Introduction
Endometrial cancer accounts for approximately 4% of
all cancers in women and occurs predominantly after menopause. Some
of the highest incidence rates worldwide are found in the US and
European populations (1). Clinical
signs and symptoms associated with endometrial cancer include
postmenopausal bleeding and perimenopausal meno-metrorrhagia
(2). Women presenting with these
conditions undergo histological evaluation of the endometrium.
Typically, endometrial tissue sampling is combined with
hysteroscopy, i.e., the optical visualization of the endometrial
cavity via an endoscope (2–5).
Hysteroscopy is a highly accurate method and is useful in
diagnosing, rather than excluding endometrial cancer in women with
abnormal uterine bleeding (6).
This technique, however, flushes fluid and endometrial cells into
the abdomen via the fallopian tubes, thus potentially spreading
malignant cells. For example, in a prospective randomized study,
Nagele et al examined 30 women undergoing hysteroscopy and
concomitant laparoscopy for infertility. Endometrial cells were
present in the peritoneal fluid in 6% of patients before
hysteroscopy and in 25% after hysteroscopy (7). A number of studies have associated
hysteroscopy with an increased risk of positive peritoneal cytology
in women with endometrial cancer (8,9),
while certain studies have raised the possibility of an adverse
impact of hysteroscopy on prognosis in women with endometrial
cancer (10–13).
Despite these concerns, however, hysteroscopy is
considered a safe and acceptable procedure and is used extensively
for the evaluation of women with postmenopausal bleeding or
perimenopausal meno-metrorrhagia (14,15).
Whether or not the duration of hysteroscopy is a matter of concern
in women with suspected endometrial cancer is yet unknown. If
prolonged hysteroscopy increased the risk of positive peritoneal
cytology and disease recurrence, this would have clinical
implications regarding time restriction or avoidance of
hysteroscopy in women with suspected endometrial cancer.
To investigate this issue, we performed a
retrospective multi-centre study in a large series of women with
endometrial cancer who underwent pre-operative hysteroscopy. The
aim of our study was to investigate whether the duration of
hysteroscopy is associated with positive peritoneal cytology at
surgery and adverse prognosis in patients with endometrial
cancer.
Materials and methods
Five hundred and fifty-two patients with endometrial
cancer, treated at the Departments of Obstetrics and Gynecology of
the Medical University of Vienna, Austria (n=220), the Medical
University of Innsbruck, Austria (n=204), the Landeskrankenhaus
Klagenfurt, Austria (n=87), the Landeskrankenhaus Wiener Neustadt,
Austria (n=39) and the Department of Gynecologic Oncology, Royal
Adelaide Hospital, Adelaide, Australia (n=2), between February 1996
and July 2009, were included in the present study. Clinical and
laboratory data were extracted retrospectively from patient
files.
Diagnosis of endometrial cancer was established by
diagnostic hysteroscopy and dilation and curettage. Hysteroscopy
was performed with saline as the distension medium. Duration of
hysteroscopy was defined as the time between the start and the end
of the surgical procedure. The duration of hysteroscopy was noted
by the nursing staff on a surgical procedure documentation sheet.
Patients were surgically staged according to the International
Federation of Gynaecology and Obstetrics (FIGO)/American Joint
Committee on Cancer (AJCC) classification system. Hysterectomy,
bilateral salpingo-oophorectomy, cytological examination of
peritoneal fluid and biopsy of any suspicious intraperitoneal or
retroperitoneal lesions were performed. Pelvic and paraaortic
lymphadenectomy were performed, except for tumour stages FIGO Ia
and Ib (using the 1989 FIGO classification for endometrial cancer)
with histological grades 1 and 2 and endometrioid histology. In
patients with intermediate- or high-risk disease, adjuvant
radiotherapy was provided according to standardized treatment
protocols (16). A regimen of
adjuvant chemotherapy using carboplatin/ paclitaxel was used in
selected patients with advanced disease.
Three months after completion of primary therapy,
the first follow-up visit was scheduled. Patients had follow-up
visits at 3-month intervals for the following 2 years, including
inspection and vaginal-rectal palpation. In the third and fourth
year, visits were scheduled bi-annually and once a year from the
fifth year on. When patients did not present for the scheduled
follow-up visits, they were contacted by administrative personnel.
In the event of any clinically suspicious symptoms and/or elevation
of tumour markers, computed tomography was performed.
Values represent the means [standard deviation (SD)]
in the case of a normal distribution, or the medians (range) in the
case of a skewed distribution. T-tests and one-way ANOVA were used
to compare the duration of hysteroscopy and clinicopathological
parameters. Correlations are described by Spearman’s correlation
coefficient. Survival probabilities were calculated by the product
limit method of Kaplan and Meier. Differences between groups were
tested using the log-rank test. The results were analyzed for the
endpoint of disease-free survival. Events were defined as
recurrence, death or progression at the time of last follow-up.
Survival times of disease-free patients or patients with stable
disease were censored with the last follow-up date. Survival times
of patients who died due to other causes were censored with the
date of death. Univariate analysis and a multivariate Cox
regression model for disease-free survival were performed using
duration of hysteroscopy (≤15 vs. >15 min), FIGO tumour stage
(FIGO I vs. FIGO II–IV), histological grade (G1+G2 vs. G3) and
histological subtype (type I vs. type II). p-values <0.05 were
considered statistically significant. The statistical software SPSS
16.0 for Mac (SPSS 16.0.1; SPSS Inc., Chicago, IL, USA) was used
for statistical analysis.
The present study was approved by the Ethics
Committee of the Medical University of Vienna and the General
Hospital of Vienna.
Results
Patient characteristics are provided in Table I. Lymph node status was available
in 147/552 (27%) patients. Lymph node involvement was noted in
27/552 (5%) patients. Adjuvant radiotherapy was administered to
298/552 (54%) patients and 45/552 (8%) patients received adjuvant
chemotherapy.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Parameter | |
|---|
| Total no. of patients
enrolled | 552 |
| Age at diagnosis in
years, mean (SD) | 66.7 (10.9) |
| Histological type, n
(%) | |
| Type I | 520 (94.2) |
| Type II | 32 (5.8) |
| Tumour stage, n
(%) | |
| FIGO IA | 78 (14.1) |
| FIGO IB | 259 (46.9) |
| FIGO IC | 91 (16.5) |
| FIGO IIA | 24 (4.3) |
| FIGO IIB | 27 (4.9) |
| FIGO III | 59 (10.8) |
| FIGO IV | 14 (2.5) |
| Histological grade, n
(%) | |
| G1 | 236 (42.8) |
| G2 | 214 (38.8) |
| G3 | 102 (18.4) |
| Peritoneal cytology,
n (%) | |
| No. of patients
with positive peritoneal cytology | 109 (19.7) |
| Recurrence status, n
(%) | |
| No. of patients
with recurrent disease | 61 (11.1) |
| Time to recurrent
disease in months (SD) | 35.5 (28.9) |
| Status at last
observation, n (%) | |
| Alive with no
evidence of disease | 439 (79.5) |
| Progressive
disease | 23 (4.2) |
| Tumour-related
death | 38 (6.9) |
| Death due to other
causes | 52 (9.4) |
The mean (SD) duration of hysteroscopy in all
patients was 18.2 (10.5) min. The mean (SD) duration of
hysteroscopy in patients with positive (n=109) and negative
peritoneal cytology (n=443) was not statistically different [17.9
(10.1) min vs. 17.9 (10.2) min, respectively; p=0.9]. There were no
statistically significant correlations between duration of
hysteroscopy (as a continuous variable) and positive peritoneal
cytology (p=0.6; rho=−0.028), FIGO stage (p=0.2; rho=−0.080), lymph
node involvement (p=0.2; rho=0.106) and patient age (p=0.5;
rho=0.033). The associations between duration of hysteroscopy and
clinicopathological parameters are provided in Table II. Longer duration of hysteroscopy
was not associated with positive peritoneal cytology, advanced
tumour stage, histological grade, histological type, lymph node
involvement or advanced patient age.
 | Table II.Relationship between
clinicopathological parameters and duration of hysteroscopy in 552
patients with endometrial cancer. |
Table II.
Relationship between
clinicopathological parameters and duration of hysteroscopy in 552
patients with endometrial cancer.
| HSC ≤15 min
(n=312) | HSC >15 min
(n=240) | p-valuea |
|---|
| Tumour stage | | | |
| FIGO I | 234 (75%) | 194 (81%) | 0.3 |
| FIGO II–IV | 78 (25%) | 46 (19%) | |
| Age at first
diagnosis | | | |
| ≤65 years | 154 (49%) | 107 (45%) | 0.4 |
| >65 years | 158 (51%) | 133 (55%) | |
| Peritoneal
cytology | | | |
| Yes | 62 (20%) | 47 (20%) | 0.8 |
| No | 208b (67%) | 170b (71%) | |
| Histological
grade | | | |
| G1+2 | 249 (80%) | 201 (84%) | 0.5 |
| G3 | 63 (20%) | 39 (16%) | |
| Histological
type | | | |
| Type I | 297 (95%) | 223 (93%) | 0.5 |
| Type II | 15 (5%) | 17 (7%) | |
| Lymph node
involvement | | | |
| Yes | 11 (4%) | 16 (7%) | 0.1 |
| No | 301c (96%) | 224c (93%) | |
In a univariate analysis, tumour stage (FIGO I vs.
II–IV), histological grade (G1+G2 vs. G3), histological type (type
I vs. II), lymph node involvement (yes vs. no) and patient age (as
a continuous variable), but not duration of hysteroscopy (as a
continuous variable), were associated with disease-free survival.
In a multivariate analysis, tumour stage, histological grade,
histological subtype and lymph node involvement were associated
with disease-free survival. Results of the univariate and
multivariate Cox-regression models and log-rank tests with respect
to disease-free survival are shown in Table III. When patients were grouped
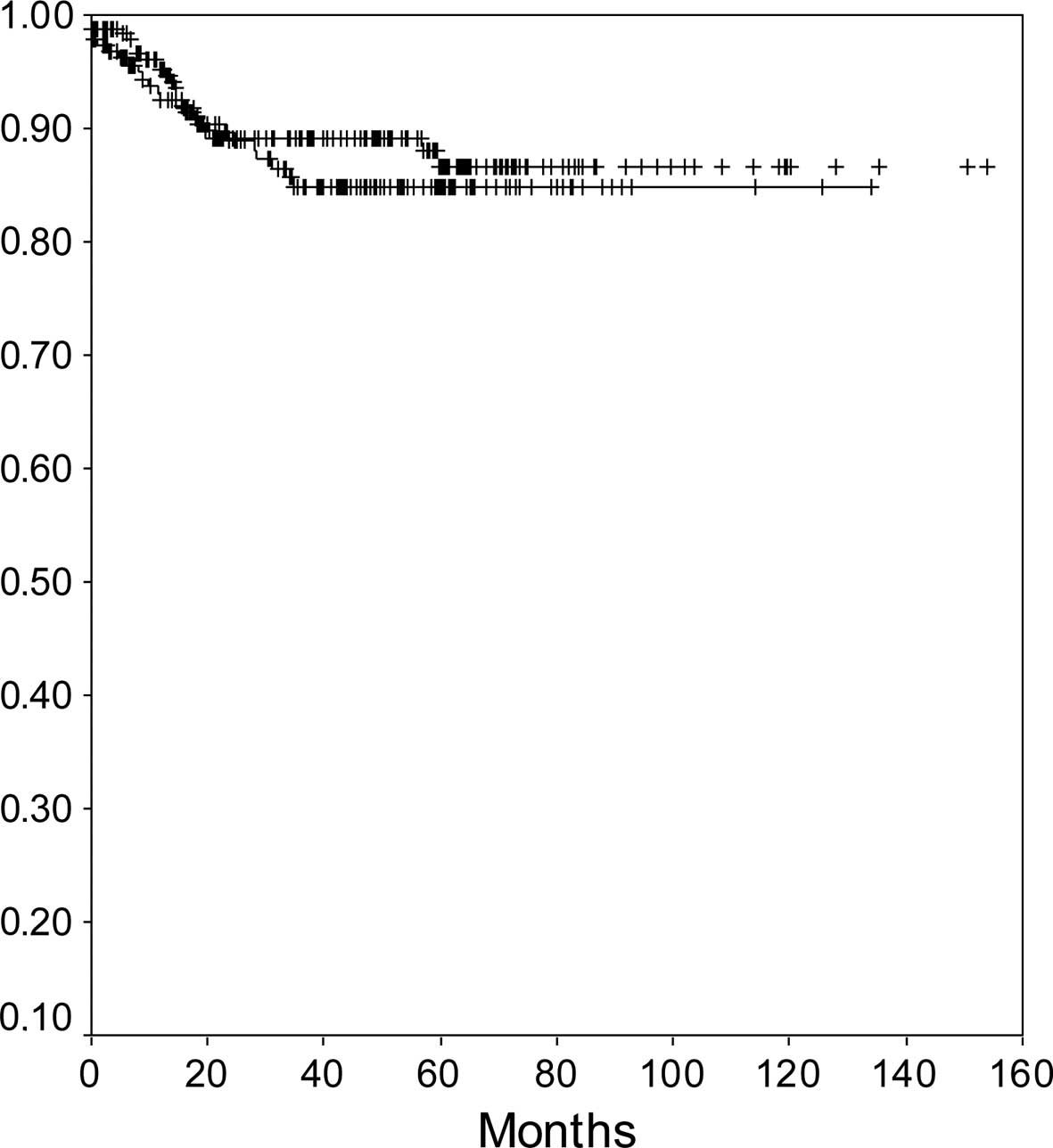
according to the duration of hysteroscopy, patients with a duration
of hysteroscopy ≤15 and >15 min had a 5-year recurrence-free
survival rate of 87 and 85%, respectively [p=0.4; hazard ratio
(HR)=1.2, 95% confidence interval (CI) 0.7–2.2] (Fig. 1).
 | Table III.Univariate Kaplan Meier analysis and
multivariate Cox regression model of prognostic covariates and
recurrence-free survival in 552 patients with endometrial
cancer. |
Table III.
Univariate Kaplan Meier analysis and
multivariate Cox regression model of prognostic covariates and
recurrence-free survival in 552 patients with endometrial
cancer.
| Univariatea
| Multivariateb
|
|---|
| p-value | HR (95% CI) | p-value |
|---|
| FIGO stage (I vs.
II–IV) | <0.00001 | 5.1 (2.5–10.2) | <0.0001 |
| Tumour grading (G1+2
vs. G3) | 0.05000 | 1.7 (0.9–3.3) | 0.0800 |
| Age (>65 vs. ≤65
years) | 0.00200 | 2.4 (1.3–4.2) | 0.0030 |
| Lymph node
involvement (yes vs. no) | 0.01000 | 3.2 (1.2–8.8) | 0.0200 |
| Histological type
(type I vs. II) | 0.10000 | 1.2 (0.8–1.8) | 0.3000 |
| Duration of HSC (≤15
vs. >15 min) | 0.40000 | 1.2 (0.7–2.2) | 0.4000 |
Discussion
In the present study, we investigated whether a
prolonged duration of hysteroscopy leads to a higher risk of
positive peritoneal cytology and an increased risk of recurrence in
women with endometrial cancer. We found that the duration of
hysteroscopy was not associated with positive peritoneal cytology
nor was it a prognostic parameter for recurrence-free survival. We
conclude from these data that longer duration of hysteroscopy in
patients with endometrial cancer is oncologically safe. It does not
increase the risk of positive peritoneal cytology and does not
adversely impact prognosis.
A number of studies have demonstrated that
hysteroscopy increases the risk of positive peritoneal cytology and
upstaging among women with endometrial cancer. For example, in a
recent meta-analysis of 9 studies and 1,015 patients, Polyzos et
al reported a significantly higher rate of malignant peritoneal
cytology after hysteroscopy with an odds ratio of 1.78 (9). Thus, even though there is no clear
evidence of an adverse prognostic effect of hysteroscopy and
positive peritoneal cytology is no longer part of the FIGO staging
system (9–13), concerns remain. Since endometrial
cancer in general is associated with a favorable prognosis, subtle
effects may be difficult to detect. Also, relevant confounders,
such as the histological type of endometrial cancer or the duration
of hysteroscopy, may influence the effect of this procedure on
cancer cell spread and prognosis. These issues are of clinical
relevance, since the duration of hysteroscopy is a potential risk
factor under the control of the treating physician. Therefore, we
investigated the influence of the duration of hysteroscopy on the
risk of positive peritoneal cytology and recurrence-free survival
in a large retrospective cohort study using a multi-centre study
approach. We found that duration of hysteroscopy, both as a
continuous variable and as a dichotomous variable with an arbitrary
cutoff point of 15 min, was not associated with the rate of
positive peritoneal cytology at the time of surgery or with the
duration of disease-free survival. The results of our study have
clinical implications, since they demonstrate that hysteroscopy can
be used safely in women with suspected endometrial cancer even when
a prolonged procedure is necessary to obtain an optimal diagnostic
result. Also, these data support the new 2009 FIGO staging revision
which omits stage IIIA based on positive peritoneal cytology
only.
Our study has limitations which include the
retrospective study design and the inclusion of multiple surgeons
and centers. Thus, we cannot exclude selection and ascertainment
bias. For example, technical expertise and equipment standards may
vary among surgeons and centers as well as over time, and specific
patient populations may or may not preferentially choose the
centers included in this study. Also, hydrostatic pressure during
hysteroscopy was not assessed. On the other hand, hysteroscopy is a
relatively simple and well-standardized procedure, thus minimizing
these effects. The strength of our study is the large number of
patients allowing for an accurate estimate of the association
between duration of hysteroscopy and clinicopathological parameters
and prognosis.
In summary, our results demonstrate that prolonged
duration of hysteroscopy is not associated with an increased risk
of positive peritoneal cytology and it does not adversely affect
the extent of recurrence-free survival. Therefore, hysteroscopy may
be used safely in women with suspected endometrial cancer, even
when the procedure requires an extended duration due to technical
or other circumstances.
References
|
1.
|
Parkin DM, Whelan SL, Ferlay J, Teppo L
and Thomas DB: Cancer Incidence in Five Continents. VIII. IARC
Press; Lyon: 2002
|
|
2.
|
Creasman WT, Odicino F, Maisonneuve P,
Beller U, Benedet JL, Heintz AP, Ngan HY, Sideri M and Pecorelli S:
Carcinoma of the corpus uteri. J Epid Biostat. 6:47–86.
2001.PubMed/NCBI
|
|
3.
|
Coulter A, Kelland A and Long A: The
management of menorrhagia. Effective Health Care Bull. 9:1–14.
1995.
|
|
4.
|
Spencer CP and Whitehead MI: Endometrial
assessment revisited. Br J Obstet Gynaecol. 106:623–632. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Mencaglia L: Hysteroscopy and
adenocarcinoma. Obstet Gynecol Clin North Am. 22:573–579.
1995.PubMed/NCBI
|
|
6.
|
Clark TJ, Voit D, Gupta JK, Hyde C, Song F
and Khan KS: Accuracy of hysteroscopy in the diagnosis of
endometrial cancer and hyperplasia: a systemic quantitative review.
JAMA. 288:1610–1621. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Nagele F, Wieser F, Deery A, Hart R and
Magos A: Endometrial cell dissemination at diagnostic hysteroscopy:
a prospective randomized cross-over comparison of normal saline and
carbon dioxide uterine distension. Hum Reprod. 14:2739–2742. 1999.
View Article : Google Scholar
|
|
8.
|
Obermair A, Geramou M and Gucer F: Does
hysteroscopy facilitate tumor cell dissemination? Incidence of
peritoneal cytology from patients with early stage endometrial
carcinoma following dilatation and curettage versus hysteroscopy
and dilatation and curettage. Cancer. 88:139–143. 2000.
|
|
9.
|
Polyzos NP, Mauri D, Tsioras S, Messini
CI, Valachis A and Messinis IE: Intraperitoneal dissemination of
endometrial cancer cells after hysteroscopy: a systematic review
and meta-analysis. Int J Gynecol Cancer. 20:261–267. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Obermair A, Geramou M, Tripcony L, Nicklin
JL, Perrin L and Crandon AJ: Peritoneal cytology: impact on
disease-free survival in clinical stage I endometrioid
adenocarcinoma of the uterus. Cancer Lett. 164:105–110. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Selvaggi L, Cormio G, Ceci O, Loverro G,
Cazzolla A and Bettocchi S: Hysteroscopy does not increase the risk
of microscopic extrauterine spread in endometrial carcinoma. Int J
Gynecol Cancer. 13:223–227. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Cicinelli E, Tinelli R, Colafiglio G,
Fortunato F, Fusco A, Mastrolia S, Fucci AR and Lepera A: Risk of
long-term pelvic recurrences after fluid minihysteroscopy in women
with endome-trial carcinoma: a controlled randomized study.
Menopause. 17:511–515. 2010.PubMed/NCBI
|
|
13.
|
Ben-Arie A, Tamir S, Dubnik S, Gemer O,
Ben Shushan A, Dgani R, Peer G, Barnett-Griness O and Lavie O: Does
hysteroscopy affect prognosis in apparent early-stage endometrial
cancer? Int J Gynecol Cancer. 18:813–819. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Serden SP: Diagnostic hysteroscopy to
evaluate the cause of abnormal uterine bleeding. Obstet Gynecol
Clin North Am. 27:277–286. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
O’Rogerson L and Duffy S: A national
survey of outpatient hysteroscopy. Gynecol Endosc. 10:343–348.
2001.
|
|
16.
|
Nag S, Erickson B, Parikh S, Gupta N,
Varia M and Glasgow G: The American Brachytherapy Society
recommendations for high-dose-rate brachytherapy for carcinoma of
the endometrium. Int J Radiat Oncol Biol Phys. 48:779–790. 2000.
View Article : Google Scholar : PubMed/NCBI
|