Introduction
Esophageal cancer is a common type of malignant
cancer in China. The 5-year survival rate of patients diagnosed
with esophageal cancer is less than 15%. Failure to make an early
diagnosis is normally attributed to the absence of symptoms or the
presence of mild symptoms during the early stages of the disease.
Most of the patients are diagnosed in the intermediate or late
stage of the disease. Although radical esophagectomy remains the
primary treatment for esophageal cancer, it is quite difficult to
treat patients with distant or lymph-node metastasis because of a
tendency of postsurgical recurrence. Therefore, identification of
biomarkers that improve the rates of early diagnosis of esophageal
cancer or precursor lesions can significantly reduce mortality in
esophageal cancer patients.
Similar to other solid tumors, chromosome loss is a
common molecular defect in esophageal cancer. Chromosome regions
with a high frequency of allelic loss indicate the presence of
tumor-associated genes (such as tumor suppressors). A study of
chromosome loss in dysplasia and early-stage esophageal cancer will
aid in the discovery of major tumor-associated genes and will lead
to the understanding of the tumorigenesis of esophageal cancer.
They may also be used as potential biomarkers for early detection
of esophageal cancer. Although extensive genomic instability has
been found in patients with esophageal cancer, the molecular
alterations that are closely related to the different stages of
tumorigenesis have not been fully understood.
In order to further clarify the molecular
alterations during the early-stage tumorigenesis of esophageal
cancer, and to identify potential biomarkers for early detection of
the disease, we analyzed allelic losses at a total of 16
microsatellite loci selected from chromosome regions 3p, 4p, 5q,
8p, 9p, 9q, 11p, 13q and 17p in matched squamous dysplasia and
squamous cancer tissues resected from 34 esophageal cancer patients
in Chongqing, southern China.
Materials and methods
Sample collection
Resection specimens from a total of 34 esophageal
cancer patients were obtained from the Department of Thoracic
Surgery, South-West Hospital, Chongqing, China. The patients
included 23 men and 11 women with an average age of 58.6 years
(range 44–74). All of the patients were newly diagnosed with
esophageal cancer, had no previous history of any other malignant
cancer and had not undergone previous radiotherapy or chemotherapy.
The patients were Han Chinese who had lived in Chongqing for more
than 20 years and did not have any direct kinship with each other.
The resected samples were placed in liquid nitrogen immediately
after the surgical removal and were preserved at −80°C before use.
In addition to the cancerous foci, surrounding tissues were also
collected from the 34 patients. The tissue samples were
pathologically diagnosed with mild, medium and severe dysplasia and
squamous cell carcinoma (SCC) (1).
This study classified mild and medium dysplasia as low-grade
dysplasia (LGD), and severe dysplasia as high-grade dysplasia
(HGD). In addition, peripheral blood samples were collected prior
to surgery. Informed consent was obtained from all of the study
subjects in accordance with the standards established by the local
institutional review boards.
Tissue cell acquisition using
microdissection
The frozen sections of squamous cancer tissue and
dysplastic tissue (5 μm) were stained with H&E and subjected to
microdissection under a x40 dissecting microscope to ensure that
the proportion of tumor cells and dysplastic cells was >80%,
which significantly reduced the rates of false positive or negative
results during lthe oss of heterozygosity (LOH) analysis.
Extraction of genomic DNA
Genomic DNA was extracted from the patient blood
samples and tissues using the DNeasy Blood and Tissue kit
(Qiagen).
Analysis of loss of heterozygosity
A total of 16 microsatellite loci were chosen from
the 3p, 4p, 5q, 8p, 9p, 9q, 11p, 13q and 17p chromosome regions.
The resected samples and paired blood samples from the 34
esophageal cancer patients were analyzed for LOH at the 16 loci.
Information concerning these markers and primer sequences is
available on the Genome Database (http://www.gdb.org/) and the NCBI genome database
(http://www.ncbi.nlm.nih.gov/). The
primers were obtained as labeled primers with fluorescent dye at
the 5-terminus (MWG Biotech, Ebersberg, Germany).
LOH analysis was performed as described in our
previous study (2). In brief, the
5-μl volume of microsatellite DNA amplification included 40 ng of
DNA template, 1X Buffer, 200 μM dNTPs, 250 nM microsatellite
primer, 2.5 mM MgCl2 and 0.25 units of Taq DNA
polymerase. The PCR conditions were as follows: pre-denaturation at
94°C for 3 min, followed by 30 cycles of 94°C for 30 sec, 55–60°C
for 30 sec, 72°C for 1 min and a final elongation step at 72°C for
4 min. PCR products were loaded on a fluorescent sequencer gel (ABI
PRISM 377). The images were captured, converted and analyzed using
GeneScan software, and the size of each allele fragment was
automatically calculated.
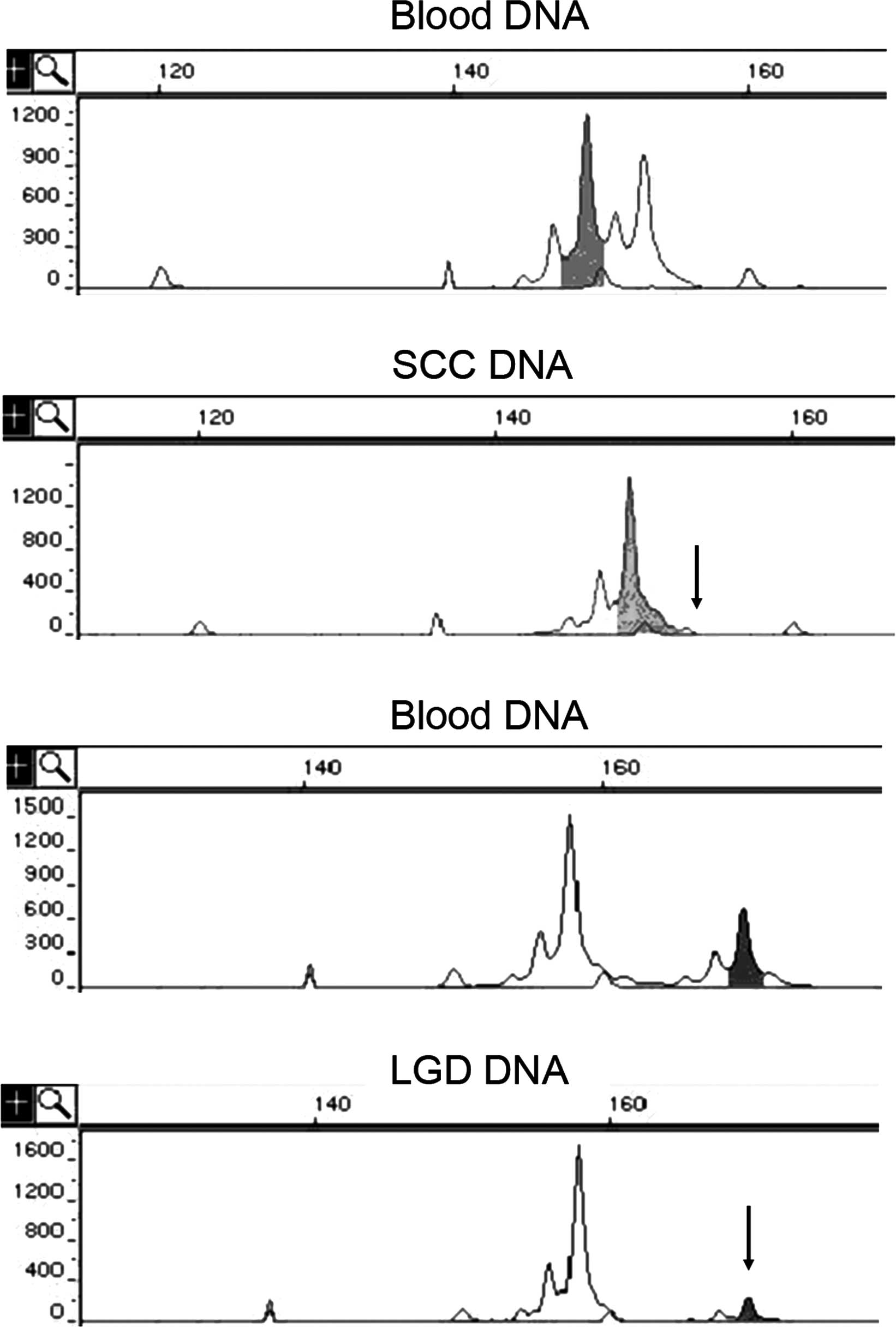
When the peripheral blood DNA of a patient showed
heterozygosity at a certain locus, the locus was marked as
informative. The tissue sample of the patient was considered to
show LOH when the DNA from the sample showed homozygosity at the
informative locus or when the fluorescence intensity of one of the
two alleles was <30% of that in the blood DNA of the patient
(Fig. 1). The frequency of LOH at
each locus was defined as the total number of tissue samples with
LOH at this locus/the total number of informative samples.
Statistical analysis
The LOH frequencies at each locus between the
different groups were compared using the χ2 test, and a
P-value <0.05 was considered statistically significant.
Results
The results of the present study are shown in
Table I. The overall frequencies
of LOH at the 16 microsatellite loci significantly increased as the
pathological status of the resection samples changed (from LGD to
HGD and to SCC). The overall frequency of LOH in the LGD samples
(9.8%) was significantly lower than that in the HGD (48.6%) and SCC
(58.5%) samples (P<0.001). Although the LOH frequency at each
locus showed an increasing trend with increasing disease severity,
the difference was not statistically significant. Eight loci
(D3S1597, D3S2452, D3S1285, D4S174, D5S2501, D9S125, D13S153 and
D17S786) showed LOH in the informative LGD samples, and another 8
loci were found to present LOH in the informative HGD specimens.
All 16 loci were found to have LOH in the SCC samples. Furthermore,
by comparing the occurrence of LOH in samples with different
pathological statuses from the same patient, we found regain of
heterozygosity at loci D3S2452, D4S174, D9S125 and D17S261, in the
SCC samples of 4 patients, respectively, compared to the HGD
specimens which showed LOH at the corresponding loci.
 | Table I.Frequencies of LOH at 16
microsatellite loci in the squamous dysplastic tissues and ESCC
tissues. |
Table I.
Frequencies of LOH at 16
microsatellite loci in the squamous dysplastic tissues and ESCC
tissues.
| Marker | Location | LGD sample
(%)a | HGD sample
(%)a | SCC sample
(%)a |
|---|
| D3S1597 | 3p25 | 4/21 (19.0) | 12/22 (54.5) | 18/22 (81.8) |
| D3S2452 | 3p21-p14 | 7/25 (28.0) | 20/25 (80.0) | 18/25 (72.0) |
| D3S1285 | 3p14 | 3/19 (15.8) | 14/21 (66.7) | 16/21 (76.2) |
| D4S174 | 4p14-p13 | 2/27 (7.4) | 7/27 (25.9) | 12/27 (44.4) |
| D5S409 | 5q14-q15 | 0/16 (0.0) | 7/16 (43.7) | 8/16 (50.0) |
| D5S2501 | 5q21-q23.3 | 1/13 (7.7) | 7/13 (53.8) | 7/13 (53.8) |
| D8S261 | 8p22-p21.3 | 0/18 (0.0) | 5/18 (27.8) | 6/18 (33.3) |
| D9S157 | 9p23-p22 | 0/23 (0.0) | 16/23 (69.5) | 15/23 (65.2) |
| D9S111 | 9q12-q21.1 | 0/25 (0.0) | 9/25 (36.0) | 15/25 (60.0) |
| D9S125 | 9q34-q34 | 9/27 (33.3) | 21/27 (77.8) | 23/27 (85.2) |
| D11S1338 | 11p15.5 | 0/29 (0.0) | 10/29 (34.5) | 11/29 (37.9) |
| D13S175 | 13q13 | 0/22 (0.0) | 10/23 (43.5) | 12/23 (52.2) |
| D13S153 | 13q14.2 | 4/24 (16.7) | 10/24 (41.7) | 13/24 (54.2) |
| D13S173 | 13q32-q34 | 0/24 (0.0) | 5/24 (20.8) | 11/24 (45.8) |
| D17S786 | 17p13.1 | 5/23 (21.7) | 13/23 (56.5) | 16/23 (69.5) |
| D17S261 | 17p11.2 | 0/22 (0.0) | 10/22 (45.4) | 11/22 (50.0) |
| Totalb | | 35/358 (9.8) | 176/362 (48.6) | 212/362 (58.5) |
Discussion
A total of 16 highly polymorphic microsatellite
markers from 9 chromosome regions with a high frequency of allelic
loss in esophageal cancer were selected. Surgically resected
squamous dysplasia and SCC samples from 34 esophageal cancer
patients were subjected to LOH analysis at these loci. The results
showed that the overall frequencies of LOH at the 16 microsatellite
loci significantly increased as the pathological status of the
resection specimens deteriorated (i.e., from LGD to HGD and to
SCC). These results indicated that tumorigenesis of esophageal
squamous epithelia is a progressive process involving a series of
molecular alterations. As the alterations accumulate to a certain
degree, the cell morphology and behavior undergo a radical change,
leading to malignancy (3). This
finding was consistent with previous studies involving other tumor
types, such as prostate, colon and breast cancer (4–7).
Moreover, the results were also in accordance with those of a
previous study showing that increasing grades of dysplasia were
associated with an increased risk of developing ESCC (8).
Of the 16 loci, 8 loci were found to show LOH in
LGD, indicating that LOH at these 8 loci may be involved in the
early-stage tumorigenesis of ESCC, and that these specific loci may
be used as markers for the screening of esophageal cancer. At
present, certain tumor-associated genes have been identified near
these loci, such as FHIT (3p), RASSF1A (3p),
APC (5q), ANXI (9q), DEC1 (9q), RB1
(13q), BRCA2 (13q), ING1 (13q) and TP53 (17p).
The expression of ANXI in chromosome 9q has been reported to be
closely related to tumor progression in patients with breast cancer
and possibly plays an important role in the early stage of
tumorigenesis (9). Study
concerning this gene in esophageal cancer showed that its
expression at the protein level in cancer tissues was significantly
lower than that in normal tissues (10). Another candidate gene, DEC1
at chromosome 9q, has been reported to be down-regulated in
esophageal cancer, and transfection of the cDNA of DEC1 may
inhibit the proliferation of various types of cancer cells,
suggesting that it may participate in the development of esophageal
cancer (11). The chromosome 13q
region has a high frequency of allelic loss in esophageal cancer.
However, the identified candidate genes within this region, such as
RB1, BRCA2 and ING1, seldom show mutations in
esophageal cancer, implying that other unknown tumor-suppressor
genes within this region may participate in the tumorigenesis of
this cancer (12). The chromosome
17p region involving the P53 locus also shows a high
frequency of allelic loss in esophageal cancer. Studies of the
P53 gene in esophageal cancer have shown that LOH and
mutations are the leading causes of its inactivation, which is in
accordance with the ‘two-hit’ model of tumorigenesis indicating
that P53 is a major tumor suppressor in esophageal cancer
(13).
In addition, 8 other loci were found to show LOH in
the HGD samples, indicating that LOH at these loci may be involved
in the late stages of tumorigenesis (such as invasion and
metastasis) in esophageal cancer.
This study also compared the occurrence of LOH in
samples of different pathological statuses from the same patient.
Notably, in 4 patients LOH was found at certain loci in the HGD
samples, whereas heterozygosity was regained at the same loci in
matched SCC samples. The regain of heterozygosity at some loci in
tumor tissues indicated that the tumorigenesis of esophageal cancer
may show genetic heterogeneity, i.e., the HGD and SCC samples of
the same patient may have been derived from different tumor-cell
clones. Cells with different molecular defects may have distinct
tumorigenesis processes, leading to different tumor-cell clones. A
similar result has been found in adenocarcinoma of the esophagus;
the molecular defects found in the severe dysplastic lesions were
not detected in the invasive adenocarcinoma lesions of the same
patient (14). A study involving
patients with oral squamous cancer has also shown that most of the
molecular alterations found in the precursor lesions were not
revealed in the matched tumor tissues (15). Similar observations have been
reported in studies on other cancers, such as breast and prostate
cancer (16,17).
Finally, it is worth mentioning that in several LGD
samples from our subjects, there were already multiple loci
exhibiting allelic loss, indicating that the earliest molecular
event in esophageal cancer may occur in histologically normal
squamous epithelia. In a recent study on esophageal cancer, 19% of
the histologically normal epithelia within the abnormal mucosal
region (unstained with iodine) already showed LOH alterations,
suggesting that the histologically normal epithelia adjacent to the
tumor tissues may have early-stage LOH alterations, which may be
necessary for the tumorigenesis of esophageal cancer (18). Although, at present, histological
examination remains an effective method for the early detection of
cancer, molecular tests, such as LOH analysis of certain specific
loci at the early stage, can be a promising and rational strategy
for the early diagnosis or prediction of esophageal cancer.
Acknowledgements
This study was supported, in part, by
a grant from the Chongqing Local Natural Sciences Foundation of
China (2010BB5192).
References
|
1.
|
Dawsey SM, Lewin KJ, Liu FS, Wang GQ and
Shen Q: Esophageal morphology from Linxian, China. Squamous
histologic findings in 754 patients. Cancer. 73:2027–2037. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Zhang XL, Fu WL, Zhao HX, Zhou LX, Huang
JF and Wang JH: Molecular studies of loss of heterozygosity in
Chinese sporadic retinoblastoma patients. Clin Chim Acta.
358:75–80. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Maley CC, Galipeau PC, Li X, et al: The
combination of genetic instability and clonal expansion predicts
progression to esophageal adenocarcinoma. Cancer Res. 64:7629–7633.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Maitra A, Wistuba II, Washington C, et al:
High-resolution chromosome 3p allelotyping of breast carcinomas and
precursor lesions demonstrates frequent loss of heterozygosity and
a discontinuous pattern of allele loss. Am J Pathol. 159:119–130.
2001. View Article : Google Scholar
|
|
5.
|
Wang Z and Lai FM: [Analysis of loss of
heterozygosity on chromosome 8 in human prostate carcinoma and high
grade prostatic intraepithelial neoplasia]. Zhonghua Nan Ke Xue.
10:26–28. 31:2004.
|
|
6.
|
Karakosta A, Golias C, Charalabopoulos A,
Peschos D, Batistatou A and Charalabopoulos K: Genetic models of
human cancer as a multistep process. Paradigm models of colorectal
cancer, breast cancer, and chronic myelogenous and acute
lymphoblastic leukaemia. J Exp Clin Cancer Res. 24:505–514.
2005.
|
|
7.
|
Ha PK, Pilkington TA, Westra WH, Sciubba
J, Sidransky D and Califano JA: Progression of microsatellite
instability from premalignant lesions to tumors of the head and
neck. Int J Cancer. 102:615–617. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Dawsey SM, Lewin KJ, Wang GQ, et al:
Squamous esophageal histology and subsequent risk of squamous cell
carcinoma of the esophagus. A prospective follow-up study from
Linxian, China. Cancer. 74:1686–1692. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Shen D, Nooraie F, Elshimali Y, et al:
Decreased expression of annexin A1 is correlated with breast cancer
development and progression as determined by a tissue microarray
analysis. Hum Pathol. 37:1583–1591. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Zhou G, Li H, DeCamp D, et al: 2D
differential in-gel electrophoresis for the identification of
esophageal scans cell cancer-specific protein markers. Mol Cell
Proteomics. 1:117–124. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Yang L, Leung AC, Ko JM, et al: Tumor
suppressive role of a 2.4 Mb 9q33-q34 critical region and DEC1 in
esophageal squamous cell carcinoma. Oncogene. 24:697–705. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Huang XP, Wei F, Liu XY, et al: Allelic
loss on 13q in esophageal squamous cell carcinomas from northern
China. Cancer Lett. 185:87–94. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Hu N, Huang J, Emmert-Buck MR, et al:
Frequent inactivation of the TP53 gene in esophageal squamous cell
carcinoma from a high-risk population in China. Clin Cancer Res.
7:883–891. 2001.PubMed/NCBI
|
|
14.
|
Koppert LB, Wijnhoven BP, van Dekken H,
Tilanus HW and Dinjens WN: The molecular biology of esophageal
adenocarcinoma. J Surg Oncol. 92:169–190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Partridge M, Pateromichelakis S, Phillips
E, Emilion G and Langdon J: Profiling clonality and progression in
multiple premalignant and malignant oral lesions identifies a
subgroup of cases with a distinct presentation of squamous cell
carcinoma. Clin Cancer Res. 7:1860–1866. 2001.PubMed/NCBI
|
|
16.
|
Cheng L, Bostwick DG, Li G, et al: Allelic
imbalance in the clonal evolution of prostate carcinoma. Cancer.
85:2017–2022. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Heim S, Teixeira MR, Dietrich CU and
Pandis N: Cytogenetic polyclonality in tumors of the breast. Cancer
Genet Cytogenet. 95:16–19. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
He S, Guo GM, Liu FX, et al: Molecular
analysis in combination with iodine staining may contribute to the
risk prediction of esophageal squamous cell carcinoma. J Cancer Res
Clin Oncol. 134:307–315. 2008. View Article : Google Scholar : PubMed/NCBI
|