Introduction
Bladder cancer is the ninth most common malignancy
worldwide; an estimated 386,300 new cases and 150,200 deaths from
bladder cancer occurred in 2008 worldwide (1,2). The
majority of bladder cancer occurred in males and among them,
non-muscle invasive bladder cancer (NMIBC) accounted for 75–85% and
the incidence rate was closely correlated to age (1,2).
Benign prostate hyperplasia (BPH) is the most common cause of
urination obstacles in elderly men; the incidence is also rising
with the aging population (3). It
is not unusual to encounter the clinical scenario of a male patient
undergoing endoscopic treatment for bladder cancer (TURBT) who also
requires transurethral resection of prostate (TURP). It was unclear
whether it was safe to combine the two procedures since there was a
risk of circulating cancer cells that may implant into the raw
prostatic fossa and thereby enhance the risk of subsequent
recurrences. In 1953 and 1956, simultaneous resection was first
reported by Kiefer (4) and Hinman
(5) based on four and three
patients, respectively. The results indicated that simultaneous
resection was inadvisable due to the high recurrence (100%) in the
vesical neck or prostatic urethra. However, Greene and Yalowitz
(6) in 1972 studied 100 patients
who underwent simultaneous transurethral resection and the authors
observed that simultaneous resection was preferable without
increasing the risk of tumor recurrence. Since then, numerous
studies on this issue have been conducted, however, the results of
these studies were different or even contradictory (7). A previous meta-analysis (8), based on five pooled non-randomized
concurrent controlled trials (NRCCTs) and one randomized controlled
trial (RCT), reported a statistically significant result. NRCCT
suffers more confounding factors and biases than RCT and they are
not suitable for pooling, so the results were unconvincing.
It was unclear whether simultaneous resection of
bladder tumor and prostate were safe and preferable for patients
with NMIBC and BPH. An in depth reassessment of this issue may have
important public health and clinical implications, so we performed
this systematic review and meta-analysis to examine all the
published evidence involving NRCCTs and RCTs, to provide
unambiguous evidence whether simultaneous TURBT/TURP in the
treatment of NMIBC with BPH was feasible.
Materials and methods
Literature search
A systematic search of the Cochrane Central Register
of Controlled Trials (CENTRAL), PubMed, EMBASE and the ISI Web of
Knowledge databases for the relevant published studies was
conducted from their establishment to March 21, 2012. The relevant
search terms were (‘prostatic hyperplasia’ OR ‘Benign Prostate
Hyperplasia’ OR ‘prostate’) AND (‘simultaneous’ OR ‘simultaneously’
OR ‘synchronous’ OR ‘coinstantaneous’) AND (‘bladder tumor’ OR
‘bladder tumour’ OR ‘bladder cancer’ OR ‘bladder neoplasm’ OR
‘bladder carcinoma’ OR ‘vesical neoplasma’) AND (‘recurrence’ OR
‘relapse’). References were explored to identify relevant
manuscripts. Only studies published in English were included.
Study selection
A study was included in this systematic review when
the following criteria were met: i) type of research: published RCT
or NRCCTs; ii) participants: patients with NMIBC (including Ta, T1)
combining benign prostatic hyperplasia (regardless of the severity,
but excluding prostate cancer), and including information about
patient age, length of follow-up and tumor stage; iii)
interventions: simultaneous group (TURBT+TURP, resection of bladder
tumor first, then prostate resection); control group (TURBT only),
regardless of whether adjuvant chemotherapy was administered; iv)
outcomes: overall tumor recurrence rates, recurrence rate at the
prostatic urethra and/or bladder neck, and tumor progression and v)
it was possible to obtain full texts.
Methodological quality assessment
The methodological quality of each RCT was assessed
using the Cochrane collaboration's tool for assessing risk of bias
(9), which utilizes seven aspects:
i) details of randomization method, ii) allocation concealment,
iii) blinding of participants and personnel, iv) blinding of
outcome assessment, v) incomplete outcome data, vi) selective
outcome reporting and vii) other sources of bias, to provide a
qualification of risk of bias.
For NRCCTs, we used MINORS (Methodological Index for
Non-Randomized Studies) guidelines (10) to assess the methodological quality.
MINORS guidelines consisted of 12 indexes: i) a clearly stated aim,
ii) inclusion of consecutive patients, iii) prospective collection
of data, iv) endpoints appropriate to the aim of the study, v)
unbiased assessment of the study endpoint, vi) follow-up period
appropriate to the aim of the study, vii) loss to follow-up less
than 5%, viii) prospective calculation of the study size, ix)
adequate control group, x) contemporary groups (control and studied
group should be managed during the same time period, no historical
comparison), xi) baseline equivalence of groups and xii) adequate
statistical analyses, every item has two scores and the total score
is 24; when the score is ≥16 points this indicates high quality,
otherwise the quality is low (<16 points).
Data extraction
Two researchers read the full texts independently
and extracted the contents as follows: the sample inclusion
criteria and sample size, methods and processes of sampling and
grouping, basic information, interventions, outcome, length of
follow-up, loss rates and reasons for the loss, and statistical
methods of the studies. To obtain the missing information, authors
were contacted by phone or e-mail. In studies involving RCT with
multiple groups or non-randomized clinical trials, only the
experimental and control groups associated with this study were
extracted.
Level of evidence
We evaluated the level of evidence by using the
GRADE (Grades of Recommendation, Assessment, Development and
Evaluation) approach (11). In
addition, the GRADEprofiler 3.6 software (12) was used to create the evidence
profile.
The GRADE system included: level of evidence: i)
high quality (or A); further research is extremely unlikely to
change our confidence in the estimate of effect, ii) moderate
quality (or B); further research is likely to have an important
impact on our confidence in the estimate of effect and may change
the estimate, iii) low quality (or C); further research is
extremely likely to have an important impact on our confidence in
the estimate of effect and is likely to change the estimate and iv)
very low quality (or D); we are extremely uncertain about the
estimate.
Statistical analysis
We proposed to pool results from single studies by
meta-analysis where this was identified to be both clinically and
statistically appropriate. We computed pooled ORs and 95% CIs using
the Cochrane Review Manager 5.1 software (version 5.1.6) to
generate forest plots and to assess the heterogeneity of the
included studies. Heterogeneity was quantified by using the
I2 statistic; low, moderate and high represented
I2 values of 40, 70 and 100%, respectively. Where
I2≤40% indicates there was no evidence of heterogeneity,
the fixed-effects model was used, otherwise the random-effects
model was used. In the presence of heterogeneity, we performed
sensitivity analyses to explore possible explanations for
heterogeneity and to examine the influence of various exclusion
criteria on the overall risk estimate. We also investigated the
influence of a single study on the overall risk estimate by
removing each study in each turn, to test the robustness of the
main results. Subgroup analysis was also conducted if significant
heterogeneity was identified, according to methodological quality
(low-quality studies vs. high-quality studies). Where possible,
potential publication bias was assessed by visual inspection of the
funnel plots of the primary outcome.
Results
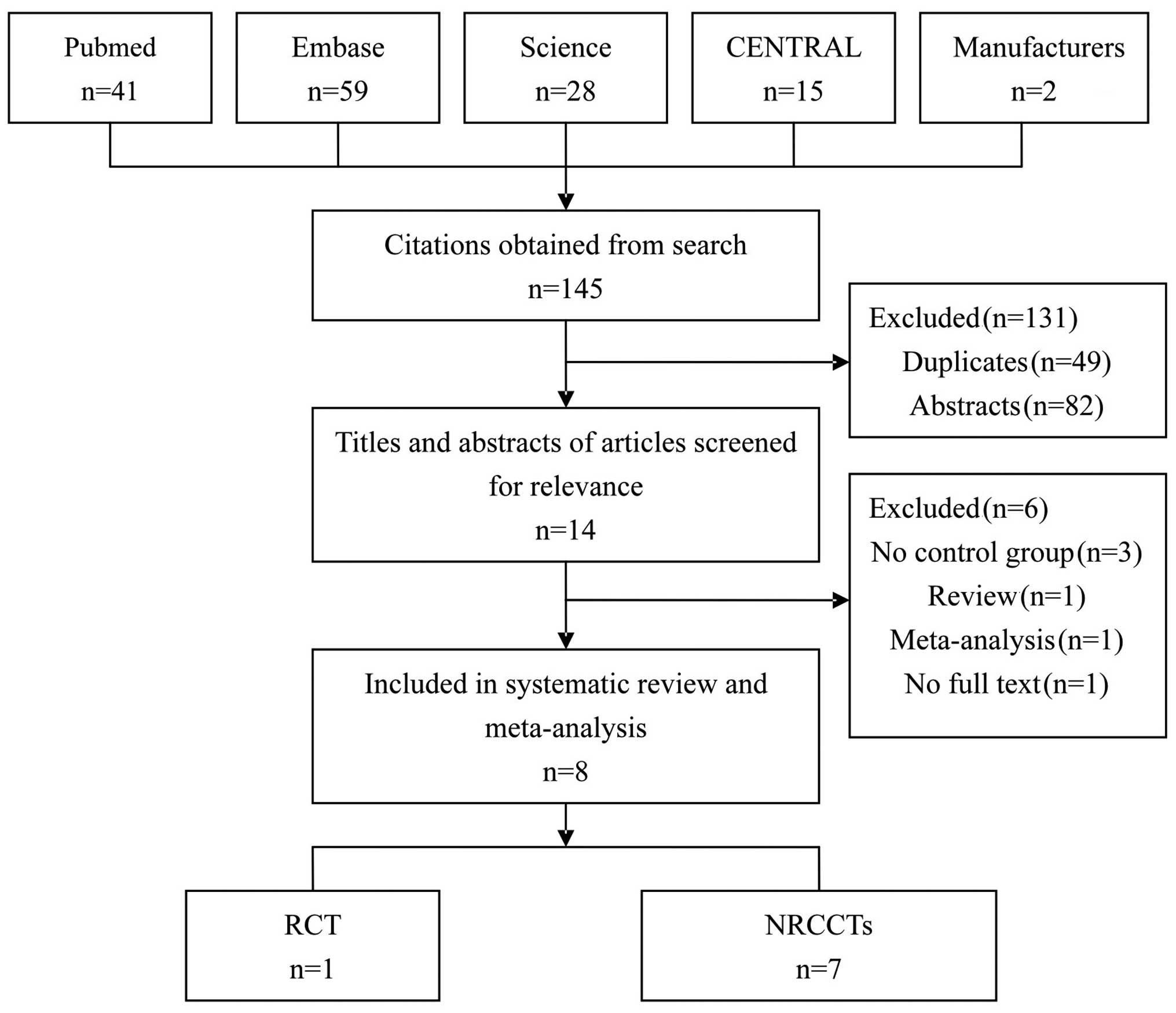
Search results
The initial search obtained 145 articles. After
reading the abstracts and the full texts, 8 were selected for this
study, including 1 RCT (13) and 7
NRCCTs (6,14–19).
Fig. 1 shows the process of
selection.
Characteristics and quality of included
studies
Table I shows the
characteristics and quality points of each included study. Of the 8
studies, 3 were performed in the USA (6,14,15),
2 in Korea (17,18) and the remaining (13,16,19)
in India, Turkey and Tunisia, respectively, during the period
between 1972 and 2010, the total number of patients in each study
ranged from 48 to 287. The baselines of 7 NRCCTs were similar and
individual results are shown in Tables
II and III. According to
MINORS evaluation criteria (10),
one study scored 22 points, 2 studies scored 20 points and 4
studies scored 19 points (Table
I). The quality of RCTs according to the Cochrane Collaboration
guidelines, provided a qualification of risk of bias. It refered to
randomization only, lacking information with regard to allocation
concealment and blind measurement; but no incomplete outcome data,
no selective outcome reporting and other sources of bias, therefore
there was a moderate risk of bias.
 | Table ICharacteristics of each primary
study. |
Table I
Characteristics of each primary
study.
| Study (ref.) | Year | Country of
origin | Study type | Patients (n)
| Mean age | Mean follow-up
(months) | Outcome | Quality (points) |
|---|
| Simultaneous
group | Control group |
|---|
| Greene and Yalowitz
(6) | 1972 | USA | NRCCT | 100 | 100 | NA | 132/132 | 1, 2,
4 | High (19) |
| Laor et
al(14) | 1981 | USA | NRCCT | 137 | 150 | 71/60 | 69/96 | 1, 2,
4 | High (19) |
| Vicente et
al(15) | 1988 | USA | NRCCT | 100 | 100 | 69/60 | 47/46 | 1, 2 | High (20) |
| Ugurlu et
al(16) | 2007 | Turkey | NRCCT | 31 | 34 | 55.97/68.22 | 30.6/27.4 | 1, 2,
3 | High (19) |
| Kim et
al(17) | 2009 | Korea | NRCCT | 24 | 165 | 70/64.1 | 52.2/43.8 | 1, 2,
3 | High (19) |
| Ham et
al(18) | 2009 | Korea | NRCCT | 106 | 107 | 66.7/65.5 | 50.1/54.3 | 1, 2,
3, 4, 5 | High (22) |
| Jaidane et
al(19) | 2010 | Tunisia | NRCCT | 85 | 85 | 71/71 | 35.2/33.1 | 1, 2,
3 | High (20) |
| Singh et
al(13) | 2009 | India | RCT | 24 | 24 | 56.06/57.36 | 35.7/37.6 | 1, 2,
3 | Moderate |
 | Table IICharacteristics of the simultaneous
groups. |
Table II
Characteristics of the simultaneous
groups.
| Study | Year | Patients (n) | Total recurrence n
(%) | Recurrence in
bladder neck and/or prostatic fossa, n (%) | Progression
(%) | Single/multiple
(n) | Ta/T1 (n) | Grade (n) | Adjuvant
chemotherapy |
|---|
| Greene and Yalowitz
(6) | 1972 | 100 | 54 (54) | 17 (17) | NA | 81/19 | NA | 57/29/14a | NA |
| Laor et
al(14) | 1981 | 137 | 77 (56.2) | 21 (15) | NA | 112/25 | NA | 34/35/51a | NA |
| Vicente et
al (15) | 1988 | 100 | 55 (55) | 10 (10) | NA | 58/42 | 21/79 | 4/78/18a | NA |
| Ugurlu et al
(16) | 2007 | 31 | 11 (35.5) | 1 (3.2) | 3 (9.7) | 31/0 | 25/6 | 26/3/2a | N |
| Kim et al
(17) | 2009 | 24 | 9 (37.5) | 1 (4.2) | 2 (8.3) | NA | 8/16 | 13/11b | NA |
| Ham et al
(18) | 2009 | 106 | 31 (29.2) | 0 | 10 (9.4) | 58/48 | 21/85 | 60/46b | Y |
| Jaidane et
al (19) | 2010 | 85 | 17 (20) | 1 (1.2) | 2 (2.3) | 70/15 | 9/76 | 32/45/8a | Y |
| Singh et al
(13) | 2009 | 24 | 12 (50) | 4 (16.2) | 3 (12.5) | 24/0 | 17/7 | 10/11/3a | N |
 | Table IIICharacteristics of the control
groups. |
Table III
Characteristics of the control
groups.
| Study | Year | Patients (n) | Total recurrence n
(%) | Recurrence in
bladder neck and/or prostatic fossa, n (%) | Progression
(%) | Single/multiple
(n) | Ta/T1(n) | Grade (n) | Adjuvant
chemotherapy |
|---|
| Greene and Yalowitz
(6) | 1972 | 100 | 54 (54) | 16 (16) | NA | 77/23 | NA | 59/23/18a | NA |
| Laor et
al(14) | 1981 | 150 | 92 (61.3) | 27 (18) | NA | 124/26 | NA | 35/7/57a | NA |
| Vicente et
al(15) | 1988 | 100 | 73 (73) | 10 (10) | NA | 52/48 | 24/76 | 18/73/9a | NA |
| Ugurlu et
al(16) | 2007 | 34 | 14 (41.2) | 1 (2.9) | 3 (8.8) | 34/0 | 25/9 | 31/3/0a | N |
| Kim et
al(17) | 2009 | 165 | 37 (22.4) | 3 (1.8) | 10 (6.1) | NA | 43/109 | 81/84b | NA |
| Ham et
al(18) | 2009 | 107 | 46 (43.0) | 0 | 12 (11.2) | 56/51 | 19/88 | 59/48b | Y |
| Jaidane et
al(19) | 2010 | 85 | 20 (23.5) | 1 (1.2) | 2 (2.3) | 65/20 | 11/74 | 33/44/8a | Y |
| Singh et al
(13) | 2009 | 24 | 11 (42.8) | 3 (12.5) | 2 (8.3) | 24/0 | 18/6 | 9/11/4a | N |
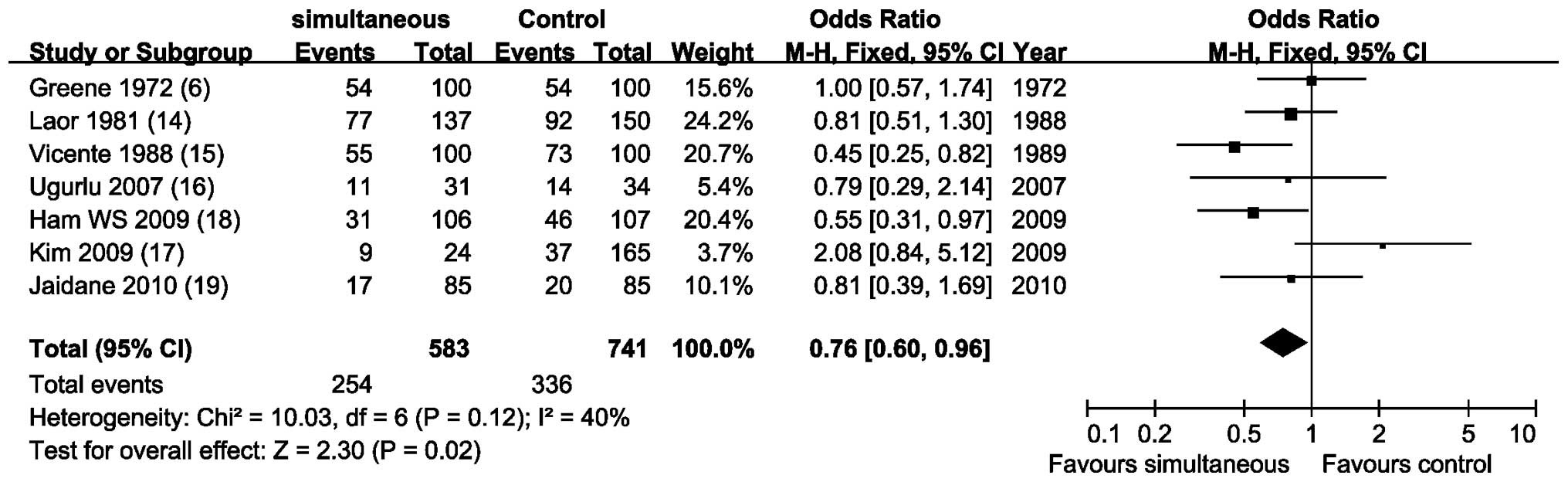
Overall tumor recurrence rates
Meta-analysis of 7 NRCCTs (6,14–19)
by a fixed-effects model (P=0.12; I2, 40%) revealed that
simultanuous resection did not increase the recurrence rate of
bladder tumor, on the contrary, recurrence rate was statistically
lower than that in the control group (OR, 0.76; 95% CI, 0.60–0.96;
P=0.02; Fig. 2).
The overall recurrence rate in simultanuous and
control groups was 50 and 42.8%, respectively, (P>0.05) in the
study by Singh et al (13).
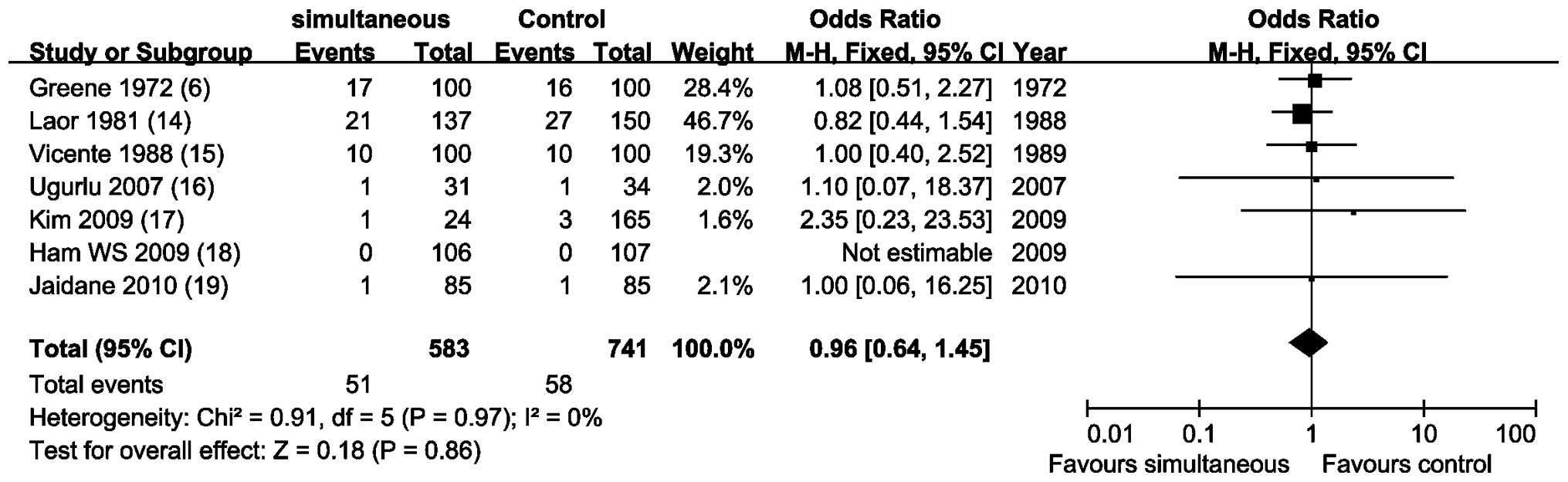
Recurrence rate at the prostatic urethra
and/or bladder neck
Meta-analysis of 7 NRCCTs (6,14–19)
by a fixed-effects model (P=0.97; I2, 0%) showed that
there was no statistical difference to compare recurrence rate to
the prostatic urethra and/or bladder neck (OR, 0.96; 95% CI,
0.64–1.45; P=0.86; Fig. 3).
The recurrence rate at the prostatic urethra and/or
bladder neck was 16.2 and 12.5%, respectively, (P>0.05) in the
study by Sing et al (13).
Tumor progression rates
Meta-analysis of 4 NRCCTs (16–19)
by a fixed-effects model (P=0.95; I2, 0%) showed that
the tumor progression rates were similar and there was no
statistical difference (OR, 0.96; 95% CI, 0.49–1.87; P=0.91;
Fig. 4).
The tumor progression rate was 12.5 and 8.3%,
respectively, (P=0.05) in the study by Singh et al (13).
GRADE profile evidence
The included NRCCTs had the same three outcome
indicators, they were the overall tumor recurrence rates,
recurrence rate at bladder neck/prostatic fossa and tumor
progression. The GRADE system evidence for each outcome level and
reasons for upgrade and downgrade are shown in Table IV. Table IV also shows the GRADE quality of
evidence for the included RCT.
 | Table IVGRADE profile evidence of the
included studies. |
Table IV
GRADE profile evidence of the
included studies.
Quality assessment
| No. of patients
| Effect
| | |
|---|
| No. of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other
considerations | Simultaneous | Control | Relative (95%
CI) | Absolute | Quality | Importance |
|---|
| Recurrence |
| 7 | NRCCT | Seriousa | No serious
inconsistency | No serious
indirectness | No serious
imprecision | Strong
associationb | 254/583
(43.6%) | 336/741
(45.3%) | OR 0.76
(0.6–0.96) | 67 fewer/1000 (from
10 fewer to 121 fewer) | ⊕⊕○○
Low | Critical |
| 1 | RCT | Seriousa | No serious
inconsistency | No serious
indirectness | No serious
imprecision | None | 12/24 (50%) | 11/24 (45.8%) | RR 1.09
(0.6–1.97) | 41 more/1000 (from
183 fewer to 445 more) | ⊕⊕⊕○
Moderate | Critical |
| Recurrence rate at
the prostatic urethra and/or bladder neck |
| 7 | NRCCT | Seriousa | No serious
inconsistency | No serious
indirectness | No serious
imprecision | Strong
associationb,
increased effect for RR ∼1c | 51/583 (8.7%) | 58/741 (7.8%) | OR 0.96
(0.64–1.45) | 3 fewer/1000 (from
27 fewer to 31 more) | ⊕⊕⊕○
Moderate | Critical |
| 1 | RCT | Seriousa | No serious
inconsistency | No serious
indirectness | No serious
imprecision | None | 4/24 (16.7%) | 3/24 (12.5%) | RR 1.33
(0.33–5.33) | 41 more/1000 (from
84 fewer to 541 more) | ⊕⊕⊕○
Moderate | Critical |
| Progression |
| 4 | NRCCT | Seriousa | No serious
inconsistency | No serious
indirectness | No serious
imprecision | Strong
associationb
increased effect for RR ∼1c | 17/246 (6.9%) | 27/391 (6.9%) | OR 0.96
(0.49–1.87) | 3 fewer/1000 (from
34 fewer to 53 more) | ⊕⊕⊕○
Moderate | Important |
| 1 | RCT | Seriousa | No serious
inconsistency | No serious
indirectness | No serious
imprecision | None | 3/24 (12.5%) | 2/24 (8.3%) | RR 1.5
(0.27–8.19) | 42 more/1000 (from
61 fewer to 599 more) | ⊕⊕⊕○
Moderate | Important |
Discussion
Previous data have demonstrated that benign
prostatic hyperplasia and other lower urinary tract obstructions
were important factors in the pathogenesis of bladder cancer
(20,21). In patients with benign prostatic
hyperplasia, the retention of urine prolonged the duration of
chemical carcinogens in bladder, and increasing the incidence of
bladder cancer. Melicow et al (22) suggested that 4-aminobiphenyl and
benzidine were decomposed into carcinogens, since the activity of
urinary β-glucuronidase increased in patients with prostatic
hyperplasia. This would cause bladder cancer. Due to the lower
urinary tract obstruction, the bladder is susceptible to become
infected, form stones and diverticulitis. Long-term and chronic
irritation would cause the formation of epithelial hyperplasia and
cystic or glandular cystitis. Part of the epithelium extended to
the submucosal connective tissue formed von Brunn nests, this may
become adenocarcinoma. As reported previously, these complications
also stimulate transitional metaplasia and lead to squamous cell
carcinoma. Therefore, early surgery over the same period to remove
the lower urinary tract obstruction, not only does not increase the
overall recurrence rate of bladder cancer, but also has the
potential to reduce the recurrence rate (6,23).
In the past, patients with NMIBC and BPH were often
treated with open or staging surgery. However, open surgery has
certain shortcomings, including serious trauma, more postoperative
complications and a longer time to recovery, particulary unbearable
for elderly patients. Staging surgery would increase the risk of
surgery and wasted money. With the development and popularity of
urological endoscopic technology, numerous scholars now suggest
simultanuous resection. However, in theory, simultanuous resection
may increase the risk that cancer cells implant into the bladder
neck and prostatic fossa. It was controversial whether simultanuous
resection was feasible, although there were numerous associated
studies.
There was a relevant meta-analysis published by Luo
et al (8) in 2011,
involving 6 studies and 983 patients. There was evidence that
simultaneous TURBT/TURP did not increase the overall recurrence
rate or recurrence rate in bladder neck/prostatic fossa. The
shortcomings in this meta-analysis were as follows: i) incomplete
retrieval or intended selective inclusion, so the efficiency of
retrieval was low and would cause serious publication bias; ii)
performed meta-analysis misused RR to pool the NRCCTs, which is the
statistical index for prospective design (e.g. RCT); iii) failure
to provide the risk bias figure, and failure to provide complete
risk bias evaluation; iv) the methodological quality assessment
tool for RCT was misused to assess the quality of NRCCTs, and
failed to provide complete risk bias evaluation. In addition, the
author also indicated in this paper that the size of inclusive and
total samples were small. The results still require proof that
includes larger sample size controlled clinical trials in the
future, in order to obtain more accurate conclusions.
This study overcame the shortcomings of the previous
study, based on a comprehensive literature search, evaluated RCT
and NRCCTs using appropriate criteria, meta-analysis of NRCCTs,
qualitative analysis of RCT, and the use of GRADE quality of
evidence given in the standard classification. The results showed:
simultaneous resection did not increase recurrence rate, on the
contrary, the overall recurrence rate was lower than that of
control group, it also did not increase the risk of tumor
metastasis or tumor progression rate.
We also assessed the level of evidence using the
GRADE approach. According to the GRADE approach, the quality of the
evidence was only intermediate (the first two or three outcome
indicators) and low (first outcome indicators) due to the limited
evidence derived from combined NRCCT, and other reasons as follows:
i) lack of allocation concealment and blinding and ii) the study
controlled important confounding factors, but did not control
others. RCTs were generally high quality, but this included one RCT
with significant limitations of the study. Therefore, the quality
of evidence was moderate in this RCT.
However, there were the following limitations in
this meta-analysis. Firstly, we included only one RCT, so high
quality meta-analysis of RCT could not be performed. Secondly, for
non-randomized trials, the possibility of other bias reflected in
the tumor status (single/multiple, tumor grade, associated with
carcinoma in situ, etc.), postoperative bladder perfusion,
technical surgical differences and transurethral tumor samples for
inspection and other aspects of quality problems. Thirdly, the lack
of long-term assessment of key indicators, such as the 5- or
10-year survival rate of patients. Lastly, the study sample size
and overall sample size were small.
In summary, current evidence suggests that: for
patients with NMIBC and BPH, simultanuous resection relief of the
lower urinary tract obstruction, did not increase the overall
recurrence rate of bladder tumors, but also did not cause
metastasis and tumor progression, reduced expenses and shortened
hospital stay and may reduce the relapse rate and improve the
quality of life of patients. Based on the GRADE system, the quality
of evidence, the recommended level was 2B. Due to the lack of
evaluation of the system, further studies are required to be
designed strictly according to CONSORT criteria (24), to design larger sample,
high-quality, multi-center RCT, and include long-term key outcome
indicators (such as 5- or 10-year survival rate of patients), in
order to further evaluate the efficacy and safety of simultanuous
resection.
Acknowledgements
This study was supported by the
Foundation of Zhongnan Hospital of Wuhan University (Wuhan China;
no. 115004) for Xing-Huan Wang and the Intramural Research Program
of the Hubei University of Medcine (Shiyan China; no. 2011 CZX01)
for Xian-Tao Zeng.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Babjuk M, Oosterlinck W, Sylvester R, et
al: EAU guidelines on non-muscle-invasive urothelial carcinoma of
the bladder, the 2011 update. Eur Urol. 59:997–1008. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rowhrborm CG and McConnell JD: Etiology,
pathophysiology, epidemiology and natural history of benign
prostatic hyperplasia. Campbell's Urology. Walsh PC, Retik AB,
Vaughan ED Jr and Wein AJ: W. B. Saunders Company. (Philadelphia,
PA). 1297–1330. 2002.
|
|
4
|
Kiefer JH: Bladder tumor recurrence in the
urethra: a warning. J Urol. 69:652–656. 1953.PubMed/NCBI
|
|
5
|
Hinman F Jr: Recurrence of bladder tumors
by surgical implantation. J Urol. 75:695–696. 1956.PubMed/NCBI
|
|
6
|
Greene LF and Yalowitz PA: The
advisability of concomitant transurethral excision of vesical
neoplasm and prostatic hyperplasia. J Urol. 107:445–447.
1972.PubMed/NCBI
|
|
7
|
Kouriefs C, Loizides S and Mufti G:
Simultaneous transurethral resection of bladder tumour and
prostate: is it safe? Urol Int. 81:125–128. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luo SJ, Lin YJ and Zhang WL: Does
simultaneous transurethral resection of bladder tumor and prostate
affect the recurrence of bladder tumor? A meta-analysis. J
Endourol. 25:291–296. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Higgins JPT and Green S: Cochrane Handbook
for Systematic Reviews of Interventions Version 5.1.0 (updated
Macch 2011). The Cochrane Collaboration. 2011.Available at
https://www.cochrane-handbook.org.
|
|
10
|
Slim K, Nini E, Forestier D, Kwiatkowski
F, Panis Y and Chipponi J: Methodological index for non-randomized
studies (minors): development and validation of a new instrument.
ANZ J Surg. 73:712–716. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guyatt GH, Oxman AD, Vist GE, et al:
GRADE: an emerging consensus on rating quality of evidence and
strength of recommendations. BMJ. 336:924–926. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
GRADEpro. [Computer program]. Version 3.6
for Windows. Brozek J, Oxman A, Schünemann H, 2011.
|
|
13
|
Singh W, Sinha RJ and Sankhwar SNS:
Outcome of simultaneous transurethral resection of bladder tumor
and transurethral resection of the prostate in comparison with the
procedures in two separate sittings in patients with bladder tumor
and urodynamically proven bladder outflow obstruction. J Endourol.
23:2007–2011. 2009. View Article : Google Scholar
|
|
14
|
Laor E, Grabstald H and Whitmore WF: The
influence of simultaneous resection of bladder tumors and prostate
on the occurrence of prostatic urethral tumors. J Urol.
126:171–175. 1981.PubMed/NCBI
|
|
15
|
Vicente J, Chéchile G, Pons R and Méndez
G: Tumor recurrence in prostatic urethra following simultaneous
resection of bladder tumor and prostate. Eur Urol. 15:40–42.
1988.PubMed/NCBI
|
|
16
|
Ugurlu O, Gonulalan U, Adsan O, Kosan M,
Oztekin V and Cetinkaya M: Effects of simultaneous transurethral
resection of prostate and solitary bladder tumors smaller than 3 cm
on oncologic results. Urology. 70:55–59. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim S, Park S and Ahn H: Oncologic results
of simultaneous transurethral resection of superficial bladder
cancer and benign prostatic hyperplasia. Urology. 74:S1442009.
View Article : Google Scholar
|
|
18
|
Ham WS, Kim WT, Jeon HJ, Lee DH and Choi
YD: Long-term outcome of simultaneous transurethral resection of
bladder tumor and prostate in patients with nonmuscle invasive
bladder tumor and bladder outlet obstruction. J Urol.
181:1594–1599. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jaidane M, Bouicha T, Slama A, et al:
Tumor recurrence in prostatic urethra following simultaneous
resection of bladder tumor and prostate: a comparative
retrospective study. Urology. 75:1392–1395. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mommsen S and Sell A: Prostatic
hypertrophy and venereal disease as possible risk factors in the
development of bladder cancer. Urol Res. 11:49–52. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fellows GJ: The association between
vesical carcinoma and urinary obstruction. Eur Urol. 4:187–188.
1978.PubMed/NCBI
|
|
22
|
Melicow MM, Uson AC and Lipton R:
beta-Glucuronidase activity in the urine of patients with bladder
cancer and other conditions. J Urol. 86:89–94. 1961.PubMed/NCBI
|
|
23
|
Karaguzhin SG, Merinov DS and Martov AG:
One-stage transurethral resection of the urinary bladder and the
prostate in patients with superficial cancer of the urinary bladder
combined with benign prostatic hyperplasia. Urologiia. 17–21.
2005.(In Russian).
|
|
24
|
Schulz KF, Moher D and Altman DG: CONSORT
2010 comments. Lancet. 376:1222–1223. 2010. View Article : Google Scholar : PubMed/NCBI
|