Introduction
Approximately 80% of patients with cerebral apoplexy
sequelae are reported to have a certain degree of limb spasm
(1), with a 38% higher incidence one
year following a stroke (2).
Although spasms can help certain patients with venous reflux and
relieve edema, spasms hinder functional recovery in the majority of
patients. If active treatment is not administered, permanent
increased muscle tension and joint contracture can seriously impact
on patient quality of life. Therefore, seizures that occur
following a stroke remain an obstacle in stroke rehabilitation
therapy (3).
Gua Lou Gui Zhi decoction (GLGZD) was originally
prescribed during the Eastern Han Dynasty around 210 AD, as written
in a famous traditional Chinese medicine (TCM) book, ‘Essentials
from the Golden Cabinet’ (4). As
outlined by Zhongjing Zhang in this book, the decoction comprised
Radix Trichosanthis, Ramulus Cinnamomi, Paeonia lactiflora,
Glycyrrhiza, Zingiber officinale Roscoe and Fructus
Jujubae. As evidenced by the age of the documented prescription of
GLGZD, this decoction has been used clinically for a number of
years in China for the treatment of muscular spasticity following a
stroke, epilepsy or a spinal cord injury (5,6).
Previous studies have demonstrated that GLGZD is effective in the
treatment of post-stroke spasticity, improving the Fugl-Meyer
assessment and Barthel index scores (7,8).
However, the mechanisms underlying the efficacy of GLGZD remain
unclear.
Rat pheochromocytoma (PC12) cells have a similar
morphology and function to sympathetic neurons in the presence of
nerve growth factor (NGF), and subsequently have been widely
utilized as a model for the study of neuronal ion channels and
receptors, and responses to stress and injury (9). Oxidative stress is an important
pathological consequence of stroke and ischemia-reperfusion injury.
In addition, oxidative stress has been reported to be a core
pathological component of stroke and brain ischemia-reperfusion
injury, since oxidative stress elevates and contributes to cerebral
ischemia reperfusion injury (10–12). A
previous study found that within the brain microvasculature
following ischemia-reperfusion injury, hydrogen peroxide
(H2O2) is produced in excess, leading to an
increased susceptibility of nerve cells to damage and the
development of various neuropathies (13). The use of PC12 cells as a model of
cerebral ischemia is similar to using primary neurons as an in
vitro and in vivo model of a cerebral ischemia neural
pathology (14). Therefore, the
present study utilized H2O2 treatment of PC12
cells to stimulate oxidative stress in the neurons.
Nuclear factor (erythroid-derived 2)-like 2 (Nrf2)
is a transcription factor that is involved in cellular protection
against oxidative stress through the antioxidant response element
(ARE)-directed induction of several antioxidant enzymes, including
heme oxygenase (HO)-1 (15). HO-1 is
a novel enzyme with potent anti-inflammatory, antioxidant and
antiproliferative effects (16,17).
Nrf2 and HO-1 proteins have been shown to play important
neuroprotective roles in oxidative damage. Therefore, to clarify
the potential mechanisms underlying the antispasticity effects
mediated by GLGZD following stroke, the present study evaluated the
antioxidant effects of GLGZD and investigated the underlying
molecular mechanisms using PC12 cells injured by
H2O2 in vitro. In the investigations
of these mechanisms, the effects of GLGZD on the mRNA and protein
expression levels of Nrf2 and HO-1 in PC12 cells injured by
H2O2 treatment were analyzed.
Materials and methods
Preparation of the ethanol extract
from GLGZD
GLGZD used in the study consisted of extracts of
Trichosanthis Radix (9 g), Ramulus Cinnamomi (9 g), Paeonia
lactiflora (9 g), Glycyrrhiza (6 g), Zingiber
officinale Roscoe (9 g) and Fructus Jujubae (9 g), all in a
ratio of 3:3:3:2:3:3. Dried crude medicinal plants were supplied by
Guo Yi Tang Chinese Herbal Medicine Store (Fuzhou, Fujian, China)
for the preparation of the GLGZD ethanol extract. A total of 300 g
GLGZD was extracted with 3,000 ml ethanol (75%) using a reflux
method, after which the solution was filtered. The ethanol solvent
was evaporated on a rotary evaporator (model RE-2000; Yarong
Biochemistry Instrument Co., Shanghai, China), and the GLGZD
extract was obtained using a spraying desiccation method with a
spray dryer (model B-290; Büchi Labortechnik AG, Flawil,
Switzerland). The stock was stored at −20°C and working
concentrations of GLGZD were established by dissolving the extract
in culture media to a concentration of 8 mg/ml. Hydrogen peroxide
(H2O2) was a product of Junsei Chemical Co.,
Ltd. (Tokyo, Japan).
Cell culture and NGF-induced
differentiation of PC12 cells
A PC12 rat pheochromocytoma cell line was obtained
from the American Type Culture Collection (Rockville, MD, USA). The
cells were maintained in RPMI 1640 medium supplemented with 10%
heat-inactivated horse serum, 5% fetal bovine serum, 100 U/ml
penicillin and 100 µg/ml streptomycin, which were all purchased
from Gibco Life Technologies (Carlsbad, CA, USA), in a 37°C
humidified incubator with 5% CO2 (Thermo Fisher
Scientific, Waltham, MA, USA). After 24 h of incubation,
differentiation was induced by treatment with 50 ng/ml NGF (NGF
2.5S; EMD Millipore, Madison, WI, USA) every other day for 6
days.
Drug treatment
All the experiments were performed 24 h following
cell seeding onto plates at a density of 1×105 cells/ml.
H2O2 was freshly prepared prior to each
experiment. Cells were preincubated with GLGZD at the various
concentrations (0.5 and 1.0 mg/ml) for 24 h prior to exposure to
300 µM H2O2 for 4 h. The control and
H2O2 treatment groups were cultured in an
equal volume of RPMI 1640 medium without or with
H2O2, respectively. Subsequently, assays for
cell survival and other cellular properties were performed as
follows.
Analysis of cell viability
Cell survival was observed using an inverted
microscope (Leica Microsystems GmbH, Wetzlar, Germany). Cell
viability was simultaneously evaluated with a
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT)
assay obtained from Sigma-Aldrich (St. Louis, MO, USA). PC12 cells
were seeded into 96-well plates at a concentration of
1×105 cells/ml, and subsequently treated with or without
300 µM H2O2 for 4 h following the addition of
GLGZD at 0.5 and 1 mg/ml for 24 h at 37°C. After incubation for 3 h
with 20 µl MTT (5 mg/ml), the cells were lysed in dimethyl
sulfoxide (Sigma-Aldrich), and the amount of MTT formazan was
qualified by determining the absorbance at 570 nm using a
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Cell viability was expressed as a percentage of the control culture
value.
Cellular ATP level determination
PC12 cells were plated in a six-well cell culture
plate overnight and incubated with GLGZD ethanol extract (0.5 and 1
mg/ml) for 24 h prior to exposure to, or absence of,
H2O2 (300 µM) for 4 h. The levels of ATP in
the cells were measured using a firefly luciferase-based ATP assay
kit (Beyotime Institute of Biotechnology, Shanghai, China),
according to the manufacturer's instructions. PC12 cell culture and
GLGZD exposure were performed as aforementioned. After rinsing with
phosphate-buffered saline (PBS), the cells were disrupted in 200 µl
lysis buffer, and centrifuged at 12,000 × g at 4°C for 5 min, from
which the supernatant was collected. In a 1.5-ml tube, a 100-µl
sample of the supernatant was mixed with 100 µl ATP detection
working solution. The luminance (RLU) was immediately measured
using a Turner BioSystems luminometer (Promega Corporation,
Madison, WI, USA). Standard curves for quantification were
generated using known amounts of an ATP standard, and the protein
concentration of each treatment group was determined using a
bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology). Total ATP levels were expressed as normalized
luminance (NRLU) in nmol/mg protein (18).
Reverse transcription quantitative
polymerase chain reaction (PCR) for Nrf2 and HO-1 mRNA
expression
Total RNA was isolated from the PC12 cells using
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA),
according to the manufacturer's instructions. In total, 1 µg RNA
was reverse transcribed into cDNA using a RevertAid™ H Minus First
Strand cDNA Synthesis kit (Fermentas GmbH, St. Leon-Rot, Germany).
The total volume of the reverse transcription reaction mixture was
20 µl cDNA, then 2 µl cDNA was used for each amplification. cDNA
was amplified by quantitative PCR (50°C for 2 min, 95°C for 10 min
followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min) using
an ABI Prism 7500 Sequence Detector (Applied Biosystems Life
Technologies, Foster City, CA, USA) with SYBR Green Real-Time PCR
Master Mix Reagent (Takara Bio, Inc., Otsu, Japan). Data from the
reaction were collected and analyzed using Sequence Detector
Software (SDS version 1.6; PE Applied Biosystems, Foster City, CA
USA). Relative quantification of gene expression was calculated
using the 2−ΔΔCt data analysis method and normalized
against GAPDH for each sample. The sequences of the primers used
for PCR were as follows: Nrf2 forward, 5′-CCA TTT ACG GAG ACC
CAC-3′ and reverse, 5′-TGA GCG GCA ACT TTA TTC-3′; HO-1 forward,
5′-GAA ACA AGC AGA ACC CAG TC-3′ and reverse, 5′-AGA GGT CAC CCA
GGT AGCG-3′; GAPDH forward, 5′-ACG GAT TTG GTC GTA TTG GGC-3′ and
reverse, 5′-CTC GCT CCT GGA AGA TGG TGAT-3′. GAPDH was selected as
the housekeeping gene.
Fluorescent immunohistochemistry
Differentiated PC12 cells were seeded into
confocal-specific sterile glass-bottom cell culture Petri dishes
(15 mm diameter; Nest Biotechnology Co., Ltd., Wuxi, China) and
cultured for 24 h. The cells were treated with different
concentrations of GLGZD (0.5 and 1.0 mg/ml), or received no
treatment, for 24 h, followed by incubation with 300 µM
H2O2 for 4 h. Subsequently, the cells were
fixed with 4% paraformaldehyde in PBS (pH 7.4; Gibco Life
Technologies) for 30 min and permeabilized in 0.1% Triton X-100 in
PBS for 10 min at room temperature. Nonspecific binding was blocked
with 5% goat serum in PBS for 30 min. Next, the cells were
incubated at 4°C overnight with the following primary antibodies:
Rabbit anti-Nrf2 (1:100 polyclonal; cat. no. SC-13032; Santa Cruz
Biotechnology, Inc. Santa Cruz, CA, USA) and rabbit anti-HO-1
(1:200 polyclonal; cat. no. SC-10789; Santa Cruz Biotechnology,
Inc.). Following incubation with the primary antibodies, the cells
were washed three times in cold PBS and incubated with a goat
anti-rabbit secondary antibody conjugated to a fluorescent marker
(1:100, Alexa 488; Abcam, Cambridge, MA, USA). The cells were
mounted with 4,6-diamidino-2-phenylindole (1:1,000; Beyotime
Institute of Biotechnology) to stain the nuclei, and the samples
were analyzed using an LSM 710 laser scanning confocal microscope
under 40 × water objective (Carl Zeiss AG, Oberkochen,
Germany).
Statistical analysis
Data were analyzed using SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA). Quantitative data are expressed as the
mean ± standard deviation of at least triplicate experiments.
One-way analysis of variance was used for data comparisons between
multiple groups, where P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of GLGZD on the viability of
H2O2-treated PC12 cells
Neuroprotective effects of GLGZD were further
confirmed by the morphological observations (Fig. 1). Following incubation with 50 µg/ml
NGF for 6 days, the morphological observations indicated that the
majority of the NGF-differentiated PC12 cells had ceased division
and extended their neuritic processes (Fig. 1A). Exposure to
H2O2 for 4 h resulted in heterogeneity in
their shape, a loss of connections between neural cells and
extensive detachment from the culture plate. As shown in Fig. 1B, the PC12 cell number markedly
decreased, with the majority of the cells darkened or shrunken, and
a number of the treated cells were even fragmented and yielding
debris after 4 h exposure to 300 µM H2O2.
Pretreatment with GLGZD was shown to alleviate the damage induced
by H2O2. Based on the morphological changes,
1 mg/ml GLGZD (Fig. 1D) exerted a
greater protective effect compared with 0.5 mg/ml GLGZD (Fig. 1C). Since the PC12 cells were very
sensitive to H2O2, pretreatment with GLGZD
(0.5 and 1.0 mg/ml) for 24 h dose-dependently increased cell
viability compared with cells treated with
H2O2 alone, as determined by the MTT assay
(Fig. 2).
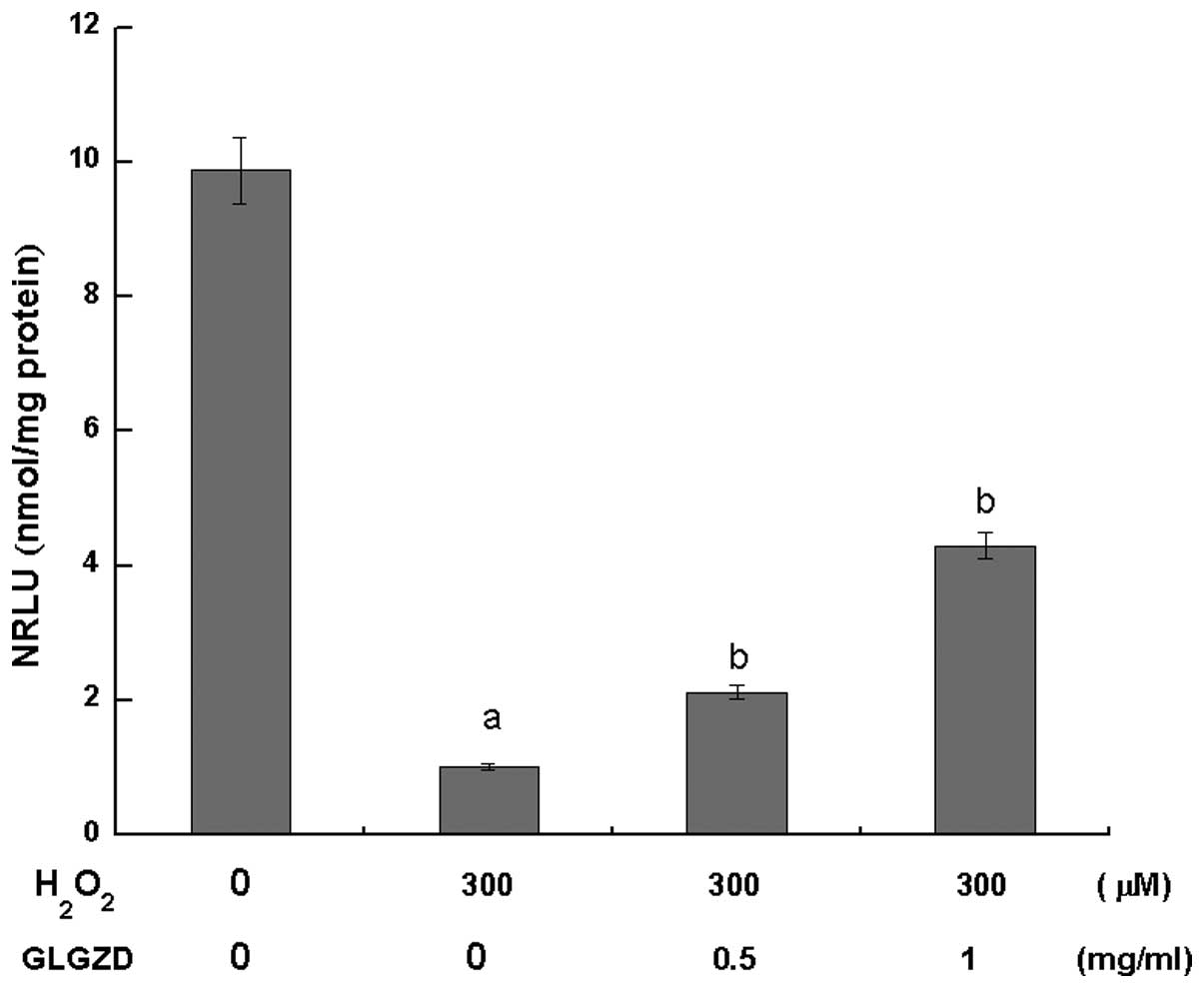
Changes in the cellular ATP
levels
After 4 h of H2O2 exposure,
the ATP levels in the two GLGZD treatment groups were significantly
higher (P<0.05) compared with the group of cells treated with
H2O2 alone. However, the ATP levels were
significantly lower in these groups when compared with the control
group (Fig. 3).
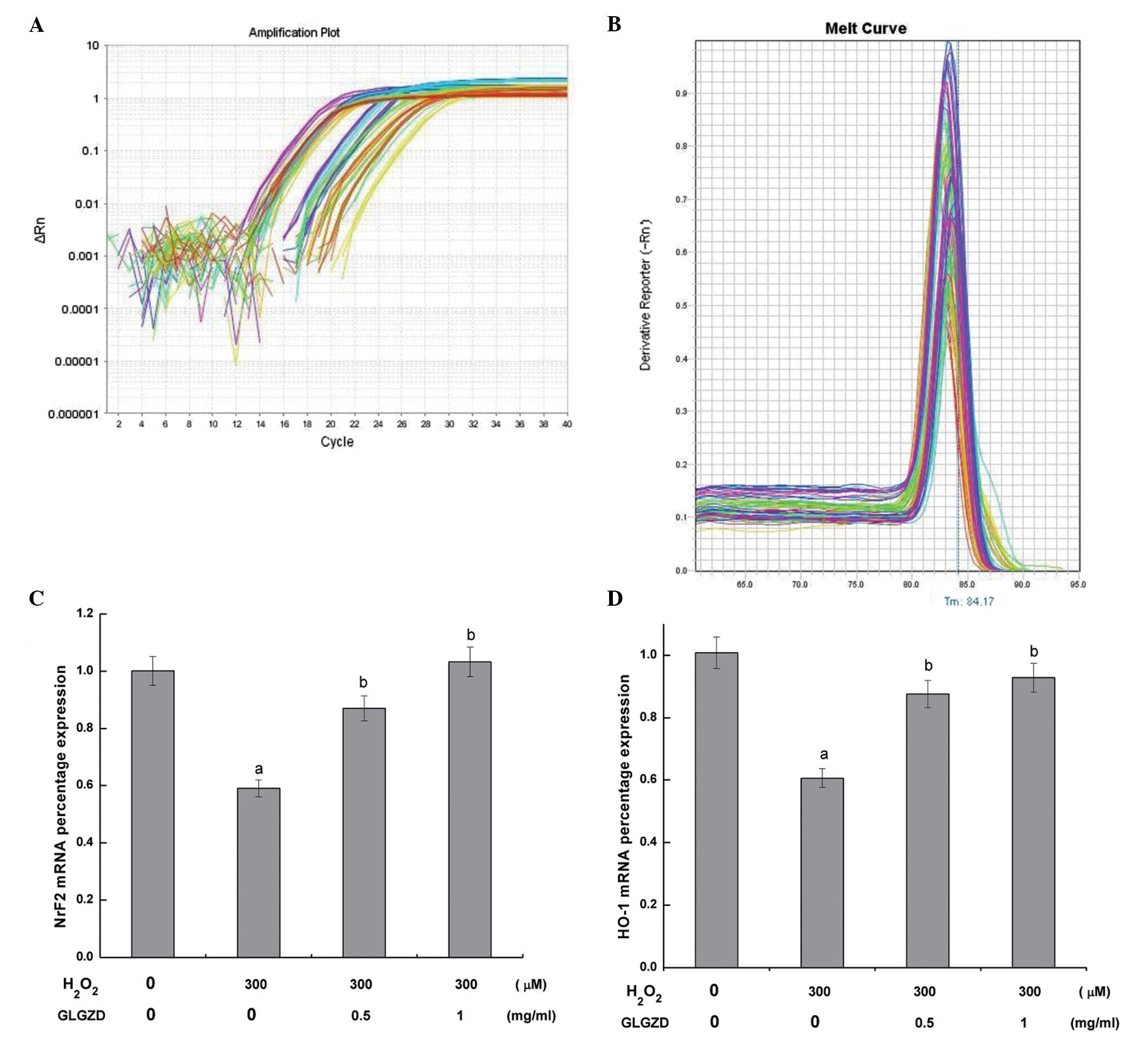
GLGZD protects PC12 cells against
H2O2-induced oxidative stress injury
To determine the mechanism by which GLGZD attenuated
H2O2-induced cell damage, the mRNA expression
levels of Nrf2 and HO-1 were determined by quantitative PCR
analysis (Fig. 4). Amplification
curves of Nrf2 and HO-1 mRNA expression in the PC12 cells are shown
in Fig. 4A. In SYBR green real-time
quantitative PCR-based melt-curve analysis each gene of real-time
fluorescent quantitative PCR is a single peak, the dissolution of
instructions are specific amplification (Fig. 4B). Statistically significant
decreases in the mRNA expression levels of Nrf2 and HO-1 were
observed in the cells treated with H2O2
alone, as compared with the control cells. Conversely, the mRNA
expression levels of Nrf2 and HO-1 were significantly increased
following treatment with 0.5 or 1.0 mg/ml GLGZD, as compared with
the cells treated with H2O2 alone.
Simultaneously, antibodies specific to the activated forms of Nrf2
and HO-1 were utilized to assess the protein expression levels,
since Nrf2 and HO-1 can protect nerves against oxidative injury
(16). Using confocal fluorescent
microscopy, fluorescent labeling of the antibodies targeting the
Nrf2 and HO-1 proteins was observed to be markedly weaker in the
group of cells treated with H2O2 alone when compared
with the control and GLGZD treatment groups. Alternatively, the
GLGZD treatment groups exhibited a higher fluorescence intensity
compared with the H2O2 treatment group (Figs. 5 and 6).
Discussion
TCMs, including Chinese herbs, such as Buyang Huanwu
decoction (19), Guizhi Fuling
capsules (20), Xiaoxuming decoction
(21), Danggui-shaoyao-san (22) and Yi-Gan-San (23), use compounds extracted from natural
products and have a relatively safe side-effect profile compared
with modern chemotherapeutics. In addition, such traditional
therapies have been used for millennia as alternative treatments
for various diseases and maladies. GLGZD is a classical TCM that
was first prescribed in the first century during the Eastern Han
Dynasty. Supporting the historic utilization and efficacy of this
traditional medicine, GLGZD was previously demonstrated to exhibit
considerable therapeutic effects on spasticity in stroke patients.
The anti-inflammatory and antiapoptotic mechanisms of GLGZD have
been shown to reduce the neurotoxicity of excitatory amino acids,
which lowers limb spasticity following a stroke (24–26). In
the assessment of cell viability, a tetrazolium salt, MTT, is
cleaved to form formazan by the mitochondrial respiratory chain
enzyme, succinate dehydrogenase, in living cells. In the present
study, an MTT assay was performed to assess the cell viability, and
the results demonstrated that following PC12 cell differentiation,
pretreatment with GLGZD at 0.5 or 1 mg/ml for 24 h clearly
protected the cells from damage caused by
H2O2, and improved the overall
cell survival rate (Fig. 2). In
addition, morphological observations using inverted microscopy
revealed that GLGZD treatment improved
H2O2-induced cell narrowing,
with the cells appearing round or polygonal, with an increased cell
diopter, cell connectivity and adherent conditions (Fig. 1). Measurement of ATP levels is a
classic method for the assessment of cell mitochondrial function
and condition, and the results of the present study revealed that
GLGZD pretreatment in the PC12 cells significantly elevated the
levels of ATP in the injury model group, indicating that GLGZD is
able to reduce the extent of
H2O2 damage inflicted on the
mitochondria in PC12 cells (Fig.
3).
Activation of the Nrf2/ARE signaling pathway has
been shown to exert a protective effect on cerebral ischemia
reperfusion injury, and high levels of Nrf2 and HO-1 resistance in
the central nervous system are known to invoke key internal and
external oxidation protection (27).
Dietz et al reported that Nrf2, through interacting with the
ARE, altered the expression of coding antioxidants and detoxifying
enzymes, which subsequently protected cells against oxidative
stress (28). Currently, the
Nrf2/ARE pathway appears to be the most important endogenous
antioxidant stress pathway (29).
Nrf2 regulates the cell oxidation stress reaction of important
transcription factors through interacting with oxidation reaction
components, such as the ARE, which subsequently affects the coding
of antioxidants, and the pathways activated can trigger the
downstream expression of HO-1 and other protective genes, fully
enhancing the cell protection activities (30,31). In
the present study, the reverse transcription quantitative PCR
results revealed that preconditioning with different concentrations
of GLGZD upregulated the mRNA expression levels of Nrf2 and HO-1 in
H2O2-damaged PC12 cells
(Fig. 4). In addition, the confocal
laser scanning microscopy data indicated that preconditioning with
GLGZD enhanced the protein expression levels of Nrf2 and HO-1 in
H2O2-injured PC12 cells
(Figs. 5 and 6).
In conclusion, the results of the present study
demonstrated that GLGZD is able to protect PC12 cells from
H2O2-induced damage, which may
be associated with the observed upregulation of the gene and
protein expression levels of Nrf2 and HO-1. Therefore, prompt
antioxidant therapy may be a therapeutic option to target one of
the important mechanisms underlying spasticity following a stroke.
To the best of our knowledge, the present study has reported for
the first time that GLGZD exerts a neuroprotective effect against
H2O2-induced nerve cell damage.
These results provide evidence that GLGZD may be a potential
therapeutic agent for the treatment of post-stroke neuronal cell
death and spasticity.
Acknowledgements
This study was supported by grants from the Guidance
Project of the Fujian Provincial Department of Science and
Technology (no. 2012D012), the Natural Science Foundation of Fujian
Province (no. 2013J01378), the Project of Fujian Education
Department (no. JK2012216) and the Project of Fujian Education
Department (no. JA2012179).
Glossary
Abbreviations
Abbreviations:
|
GLGZD
|
Gua Lou Gui Zhi decoction
|
|
Nrf2
|
nuclear factor (erythroid-derived
2)-like 2
|
|
HO
|
heme oxygenase
|
|
H2O2
|
hydrogen peroxide
|
|
PCR
|
polymerase chain reaction
|
|
NGF
|
nerve growth factor
|
|
ARE
|
antioxidant response element
|
|
TCM
|
traditional Chinese medicine
|
References
|
1
|
Elovic E: Principles of pharmaceutical
management of spastic hypertonia. Phys Med Rehabil Clin N Ant.
12:793–816. 2001.
|
|
2
|
Watkins CL, Leathley MJ, Gregson JM, Moore
AP, Smith TL and Sharma AK: Prevalence of spasticity post stroke.
Clin Rehabil. 16:515–522. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Duncan PW, Zorowitz R, Bates B, et al:
Management of adult stroke rehabilitation care: A clinical practice
guideline. Stroke. 36:e100–e143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao L: Jia Zi Shi Xiao Fang. China Press
Traditional Chinese Med. 14:2010.(In Chinese).
|
|
5
|
Zhang L and Ai H: Effects of Gua Lou Gui
Zhi Decoction on c-fos and c-jun on epileptic rats. Shi Yong Zhong
Yi Yao Za Zhi. 23:21–22. 2005.(In Chinese).
|
|
6
|
Sun X: Research on formula treating
paralysis and spasticity From ‘Treatise on febrile and
miscellaneous diseases’. Zhongguo Zhong Yi Ji Chu Yi Xue Za Zhi.
8:644–645. 2010.(In Chinese).
|
|
7
|
Chen Y, Chen L and Tao J: Clinical
research on treating limbs spasm from cerebral apoplexy with the
Gualou Guizhi decoction. Clin J Chin Med. 5:7–9. 2013.(In
Chinese).
|
|
8
|
Yang C, Chen LD and Tao J: New usage of a
classical formula - Gua Lou Gui Zhi Decoction. Liaoning J Tradit
Chin Med. 8:1599–1600. 2012.(In Chinese).
|
|
9
|
Li W and Wang X: The research progress of
PC12 cells as oxidative stress model. Zhong Guo Zhong Xi Yi Jie He
Za Zhi. 31:1575–1580. 2011.(In Chinese).
|
|
10
|
Candelario-Jalil E: Injury and repair
mechanisms in ischemic stroke: Considerations for the development
of novel neurotherapeutics. Curr Opin Investig Drugs. 10:644–654.
2009.PubMed/NCBI
|
|
11
|
Markesbery WR and Lovell MA: Damage to
lipids, proteins, DNA and RNA in mild cognitive impairment. Arch
Neurol. 64:954–956. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Adibhatla RM, Dempsy R and Hatcher JF:
Integration of cytokine biology and lipid metabolism in stroke.
Front Biosci. 13:1250–1270. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rodrigo J, Fernandez AP, Serrano J,
Peinado MA and Martinez A: The role of free radicals in cerebral
hypoxia and ischemia. Free Radic Biol Med. 39:26–50. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peruche B and Krieglstein J: Neuroblastoma
cells for testing neuroprotective drug effects. J Pharmacol Meth.
26:139–148. 1991. View Article : Google Scholar
|
|
15
|
Cui Y, Ma HY and Kong L: Research progress
on Nrf2/ARE pathway and mechanism of antioxidation. Jilin Da Xue
Xue Bao. Yi Xue Ban. 37:187–190. 2011.(In Chinese).
|
|
16
|
Tulis DA, Durante W, Peyton KJ, Evans AJ
and Schafer AI: Heme oxygenase-1 attenuates vascular remodeling
following balloon injury in rat carotid arteries. Atherosclerosis.
155:113–122. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maines MD: Heme oxygenase: Function,
multiplicity, regulatory mechanisms and clinical application. FASEB
J. 2:2557–2568. 1988.PubMed/NCBI
|
|
18
|
Li X, Jing C, Zang Q, Yang S and Wang J:
Toxic cytological alteration and mitochondrial dysfunction in PC12
cells induced by 1-octyl-3-methylimidazolium chloride. Toxicol In
Vitro. 26:1087–1092. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao LD, Wang JH, Jin GR, Zhao Y and Zhang
HJ: Neuroprotective effect of Buyang Huanwu decoction against focal
cerebral ischemia/reperfusion injury in rats - time window and
mechanism. J Ethnopharmacol. 140:339–344. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li TJ, Qiu Y, Mao JQ, Yang PY, Rui YC and
Chen WS: Protective effects of Guizhi-Fuling capsules on rat brain
ischemia/reperfusion injury. J Pharmacol Sci. 105:34–40. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu XH, Li SJ, Hu HH, Sun LR, Das M and
Gao TM: Neuroprotective effects of Xiao-Xu-Ming decoction against
ischemic neuronal injury in vivo and in vitro. J Ethnopharmacol.
127:38–46. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qian YF, Wang H, Yao WB and Gao XD:
Aqueous extract of the Chinese medicine, Danggui-Shaoyao-San,
inhibits apoptosis in hydrogen peroxide-induced PC12 cells by
preventing cytochrome C release and inactivating of caspase
cascade. Cell Biol Int. 32:304–311. 2008.PubMed/NCBI
|
|
23
|
Kawakami Z, Kanno H, Ikarashi Y and Kase
Y: Yokukansan, a kampo medicine, protects against glutamate
cytotoxicity due to oxidative stress in PC12 cells. J
Ethnopharmacol. 134:74–81. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu H, Li Z, Zhu X, Lin R, Lin J, Peng J,
Tao J and Chen L: Gua Lou Gui Zhi decoction suppresses LPS-induced
activation of the TLR4/NF-κB pathway in BV-2 murine microglial
cells. Int J Mol Med. 31:1327–32. 2013.PubMed/NCBI
|
|
25
|
Li Z, Hu H, Lin R, Mao J, Zhu X, Hong Z,
Tao J, Zhang Y and Chen L: Neuroprotective effects of Gua Lou Gui
Zhi decoction against glutamate-induced apoptosis in BV-2 cells.
Int J Mol Med. 33:597–604. 2014.PubMed/NCBI
|
|
26
|
Huang J, Tao J, Xue X, Yang S, Han P, Lin
Z, Xu W, Lin J, Peng J and Chen L: Gua Lou Gui Zhi decoction exerts
neuroprotective effects on post-stroke spasticity via the
modulation of glutamate levels and AMPA receptor expression. Int J
Mol Med. 31:841–848. 2013.PubMed/NCBI
|
|
27
|
Son TG, Camandola S, Arumugam TV, et al:
Plumbagin, a novel NrF2/ARE activator, protects against cerebral
ischemia. J Neurochem. 112:1316–1326. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dietz BM, Liu D, Hagos GK, et al:
Angelica sinensis and its alkylphthalides induce the
detoxification enzyme NAD(P)H: Quinone oxidoreductase 1 by
alkylating Keap1. Chem Res Toxicol. 21:1939–1948. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Adibhatla RM, Dempsy R and Hatcher JF:
Integration of cytokine biology and lipid metabolism in stroke.
Front Biosci. 13:1250–1270. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li MH, Cha YN and Surh YJ: Peroxynitrite
induces HO-1 expression via PI3K/Akt-dependent activation of
NF-E2-related factor 2 in PC12 cells. Free Radic Biol Med.
41:1079–1091. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee JM, Shih AY, Murphy TH and Johnson JA:
NF-E2-related factor-2 mediates neuroprotection against
mitochondrial complex I inhibitors and increased concentrations of
intracellular calcium in primary cortical neurons. J Biol Chem.
39:37948–37956. 2003. View Article : Google Scholar
|