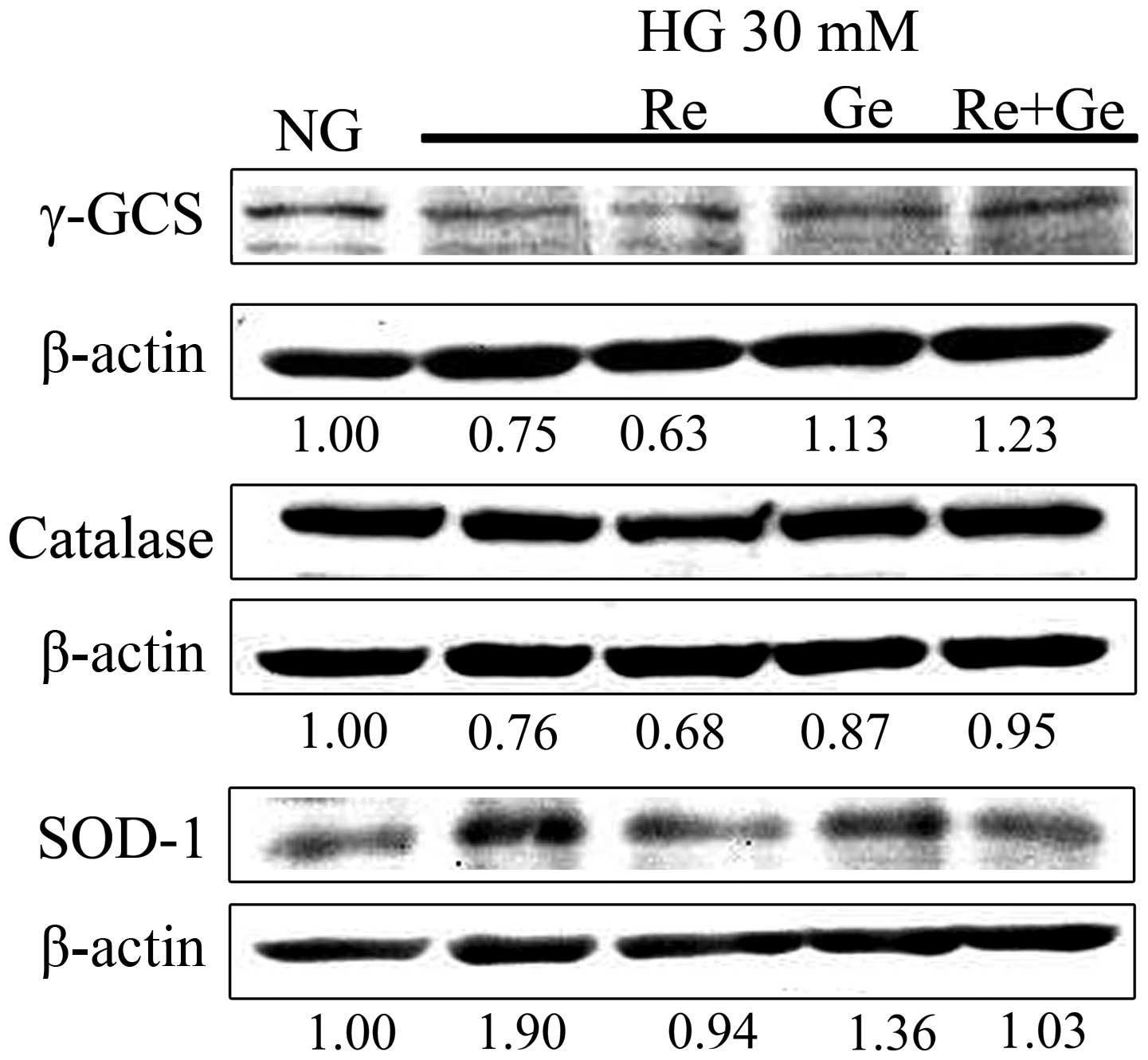 | Figure 5.Expression of antioxidant enzymes in
the Re/Ge combination-treated HG-incubated MDCK cells. MDCK cells
(4×105) were plated in 100-mm cultured dishes for 24 h.
MDCK cells were separately grown under NG and HG media for 48 h.
Except for the NG and HG groups, cells in the HG incubation were
treated with or without 3 µM Re alone, 1 µM Ge alone and the Re/Ge
combination. Following treatment, the expression levels of the
antioxidant enzymes, γ-GCS, catalase and SOD-1, were detected using
western blotting. Re, resveratrol; Ge, genistein; HG, high glucose;
NG, normal glucose; MDCK, Madin-Darby canine kidney; γ-GCS,
γ-glutamylcysteine synthetase; SOD-1, superoxide dismutase-1. |
Re/Ge synergistically inhibits
intracellular hydroxyl radials in HG treatment
The antioxidant activity on hydroxyl radicals of Re
alone, Ge alone and Re/Ge combination in the HG-treated MDCK cells
was evaluated by HPF staining and flow cytometry. HG incubation
induced a 1.5-fold increase in intracellular hydroxyl radicals as
compared with NG incubation (Fig. 6).
Re alone or Ge alone led to a significant inhibition of
intracellular hydroxyl radicals as compared with HG incubation. Of
note, the intracellular hydroxyl radicals were significantly
inhibited in the Re/Ge combination as compared with Re or Ge alone
treatment.
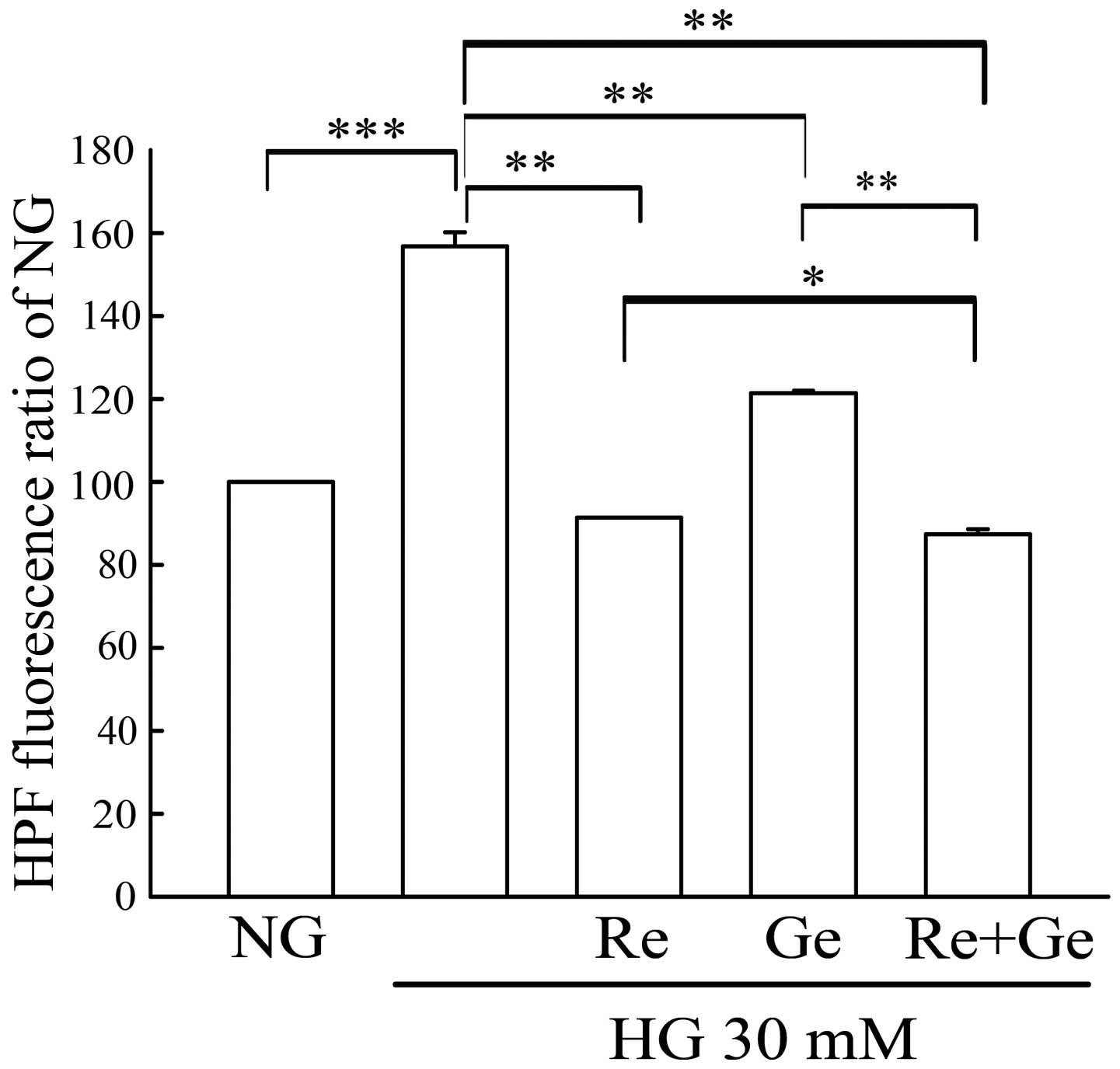 | Figure 6.Evaluation of the intracellular
hydroxyl radicals in the Re/Ge combination-treated HG-incubated
MDCK cells. MDCK cells (2×105) were plated in 60-mm
cultured dishes for 24 h. MDCK cells were separately grown under NG
and HG media for 48 h. Except for the NG and HG groups, cells in
the HG incubation were treated with or without 3 µM Re alone, 1 µM
Ge alone and the Re/Ge combination. Following treatment,
intracellular reactive oxygen species production was detected by
flow cytometry using HPF staining. The values shown are mean ±
standard deviation (n=5–8 samples/experiment). *P<0.05,
**P<0.01 and ***P<0.001, respectively. Re, resveratrol; Ge,
genistein; HG, high glucose; NG, normal glucose; MDCK, Madin-Darby
canine kidney; HPF, hydroxyphenyl fluorescein. |
Discussion
In Fig. 3, RO (a
complex I inhibitor) inhibited the largest amount of ROS in the HG
treatment, suggesting the site of the ROS source provided from HG
incubation is mitochondrial complex I. Previous studies have
indicated that the hyperglycemia characteristics of diabetes
increase the complex I substrate NADH (8), which is likely to potentiate ROS
production by the respiratory chain (9). This is in agreement with the present
finding that mitochondrial complex I generated the maximum amount
of ROS in the HG-treated MDCK cells. An important source of ROS
production is NADPH oxidase. This enzyme results in superoxides
during the respiratory burst in phagocytes. The NADPH oxidase
consists of membrane-bound subunits (gp91phox and p22phox) and
cytosolic subunits (p47phox, p40phox, p67phox and Rac) (10). When activated, p47phox is
phosphorylated, and the cytosolic components translocate to the
membrane, where they form a molecular cluster of the catalytically
active oxidase. The expression of p47phox appeared to exhibit the
largest inhibition by the Re/Ge combination as compared with Re or
Ge alone, indicating that the Ge/Re combination inhibits the
activation of NADPH oxidase in HG incubation.
Xanthine oxidase is an enzyme that converts
hypoxanthine/xanthine to uric acid in a reaction that liberates
superoxide. High activity of xanthine oxidase has been observed in
numerous pathological conditions, as the xanthine oxidase-generated
superoxide is cytotoxic. A significant increase in the activity of
xanthine oxidase was observed in diabetics (11). Inhibition of xanthine oxidase reduces
hyperglycemia-induced oxidase stress in skeletal muscle of diabetic
mice. Evidence is accumulating that some of the products derived
from the actions of lipoxygenases contribute to diabetic
nephropathy. Specifically, levels of lipoxygenases 12 and 15 are
increased in diabetic animals. In addition, HG levels increase the
expression of lipoxygenases 12 and 15 in cultured mesangial cells
(12). The results of the present
study show that Allo (a xanthine oxidase inhibitor) and Nordy (a
lipooxygenase inhibitor) inhibited a partial ROS level in
HG-incubated MDCK cells (Fig. 3)
suggesting that xanthine oxidase and lipoxygenase also have
important roles in ROS generation of kidney cells in diabetes.
Other possible mechanisms by which HG incubation
increases intracellular ROS is the upregulation of SOD-1 observed
in MDCK cells (Fig. 5). SOD-1 is a
superoxide metabolic enzyme that metabolizes superoxide to
H2O2, which is further metabolized to
H2O and O2 by catalase. HG incubation
markedly increased the expression of SOD-1, and this effect would
result in intracellular superoxide being converted to
H2O2 (Fig. 5).
Furthermore, as HG incubation significantly inhibited the
expression of catalase to 0.76-fold (Fig.
5), the elimination of intracellular H2O2
may not be executed normally in MDCK cells and may ultimately
contribute to the increase in intracellular
H2O2 after 48 h of HG incubation. By
contrast, the Re/Ge combination could recover the expression of
catalase to 0.95-fold in HG incubation suggesting that the
synergistic antioxidant activity is partially a result from the
maintenance of catalase expression.
γ-GCS is the first enzyme of the cellular
glutathione (GSH) biosynthetic pathway that catalyzes the chemical
reaction. GSH combines with hydroxyl radical, peroxynitrite, and
hydroperoxides, as well as reactive electrophiles, including
activated phosphoramide mustard (13).
The activity of γ-GCS was significantly lower in diabetics compared
to normal controls (14). A decreased
expression of γ-GCS in diabetic rats is consistent with repressed
GSH synthesis (15). These results are
in agreement with the present findings that the expression of γ-GCS
in HG incubation was decreased to 0.75-fold of NG-incubated cells.
The expression of γ-GCS was increased to 1.23-fold in the Re/Ge
combination indicating another important antioxidant mechanism of
the Re/Ge combination, which provides an enhanced GSH synthesis
function to antagonize the ROS in HG incubation.
Re has potent protective effects on diabetes and
diabetic complications including diabetic nephropathy. Xu et
al (16) demonstrated that
pretreatment with Re (10 µM) for 6 h prior to HG (30 mM) treatment
for 48 h significantly reduced the hyperglycemia-induced increase
in ROS production in rat mesangial cells. This is in agreement with
the present results, as Re (10 µM) alone decreased HG-induced ROS
in MDCK cells. In addition, another study also demonstrated that
the addition of Ge to soy protein causes improvements in the
antioxidant status of kidney tissue. The catalase activity was
significantly increased in soy/Ge treatment (17). The present findings explain the
observation that the low concentration of Re (3 µM) in combination
with Ge (1 µM) could further decrease the HG-induced ROS,
suggesting that an important synergistic antioxidant effect may be
applied in diabetic nephropathy.
Acknowledgements
The present study was supported by a grant from the
Ministry of Economic Affairs of Taiwan, R.O.C (no.
104-EC-17-A-18-S1-226).
References
|
1
|
Tisminetzky M, McManus DD, Dor A, Miozzo
R, Yarzebski J, Gore JM and Goldberg RJ: Decade-long trends
(1999-2009) in the characteristics, management, and hospital
outcomes of patients hospitalized with acute myocardial infarction
with prior diabetes and chronic kidney disease. Int J Nephrol
Renovasc Dis. 8:41–51. 2015.PubMed/NCBI
|
|
2
|
Kashihara N, Haruna Y, Kondeti VK and
Kanwar YS: Oxidative stress in diabetic nephropathy. Curr Med Chem.
17:4256–4269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de la Lastra CA and Villegas I:
Resveratrol as an antioxidant and pro-oxidant agent: mechanisms and
clinical implications. Biochem Soc Trans. 35:1156–1160. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Han RM, Tian YX, Liu Y, Chen CH, Ai XC,
Zhang JP and Skibsted LH: Comparison of flavonoids and
isoflavonoids as antioxidants. J Agric Food Chem. 57:3780–3785.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Borrás C, Gambini J, López-Grueso R,
Pallardó FV and Viña J: Direct antioxidant and protective effect of
estradiol on isolated mitochondria. Biochim Biophys Acta.
1802:205–211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ullmann K, Wiencierz AM, Müller C,
Thierbach R, Steege A, Toyokuni S and Steinberg P: A
high-throughput reporter gene assay to prove the ability of natural
compounds to modulate glutathione peroxidase, superoxide dismutase
and catalase gene promoters in V79 cells. Free Radic Res.
42:746–753. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang JT, Li ZL, Wu JY, Lu FJ and Chen CH:
An oxidative stress mechanism of shikonin in human glioma cells.
PLoS One. 9:e941802014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kabat A, Pönicke K, Salameh A, Mohr FW and
Dhein S: Effect of a beta 2-adrenoceptor stimulation on
hyperglycemia-induced endothelial dysfunction. J Pharmacol Exp
Ther. 308:564–573. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brownlee M: Biochemistry and molecular
cell biology of diabetic complications. Nature. 414:813–820. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Babior BM: NADPH oxidase. Curr Opin
Immunol. 16:42–47. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suriyajothi MA, Sangeetha R and
Venkateswari R: Activity of xanthine oxidase of diabetes: Its
correlation with aging. Pharmacologyonline. 2:128–133. 2011.
|
|
12
|
Hao CM and Breyer MD: Roles of lipid
mediators in kidney injury. Semin Nephrol. 27:338–351. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Griffith OW and Mulcahy RT: The enzymes of
glutathione synthesis: Gamma-glutamylcysteine synthetase. Adv
Enzymol Relat Areas Mol Biol. 73:209–267, xii. 1999.PubMed/NCBI
|
|
14
|
Murakami K: Glutathione metabolism in
erythrocytes from patients with diabetes mellitus. Hokkaido Igaku
Zasshi. 66:29–40. 1991.(In Japanese). PubMed/NCBI
|
|
15
|
Furfaro AL, Nitti M, Marengo B,
Domenicotti C, Cottalasso D, Marinari UM, Pronzato MA and Traverso
N: Impaired synthesis contributes to diabetes-induced decrease in
liver glutathione. Int J Mol Med. 29:899–905. 2012.PubMed/NCBI
|
|
16
|
Xu Y, Nie L, Yin YG, Tang JL, Zhou JY, Li
DD and Zhou SW: Resveratrol protects against hyperglycemia-induced
oxidative damage to mitochondria by activating SIRT1 in rat
mesangial cells. Toxicol Appl Pharmacol. 259:395–401. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Javanbakht MH, Sadria R, Djalali M,
Derakhshanian H, Hosseinzadeh P, Zarei M, Azizi G, Sedaghat R and
Mirshafiey A: Soy protein and genistein improves renal antioxidant
status in experimental nephrotic syndrome. Nefrologia. 34:483–490.
2014.PubMed/NCBI
|




















