Introduction
Anthracyclines (ANTs) are among the most effective
anticancer drugs used in the treatment of a wide spectrum of
malignancies. Regrettably, their clinical use is limited by the
occurrence of dose-related cardiotoxicity (4).
Several studies have shown that chemotherapy-induced
cardiotoxicity (CTX) produced by ANTs is at least partially
mediated by chronic inflammation and oxidative stress, with
proinflammatory cytokines, interleukin-6 (IL-6) and tumor necrosis
factor (TNF)-α and reactive oxygen species (ROS) (2) playing a central role (5,6). It
has also been shown that the use of a conventional cardioprotective
agent, such as dexrazoxane, together with chemotherapy, reduces the
expression of the NRF-2 gene (responsible for oxidative stress
response), which is overexpressed in patients receiving ANT alone
(2).
We previously identified an epirubicin (EPI)-induced
early myocardial dysfunction, detected after a low dose (200 mg/
m2) of EPI (7). This
dysfunction was shown to be correlated with a significant increase
in several biological markers of inflammation and oxidative stress,
and persisted throughout EPI treatment and up to the 18-month
follow-up (FU) (8).
Recent accumulating evidence suggests the
involvement of the renin-angiotensin system (RAS) in ANT-induced
CTX. Angiotensin II plays a crucial role, not only as a
vasoconstrictor agent, but also as a mitogenic factor by
interacting with angiotensin II type-1 receptors (AT1Rs) in
cardiovascular myocytes (9).
Cardiac dysfunction after doxorubicin has not been demonstrated in
the knockout rat for the AT1R gene, a finding confirmed by the
absence of apoptosis and myofibrillar damage (10). In a recent study, the
cardioprotective effect of the angiotensin receptor blocker
(11), telmisartan (TEL), has been
demonstrated in rats exposed to ANT (12). The authors argued that the effect
was sustained through a decrease in oxidative stress, which in turn
was able to reduce the structural damage of cardiomyocytes.
Regarding the possible role of ARBs in mitogenesis and
angiogenesis, these drugs were found to suppress the signal
transduction mediated by growth factors, such as the epidermal
growth factor (EGF), through AT1R antagonism (13). Furthermore, the ARB, telmisartan,
was shown to inhibit the proliferation of prostate cancer cells
through activation of the peroxisome proliferator-activated
receptor (PPAR)-γ (3).
In a previous phase II placebo (PLA)-controlled
study we used TEL in order to prevent EPI-induced myocardial damage
(14). We aimed to exploit the
ability of this drug to inhibit the production of superoxide
radicals by mitochondrial NADPH-dependent oxidase and xanthine
oxidase (15), and to, at least
partially, antagonize PPAR-γ activation (16). In this earlier study, we reported
that TEL was able to reduce EPI-induced oxidative stress/chronic
inflammation and to reverse early myocardial impairment (14).
The aim of the present study was to confirm a
long-lasting cardioprotective activity of TEL, up to a 12-month FU
(3), in preserving the systolic
function [assessed as strain rate (SR)] (primary endpoint) and
reducing inflammation and oxidative stress (secondary endpoint) in
a population of cancer patients treated with EPI. We also assessed
the changes in the above parameters at intermediate time points (3-
and 6-month FU) as tertiary endpoints.
Patients and methods
Patient population and study
protocol
From September 2008 to October 2009, we enrolled 49
consecutive eligible patients (male/female ratio, 12/37) with
histologically confirmed tumors at different sites, previously
untreated. All eligible patients were included. The majority of
patients (n=30) had early-stage cancer and only a minority had
oligometastatic disease. Patients were eligible for an EPI-based
chemotherapy regimen and were followed up until a maximal
cumulative dose of 400±20 mg/m2 (mean ± SD), according
to the international standardized protocols for EPI-based
administration. Inclusion criteria were the following: patients
18–70 years of age; an echocardiographic left ventricle ejection
fraction (LVEF) value ≥55%; an SR value in the normal range
(1.7–2.1 cm/ sec); an Eastern Cooperative Oncology Group (ECOG)
performance status score of 0–2 (11); normal hepatic and renal function
(bilirubin ≤1.5 mg/dl and creatinine ≤2.0 mg/dl); no concomitant
medications known to interfere with inflammatory and oxidative
stress parameters. Patients were not eligible if they had a history
of cardiac disease, hypertension, diabetes and/or had been
previously treated with mediastinal radiotherapy. The study was a
one-institution ‘independent’, randomized, PLA-controlled trial and
was approved by the Institutional Ethics Committee of the ‘Azienda
Ospedaliero Universitaria’ of Cagliari, Italy. Written informed
consent was obtained by all patients included in the study.
A blind randomization was performed: 25 patients
were randomized by a block randomization technique to the TEL
(1) arm and 24 to the PLA arm.
Clinical characteristics of patients in each arm are summarized in
Table I. Patients were treated
with 40 mg of TEL (Micardis®, Boehringer-Ingelheim,
Milan, Italy), 1 tablet/day, or PLA starting 1 week before the
beginning of EPI treatment and up to 6 months after EPI
discontinuation. The PLA tablets were supplied from the
insitutional pharmacy and were identical in appearance and taste to
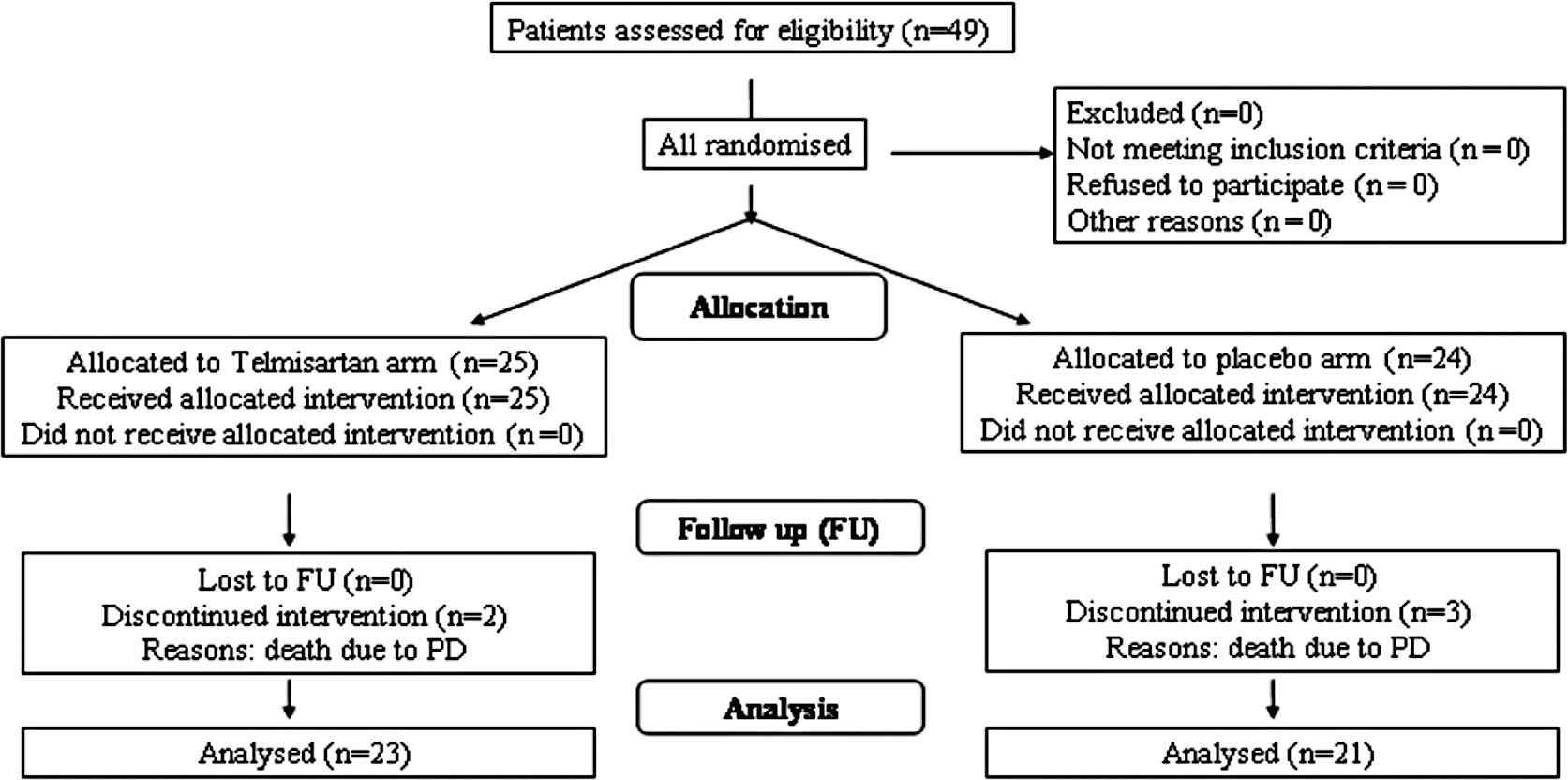
TEL. The consort diagram is shown in Fig. 1.
 | Table I.Clinical characteristics of the
patients. |
Table I.
Clinical characteristics of the
patients.
| PLA | TEL |
|---|
| Patients | 24 | 25 |
| Gender
(male/female) | 6/18 | 6/19 |
| Age, years (mean
± SD) | 53±10 | 52.9±9 |
| Tumor site | | |
| Endometrium | 9 | 12 |
| Salivary
gland | 1 | 0 |
| Non Hodgkin’s
lymphoma | 2 | 1 |
| Breast | 10 | 8 |
| Ovary | 1 | 4 |
| Lung (NSCLC) | 1 | 0 |
| Stage | | |
| I | 13 | 14 |
| II | 7 | 6 |
| III | 3 | 4 |
| IV | 1 | 1 |
| ECOG PS | | |
| 0 | 15 | 18 |
| 1 | 4 | 6 |
| 2 | 5 | 1 |
| BMI | | |
| <18.5 | 0 | 0 |
| 18.5–25 | 20 | 20 |
| >25 | 4 | 5 |
Clinical and laboratory assessments
At enrollment, before randomization as well as after
each subsequent administration of EPI, patients underwent a
physical examination, blood pressure measurement, 12-lead
electrocardiogram and echocardiographic analysis (conventional and
Tissue Doppler Imaging; TDI). The following laboratory tests were
carried out: blood and platelet count, BUN, uric acid, creatinine,
blood and urine electrolytes, direct and indirect bilirubin, AST,
ALT, γGT, alkaline phosphatase, iron, ferritinemia and blood
transferrin. In all patients, blood samples were collected for the
assessment of circulating levels of proinflammatory cytokines (IL-6
and TNF-α), ROS and antioxidant enzymes, glutathione peroxidase
(GPx) and superoxide dismutase (SOD). The instrumental and
laboratory variables were assessed at baseline (t0), 7
days after reaching the EPI dose of 100, 200, 300 and 400
mg/m2 (t1, t2, t3 and
t4, respectively) and at the 3, 6 and 12-month FU
(3). The reported doses of EPI
were always intended to be cumulative.
Conventional echocardiography and
TDI
Echocardiographic images were recorded using a
commercially available system equipped with TDI, strain (S) and
strain rate (SR) imaging (Toshiba APLIO CV ultrasound system-SSA
770A/CV; Toshiba Corp., Tochigi, Japan). LVEF was obtained from the
apical 4- and 2-chamber views according to Simpson’s rule and was
considered abnormal under 55%.
Conventional echocardiography parameters, such as
left ventricular end diastolic diameter (LVEDD) and atrial
dimensions, were assessed in both arms.
A pulsed wave Doppler (PWD) examination of the LV
inflow from the 4-chamber view was performed, with the sample
volume placed between the mitral leaflet tips and the early (E) and
late (A) diastolic peak velocities; E deceleration time (DecT) was
measured and the E/A ratio was derived. We evaluated the
longitudinal function using pulsed TDI at the mitral annulus,
placing the sample volume in the basal segment of the
interventricular septum (IVS) from the apical 4-chamber view; peak
velocities in systole (Sm), isovolumic relaxation time
(IVRT), early (Em) and late (Am) diastole
were measured. LV longitudinal function was evaluated from raw
data; myocardial S and SR were also quantified in the IVS. The same
experienced echocardiographer carried out all examinations of each
individual patient. To reduce inter-observer variability, all
echocardiographic data were randomly read by a second experienced
observer and an average value for each measurement was calculated.
Reproducibility of TDI parameters in our laboratory have been
previously documented.
Inflammatory and oxidative stress
markers
Blood samples were obtained from all patients by
venipuncture of the antecubital vein at 8 a.m., after overnight
fasting. Levels of IL-6 and TNF-α were determined by enzyme-linked
immunosorbent assay (Immunotech, Marseille, France) and expressed
in pg/ ml. Blood levels of ROS were determined using fresh
heparinized blood samples using the free oxygen radical test
(FORT). Results are expressed as FORT units (U), where 1 FORT U
corresponds to 0.26 mg/l of H2O2. The
erythrocyte antioxidant enzymes, GPx and SOD, were measured by a
photometer using a commercially available kit (Ransod; Randox
Laboratory, Crumlin, UK) and expressed as U/l and U/ml,
respectively.
Statistical analysis
Considering an α type error of 0.05, a β type error
of 0.10 and a difference in SR changes between arms of 10% of the
primary endpoint (SR change) as clinically meaningful, 50 patients
were enrolled in each arm. An interim analysis on the basis of the
early-stopping rules was planned after the enrollment of 50
patients. Treatment arms were compared by the Student’s t-test for
changes. Differences between values measured at different times
(different EPI doses) and at the 3-, 6- and 12-month FU were
calculated by the ANOVA test. The correlation between instrumental
(TDI) and laboratory variables was assessed by Pearson’s t-test (or
Spearman’s t-test for non-parametric variables). Significant
relationships were then examined by multivariate linear regression
analysis. Results were considered significant for p-values ≤0.05.
Data are reported as the means ± SD. Statistical analysis was
performed using SPSS version 14 for Windows.
Results
The two treatment arms were well-matched in terms of
age, ECOG performance status, site and stage of disease at
enrollment (Table I). All patients
reached the scheduled cumulative EPI dose of 400 mg/m2.
Regarding tumor history, it should be noted that overall 5 patients
died at 5±2 months after the end of EPI treatment due to disease
progression (PD): 2 patients in the TEL arm and 3 patients in the
PLA arm (Fig. 1). Moreover, at the
12-month FU, PD was noted in 1/23 patients in the TEL arm and in
2/21 patients in the PLA arm.
ECG monitoring
At ECG monitoring, a normal morphology was observed
throughout the treatment in 33 patients; in 16 patients (9 in the
TEL and 7 in the PLA arm) widespread and unspecified changes were
observed during the ventricular repolarization phase concurrent
with t2, with no significant differences between the TEL
and PLA arm.
Conventional echocardiography and
TDI
In the PLA arm, 1 week after t2, as well
as at t3, a significant LV diastolic impairment was
observed, represented by a reduction in the E/A ratio at PWD
examination (p<0.05; Table II).
In the TEL arm, a slight reduction in the E/A ratio was observed,
which, however, did not reach statistical significance. No
significant abnormalities of LVEF and DecT were found in any of the
two arms throughout the treatment (Table II).
 | Table II.Conventional echocardiographic
parameters of systolic and diastolic function in both arms. |
Table II.
Conventional echocardiographic
parameters of systolic and diastolic function in both arms.
| Conventional
echo | t0
(n=49) | t2
(n=49) | t3
(n=49) | t4
(n=49) | 12-month FU
(n=44) |
|---|
| LVEF | | | | | |
| PLA | 66±5% | 68±6% | 66±5% | 66±5% | 67±5% |
| TEL | 66±7% | 67±6% | 68±4% | 70±6% | 68±4% |
| DecT | | | | | |
| PLA | 0.22±0.04 | 0.24±0.05 | 0.22±0.02 | 0.23±0.04 | 0.22±0.03 |
| TEL | 0.19±0.04 | 0.21±0.04 | 0.20±0.02 | 0.21±0.03 | 0.21±0.04 |
| E/A | | | | | |
| PLA | 1.13±0.14 | 1.08±0.12 | 0.92±0.05a | 0.90±0.06a | 1.06±0.42 |
| TEL | 0.96±0.12 | 0.86±0.08 | 0.83±0.07 | 0.95±0.14 | 0.87±0.31 |
Conventional echocardiography parameters, LVEDD and
atrial dimensions, were in the normal range and did not differ
between arms at baseline; they did not change during treatment up
to the 12-month FU (data not shown).
TDI echocardiographic analysis revealed in the PLA
arm an LV diastolic impairment, indicated by a reduction in the
Em/ Am ratio measured in the basal portion of
IVS, first recognized at t2 (p<0.05; Table III). This impaired function
persisted throughout the treatment, at t3 (p<0.05)
and t4 (p<0.05; Table
III), whereas in the TEL arm this diastolic impairment did not
occur (the Em/Am ratio did not change
significantly). At the 12-month FU, the Em/Am
ratio returned within the t0 range in the PLA arm. The
other TDI parameters (Em, Sm and S) did not
show any significant changes during treatment up to the 12-month FU
in any of the two arms (Table
III).
 | Table III.TDI echocardiographic parameters of
systolic and diastolic function in both arms. |
Table III.
TDI echocardiographic parameters of
systolic and diastolic function in both arms.
| TDI echo | t0
(n=49) | t2
(n=49) | t3
(n=49) | t4
(n=49) | 12-month FU
(n=44) |
|---|
| Em | | | | | |
| PLA | 8.66±4.90 | 8.64±6.07 | 7.73±4.90 | 7.54±3.50 | 7.88±2.07 |
| TEL | 7.89±2.14 | 7.33±2.45 | 7.53±2.17 | 6.93±1.46 | 7.74±1.58 |
|
Em/Am | | | | | |
| PLA | 1.13±0.26 | 0.85±0.35a | 0.72±0.30a | 0.75±0.32a | 0.96±0.27 |
| TEL | 0.90±0.11 | 0.84±0.07 | 0.85±0.05 | 0.71±0.23 | 0.78±0.28 |
| Sm | | | | | |
| PLA | 7.15±0.65 | 7.28±1.71 | 7.28±0.82 | 6.83±0.69 | 7.25±1.37 |
| TEL | 7.33±1.76 | 7.09±1.21 | 7.04±1.12 | 7.29±1.08 | 7.33±1.22 |
| Strain | | | | | |
| PLA | 20.89±1.96 | 20.75±2.06 | 18.00±2.55 | 18.65±1.25 | 16.78±2.22 |
| TEL | 22.80±1.54 | 21.20±1.86 | 20.40±0.94 | 19.90±0.92 | 19.64±1.82 |
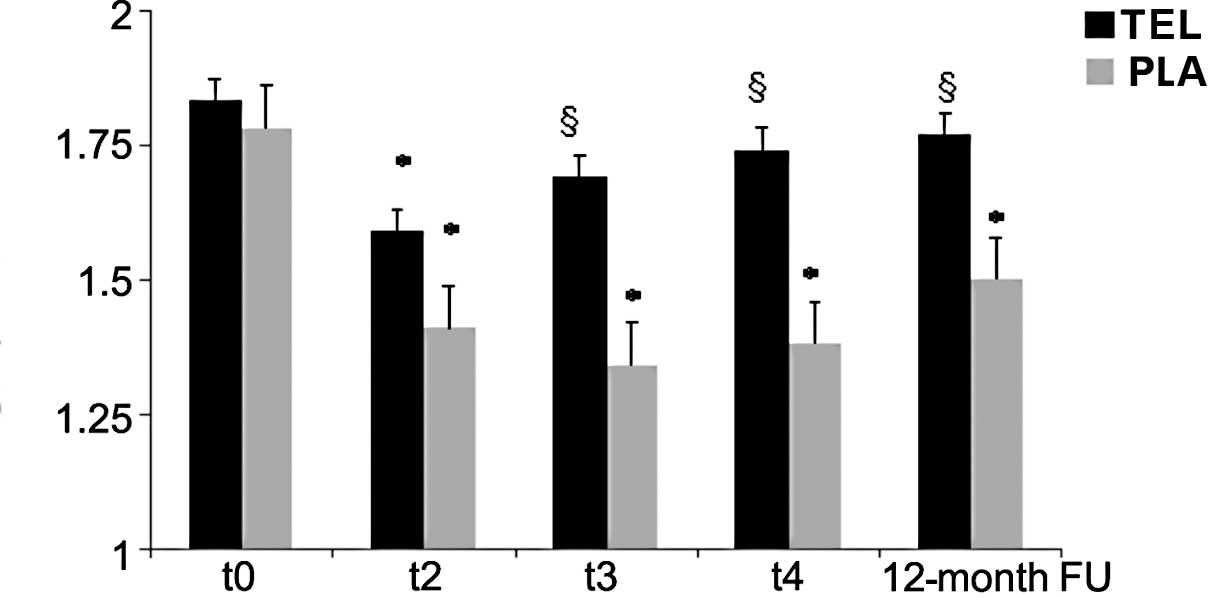
A significant reduction in the SR peak both in the
TEL and PLA arms was observed at t2 (cumulative dose of
200 mg/ m2 EPI) in comparison to t0
(1.45±0.33 vs. 1.54±0.35 s-1; NS). Conversely, at t3
(300 mg/m2 EPI), t4 (400 mg/m2
EPI) and the 12-month FU, the SR increased reaching the normal
range only in the TEL arm, while in the PLA arm the SR remained
significantly lower as compared to t0 (baseline).
The differences between SR changes in the PLA and
TEL arms were significant at t3, t4 and the
12-month FU (Fig. 2).
Inflammation and oxidative stress
markers
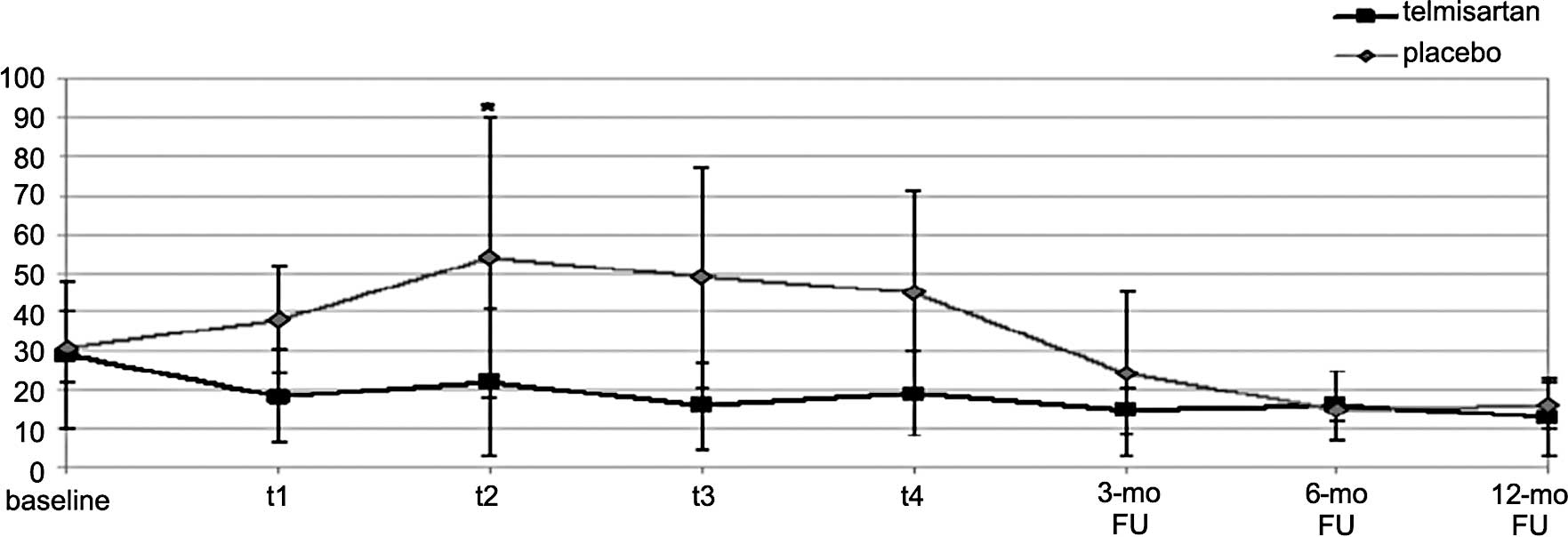
Serum levels of IL-6 increased significantly in the
PLA arm at t2 in comparison to baseline (68.8±52.6 vs.
31±8.8 pg/ml, p<0.005), but remained unchanged in the TEL arm
(28±18.1 vs. 26±18.8 pg/ ml) (Fig.
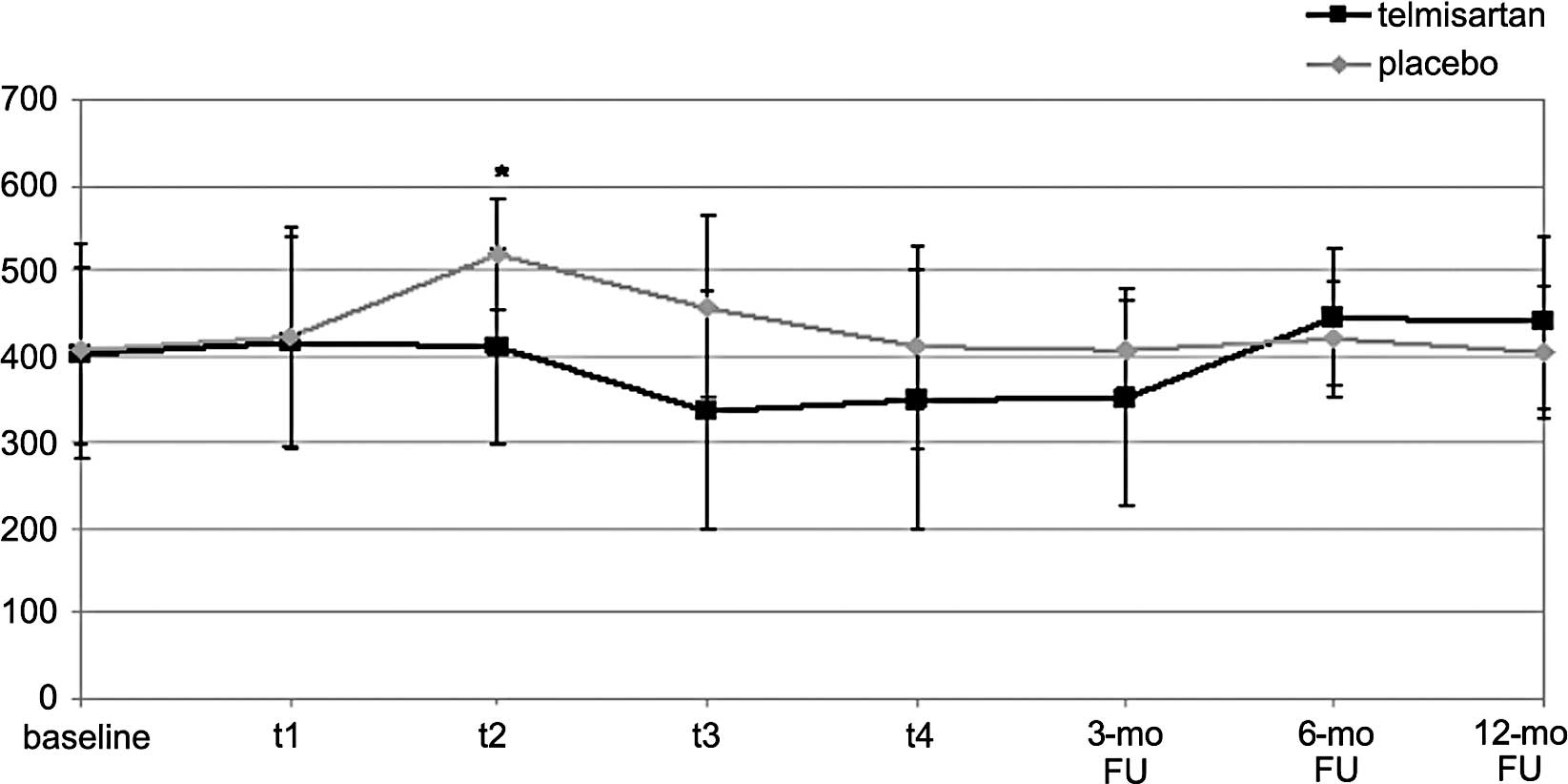
3). The same trend was shown for ROS levels which significantly
increased at t2 vs. baseline in the PLA arm (518±65 vs.
407.3±126 FORT-U, p<0.05), while they remained unchanged in the
TEL arm (438±105 vs. 452±61 FORT-U; NS) (Fig. 4). The mean change in IL-6 and ROS
at t2 was significantly different between the two arms
(Figs. 3 and 4).
The other laboratory parameters (TNF-α, GPx and SOD)
did not show significant changes in any of the two arms throughout
the study (Table IV).
 | Table IV.Inflammation and oxidative stress
markers in both arms. |
Table IV.
Inflammation and oxidative stress
markers in both arms.
| Parameters | t0
(n=49) | t2
(n=49) | t3
(n=49) | t4
(n=49) | 12-month FU
(n=44) | p-value (ANOVA
test) |
|---|
| TNF-α | | | | | | |
| PLA | 30.1±9.0 | 47.1±4.6 | 30.0±14.4 | 25.8±8.6 | 30.0±14.4 | 0.32 |
| TEL | 23.5±5.5 | 22.3±10.6 | 35.1±8.6 | 25.9±4.2 | 35.1±8.6 | 0.56 |
| GPx | | | | | | |
| PLA | 7,386±3,041 | 6,898±1,552 | 9,427±3,078 | 10,232±1,875 | 9,427±3,078 | 0.56 |
| TEL | 7,415±2,182 | 6,381±2,137 | 7,263±2,998 | 7,479±1,769 | 7,263±2,998 | 0.88 |
| SOD | | | | | | |
| PLA | 130.0±9.2 | 138.0±2.1 | 193.0±41.0 | 148.0±38.0 | 135.0±26.0 | 0.32 |
| TEL | 126.1±41.6 | 150.0±37.0 | 140.0±40.0 | 145.0±74.0 | 150.0±37.0 | 0.95 |
Correlations between echocardiographic
and laboratory data
In the PLA arm, the early impairment of the TDI
parameters (calculated as Δ by subtracting the values at
t2 from the baseline values) was also correlated with
changes in circulating levels of inflammation and oxidative stress
markers. Significant correlations were found between ΔSR, increase
in IL-6 and ROS (r=0.58, p<0.005 and r=0.51, p<0.05,
respectively) in the PLA arm.
Safety
In regards to TEL, the drug was well tolerated by
all patients throughout the study. However, a significant
hypotension episode (blood pressure values <95/50 mmHg) was
observed only once in 2 patients (approximately corresponding to
t2 and t3). For this event, the TEL dose was
reduced from 40 to 20 mg/day for 2 subsequent weeks, and thereafter
the full dose of 40 mg/day was re-established.
As for EPI-related side effects, the only
significant adverse event was a grade 3/4 neutropenia observed at
t3 in 6 patients who needed administration of
granulocyte colony-stimulating factor and a postponement of the
subsequent EPI-based cycle.
Discussion
The present study confirmed the results reported in
our previous work (14) and
various important novel findings were demonstrated: i) a reduction
in the SR peak at t2 in both treatment arms with no
statistical difference between the two arms; ii) a persistent
reduction in the SR peak in the PLA arm at t3 and
t4 and, more importantly, the SR peak reduction
persisted also at the 12-month FU; iii) a recovery of the SR peak
in the TEL arm which, starting from t3, reached values
within the range of t0 which were persistent up to the
12-month FU (data shown for the first time in the present study);
iv) serum levels of IL-6 increased significantly in the PLA arm
from t0 to t2, then decreased subsequently to
the baseline range up to the 12-month FU (the latter is a new
finding), whereas serum levels of IL-6 in the TEL arm remained
unchanged from t0 to the 12-month FU; v) blood levels of
ROS demonstrated a superimposable pattern to that of IL-6; and vi)
changes in the SR peak, an echocardiographic equivalent of early
myocardial systolic dysfunction revealed by TDI, correlated with
changes in the levels of IL-6 and ROS, which are indicative of the
body inflammatory and oxidative stress status, only in the PLA arm
at t2. The results of the present study prompted us to
discontinue the study at the planned interim analysis based on the
early-stopping rules for superiority of the TEL arm due mainly to
ethical reasons.
The findings of the present study confirmed that
AT1R blockade by TEL, administered 1 week before and throughout the
duration of EPI treatment, was able to initially (t2)
reduce and later (t3 and t4) reverse
EPI-induced cardiac abnormalities. This effect was long-lasting and
still persisted at the 12-month FU. Moreover, TEL co-administration
also prevented increases in IL-6 and ROS levels after EPI
administration.
In previous reports, we found that a measurable
decline in the SR peak, currently regarded as the earliest sign of
subclinical CTX, may be detected in EPI-treated patients long
before the clinical evidence of heart failure (7). The subtle systolic impairment
appeared after 200 mg/m2 EPI, a dose which was, until
recently, considered insufficient to induce cardiac injury
(17). Moreover, a progressive
EPI-induced myocardial dysfunction, which was present even at the
18-month FU in a population of patients not treated with the
cardioprotective drugs (8), was
not noted in the present study in the TEL arm. As reported above,
TEL was shown to reverse the early myocardial dysfunction observed
at 200 mg/m2 EPI and, importantly, its beneficial
cardioprotective effect persisted up to the 12-month FU, i.e., 12
months after discontinuation of EPI chemotherapy and 6 months after
the end of TEL coverage.
A large body of evidence has confirmed the role of
the AT1Rs in mediating the damage caused by from myocardial
ischemia/reperfusion, resulting from acute ANT-induced CTX
(18,19). Accordingly, ANTs were found to
induce myofibrillar loss, increase the number of apoptotic cells
and significantly impair cardiac function in control mice, but not
in AT1R-knockout mice or in animals treated with an AT1R antagonist
(9). This evidence suggests that
an ARB, such as TEL, may be able to prevent ANT-induced CTX.
In a recent study, a protective effect of TEL
against acute ANT-induced CTX was demonstrated in rats; it was
shown that pre-treatment with the ARB TEL elicited a normalization
of significant biochemical parameters and reduced cardiac tissue
damage (12).
Therefore, the present study supports the previously
reported role of the RAS in the pathophysiology of
chemotherapy-induced CTX; in particular, it also demonstrates for
the first time in, a clinical trial, the anti-inflammatory and
antioxidant properties of TEL, previously observed only in
pre-clinical models (20).
Moreover, the beneficial effect shown by telmisartan may be
explained by its multiple therapeutic characteristics. Indeed, TEL
is a unique ARB with selective PPAR-γ-modulating activity which
affects nitric oxide bioavailability thus leading to its
anti-inflammatory, antioxidant and antiproliferative effects on
vascular wall cells (21).
Additionally, it has also been shown to play a role in lipid and
glucose metabolism (22).
Cytokines, sensitive markers of tissue damage, are
responsible for a negative inotropic effect in the failing human
heart (1,12) and in the pathophysiology of dilated
cardiomyopathy (22). The increase
in proinflammatory cytokines (IL-6) and oxidative stress markers
(2) after EPI administration
confirms that systemic inflammation/oxidative stress plays a
central role in the cardiac damage induced by EPI. Indeed,
significant correlations between cytokines/ROS levels and SR
decline, observed in our previous study (8) and confirmed in the PLA arm of the
present trial, suggest that an increase in inflammatory/ROS markers
may be analogous to early myocardial cell dysfunction shown by
TDI.
A pathogenetic hypothesis based on oxidative stress
has gained the widest acceptance in the study of acute EPI-induced
CTX. Its molecular basis is attributable to the one-electron redox
cycling of the quinone moiety, which generates ROS in excess of
limited cardiomyocyte antioxidant defenses (23). This cellular pathway results in
severe oxidative stress and disruption of the mitochondrial
energetic machinery, ultimately leading to cardiomyocyte apoptosis
or necrosis (24). Indeed, a
relationship was found between cytokine release and ROS increase in
patients with dilated cardiomyopathy (25).
The observation that TEL is able to prevent such a
number of potentially harmful effects induced by EPI, to which,
however, its antineoplastic therapeutic efficacy is attributable,
suggests that its administration may also compromise or weaken the
antitumor efficacy of ANT. To date, however, this hypothesis is not
supported by any data in the literature.
Regarding the use of TEL as a cardioprotective drug
in our present trial, it is to be noted that very recently (July
2010), and long after the beginning of our study (end of 2008) and
concomitantly with the publication of the first results (September
2010), a meta-analysis was published by Sipahi et al
(26). The authors concluded that
when the analysis was limited to TEL, the excess in new cancer
(lung cancer) occurrence was of borderline significance (p=0.05)
and that no statistically significant difference in cancer deaths
was observed. Furthermore, the TEL dose used in the trials which
were reviewed in the meta-analysis was 80 mg/day, i.e., double the
dose used in our trial. For these reasons we believe that the
findings of Sipahi et al have no bearing on our study.
In conclusion, the present study augments the
findings of our earlier research (14) which aimed to assess the
cardioprotective effect of TEL only during the period of EPI
administration. The present study subsequently demonstrated that
the protection obtained with the AT1R blockade had a long-lasting
effect, probably by ensuring a permanent (at least up to 12-month
FU) defense against chronic or late-onset types of ANT-induced CTX.
This finding is extremely important, since ANT-induced CTX persists
for years with no clinical symptoms, whereas upon the development
of overt heart failure, the prognosis becomes extremely poor,
possibly even worse than that of ischemic or idiopathic dilated
cardiomyopathy.
Abbreviations:
|
ANTs
|
Anthracyclines
|
|
CTX
|
chemotherapy-induced
cardiotoxicity
|
|
IL-6
|
interleukin-6
|
|
TNF
|
tumor necrosis factor
|
|
ROS
|
reactive oxygen species
|
|
EPI
|
epirubicin
|
|
FU
|
follow-up
|
|
RAS
|
renin-angiotensin system
|
|
AT1Rs
|
angiotensin II type-1 receptors
|
|
TEL
|
telmisartan
|
|
EGF
|
epidermal growth factor
|
|
PPAR
|
proliferator-activated receptor
|
|
PLA
|
placebo
|
|
SR
|
strain rate
|
|
LVEF
|
left ventricle ejection fraction
|
|
ECOG
|
Eastern Cooperative Oncology Group
|
|
TDI
|
Tissue Doppler Imaging
|
|
GPx
|
glutathione peroxidase
|
|
SOD
|
superoxide dismutase
|
|
S
|
strain
|
|
LVEDD
|
left ventricular end diastolic
diameter
|
|
PWD
|
pulsed wave Doppler
|
|
E
|
early diastolic peak velocities
|
|
A
|
late diastolic peak velocities
|
|
DecT
|
deceleration time
|
|
IVS
|
interventricular septum
|
|
Sm
|
systole
|
|
IVRT
|
isovolumic relaxation time
|
|
Em
|
early diastole
|
|
Am
|
late diastole
|
|
FORT
|
free oxygen radical test
|
|
PD
|
disease progression
|
Acknowledgements
This study was partially funded by the
AIRC (Associazione Italiana Ricerca per il Cancro), project no.
8679.
References
|
1.
|
Escobar E, Rodriguez-Reyna TS, Arrieta O
and Sotelo J: Angiotensin II, cell proliferation and angiogenesis
regulator: biologic and therapeutic implications in cancer. Curr
Vasc Pharmacol. 2:385–399. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Thompson KL, Rosenzweig BA, Zhang J, et
al: Early alterations in heart gene expression profiles associated
with doxorubicin cardiotoxicity in rats. Cancer Chemother
Pharmacol. 66:303–314. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Funao K, Matsuyama M, Kawahito Y, et al:
Telmisartan is a potent target for prevention and treatment in
human prostate cancer. Oncol Rep. 20:295–300. 2008.PubMed/NCBI
|
|
4.
|
Paulides M and Wojnowski L:
[Chemotherapeutics-induced heart failure]. Med Klin. 102:574–578.
2007.(In German).
|
|
5.
|
Meldrum DR, Cleveland JC Jr, Cain BS, Meng
X and Harken AH: Increased myocardial tumor necrosis factor-alpha
in a crystalloid-perfused model of cardiac ischemia-reperfusion
injury. Ann Thorac Surg. 65:439–443. 1998. View Article : Google Scholar
|
|
6.
|
Kupatt C, Habazettl H, Goedecke A, et al:
Tumor necrosis factor-alpha contributes to ischemia- and
reperfusion-induced endothelial activation in isolated hearts. Circ
Res. 84:392–400. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Mercuro G, Cadeddu C, Piras A, et al:
Early epirubicin-induced myocardial dysfunction revealed by serial
tissue Doppler echocardiography: correlation with inflammatory and
oxidative stress markers. Oncologist. 12:1124–1133. 2007.
View Article : Google Scholar
|
|
8.
|
Mantovani G, Madeddu C, Cadeddu C, et al:
Persistence, up to 18 months of follow-up, of epirubicin-induced
myocardial dysfunction detected early by serial tissue Doppler
echocardiography: correlation with inflammatory and oxidative
stress markers. Oncologist. 13:1296–1305. 2008. View Article : Google Scholar
|
|
9.
|
Toko H, Oka T, Zou Y, et al: Angiotensin
II type 1a receptor mediates doxorubicin-induced cardiomyopathy.
Hypertens Res. 25:597–603. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Soga M, Kamal FA, Watanabe K, et al:
Effects of angiotensin II receptor blocker (candesartan) in
daunorubicin-induced cardiomyopathic rats. Int J Cardiol.
110:378–385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Oken MM, Creech RH, Tormey DC, et al:
Toxicity and response criteria of the Eastern Cooperative Oncology
Group. Am J Clin Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Iqbal M, Dubey K, Anwer T, Ashish A and
Pillai KK: Protective effects of telmisartan against acute
doxorubicin-induced cardiotoxicity in rats. Pharmacol Rep.
60:382–390. 2008.PubMed/NCBI
|
|
13.
|
Ishiguro H, Ishiguro Y, Kubota Y and
Uemura H: Regulation of prostate cancer cell growth and PSA
expression by angiotensin II receptor blocker with peroxisome
proliferator-activated receptor gamma ligand like action. Prostate.
67:924–932. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Cadeddu C, Piras A, Mantovani G, et al:
Protective effects of the angiotensin II receptor blocker
telmisartan on epirubicin-induced inflammation, oxidative stress,
and early ventricular impairment. Am Heart J. 160:e481–e487. 2010.
View Article : Google Scholar
|
|
15.
|
Wenzel P, Schulz E, Oelze M, et al:
AT1-receptor blockade by telmisartan upregulates GTP-cyclohydrolase
I and protects eNOS in diabetic rats. Free Radic Biol Med.
45:619–626. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Stephen RL, Gustafsson MC, Jarvis M, et
al: Activation of peroxisome proliferator-activated receptor delta
stimulates the proliferation of human breast and prostate cancer
cell lines. Cancer Res. 64:3162–3170. 2004. View Article : Google Scholar
|
|
17.
|
Jensen BV, Skovsgaard T and Nielsen SL:
Functional monitoring of anthracycline cardiotoxicity: a
prospective, blinded, long-term observational study of outcome in
120 patients. Ann Oncol. 13:699–709. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Jalowy A, Schulz R and Heusch G: AT1
receptor blockade in experimental myocardial ischemia/reperfusion.
Basic Res Cardiol. 93(Suppl 2): 85–91. 1998. View Article : Google Scholar
|
|
19.
|
Ferreira AL, Matsubara LS and Matsubara
BB: Anthracycline-induced cardiotoxicity. Cardiovasc Hematol Agents
Med Chem. 6:278–281. 2008. View Article : Google Scholar
|
|
20.
|
Cianchetti S, del Fiorentino A, Colognato
R, di Stefano R, Franzoni F and Pedrinelli R: Anti-inflammatory and
anti-oxidant properties of telmisartan in cultured human umbilical
vein endothelial cells. Atherosclerosis. 198:22–28. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Yamagishi S and Takeuchi M: Telmisartan is
a promising cardiometabolic sartan due to its unique
PPAR-gamma-inducing property. Med Hypotheses. 64:476–478. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Tuck ML: Angiotensin-receptor blocking
agents and the peroxisome proliferator-activated receptor-gamma
system. Curr Hypertens Rep. 7:240–243. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Minotti G, Menna P, Salvatorelli E, Cairo
G and Gianni L: Anthracyclines: molecular advances and
pharmacologic developments in antitumor activity and
cardiotoxicity. Pharmacol Rev. 56:185–229. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Conklin KA: Coenzyme q10 for prevention of
anthracycline-induced cardiotoxicity. Integr Cancer Ther.
4:110–130. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Kaur K, Sharma AK, Dhingra S and Singal
PK: Interplay of TNF-alpha and IL-10 in regulating oxidative stress
in isolated adult cardiac myocytes. J Mol Cell Cardiol.
41:1023–1030. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Sipahi I, Debanne SM, Rowland DY, Simon DI
and Fang JC: Angiotensin-receptor blockade and risk of cancer:
meta-analysis of randomised controlled trials. Lancet Oncol.
11:627–636. 2010. View Article : Google Scholar : PubMed/NCBI
|