Introduction
Morphine is a potent opioid analgesic that is widely
used for clinical pain management. However, repeated administration
of morphine leads to tolerance and hyperalgesia, which limit its
use as an antinociceptive agent. Despite extensive research efforts
in the area of morphine tolerance and hyperalgesia, the underlying
mechanisms involved in these phenomena remain largely unknown.
Previous studies showed that glial activation and subsequent immune
responses at the lumbar spinal cord contribute to the development
of morphine tolerance and withdrawal-induced hyperalgesia (1–3).
Pro-inflammatory cytokines, including interleukin (IL)-1, IL-6 and
tumor necrosis factor, have been implicated in the development of
morphine tolerance and withdrawal-induced hyperalgesia (4,5). The
complement system is a crucial effector mechanism of the innate
immune system. Components of the complement cascade are
constitutively produced in the central nervous system (6). However, whether the complement
cascade is activated in the spinal cord under conditions of chronic
morphine treatment is unknown. Furthermore, whether products of
complement cascade activation modify morphine tolerance and
withdrawal-induced hyperalgesia is also unknown.
The complement cascade, consisting of more than 30
soluble and membrane-bound proteins, is activated by four pathways;
the classical, the alternative, the mannose-binding lectin and the
extrinsic pathways (7).
Eventually, all four pathways lead to the production of the
important anaphylatoxin C5a in the terminal cascade (8,9). C5a
exerts a range of inflammatory and immune effects by binding to the
G-coupled receptor, C5aR and a second receptor, the C5a-like
receptor 2 (10). The uncontrolled
activation of the complement cascade causes harmful effects,
including immunoparalysis and organ dysfunction (11–13).
Due to its strong pro-inflammatory character, C5a is considered as
the most hazardous molecule in the excessive activation of the
complement cascade (13,14). A body of evidence suggests that
C5a-induced C5aR activation plays a deleterious role in neural
diseases including pain, Alzheimer’s disease, Huntington’s disease
and amyotrophic lateral sclerosis (6,15–18).
The inhibition of C5a or C5aR is beneficial for protection against
these diseases in animal models. Thus, the present study was
designed to evaluate the roles of C5a and C5aR in the expression of
morphine tolerance and withdrawal-induced hyperalgesia in rats.
In the present study, we examined the influence of
morphine administration on the expression levelss of C5 and C5aR in
the L5 lumbar section of spinal cords. The effects of C5a and C5aR
antagonist PMX35 on morphine tolerance and withdrawal-induced
hyperalgesia were then examined.
Materials and methods
Animal preparation and intrathecal (i.t.)
catheter implantation
Male Sprague-Dawley rats (Kunming Medical College,
Kunming, China) weighing 250-300 g were used in the experiments.
The rats were kept in the housing facilities for 1 week prior to
experiments. The implantation of i.t. catheters was performed as
described by Yaksh and Rudy (19).
The rats were anesthetized with phenobarbital (50 mg/kg,
intraperitoneally). The i.t. polyethylene catheters (PE-10) were
inserted via an incision in the cisterna magna and advanced
caudally to the lumbar enlargement of the spinal cord. The incision
site was sutured in layers and the catheter was immobilized under
the skin. Following surgery, all rats were returned to cages for
recovery, housed individually and maintained on a 12-h light-dark
cycle with food and water freely available. Rats with neurological
deficit or infection were excluded from experiments. The use of
experimental animals was in accordance with guidelines made by the
local Committee on Animal Care and Use. The use of rats was
approved by the Animal Care and Use Committees of Kunming Medical
University and the First People’s Hospital of Yunnan Province.
Behavioral tests
The antinociceptive responses were determined by
mechanical (Analgesy-Meter) and thermal (tail-flick) test
paradigms. The mechanical nociceptive thresholds were evaluated as
described by Stein et al (20). Rats were gently held and increasing
pressure (maximum 250 g) was applied onto the dorsal surface of the
ipsilateral hind paw. The paw pressure thresholds (PPT), the
pressure required to elicit paw withdrawal, were determined. The
thermal nociceptive thresholds were determined by the hot water
tail-flick test. The tails of rats were immersed into water
(49±0.5°C) and the latency to a rapid flick was recorded. The
morphine-induced antinociceptive responses in mechanical and
thermal tests were expressed as the % maximum possible effect
(%MPE) using the formula (21):
%MPE = (WT − CT)/(CO − CT) x 100; where WT is the withdrawal
latency (sec) or threshold (g) following morphine/saline treatment,
CT is the latency prior to morphine/saline treatment and CO is the
cut-off value (i.e. 250 g for the mechanical test and 15 sec for
the tail-flick test). Behavioral tests were carried out in a
blinded manner with respect to the groups.
Experimental design
The i.t. drug administration was accomplished using
a microinjection syringe connected to the i.t. catheter in
conscious rats. Rats received either saline or morphine (10 mg/kg,
s.c., twice daily for 5 days) and were treated once daily (11 a.m.)
with C5a (10 ng, R&D Systems, Inc., Minneapolis, MN, USA),
PMX53 (200 ng) or saline via i.t. catheter during induction of
morphine tolerance. Chronic morphine withdrawal-induced
hyperalgesia and allodynia in rats were examined 16 h after the
last injection of s.c. morphine. The animals were treated with
morphine (5 μg) acutely via i.t. catheter to study the expression
of morphine tolerance after the recording of morphine
withdrawal-induced hyperalgesia and allodynia. The acute
antinociceptive activity of i.t. administered morphine in these
rats was evaluated by an Analgesy-Meter and tail-flick test. The
behavior recorded prior to the acute administration of i.t.
morphine served as the basal latency.
Spinal cord sample preparation
After behavioral testing, the heart perfusion of
rats was performed using saline under isoflurane anesthesia. A
laminectomy was performed from the lower edge of the 12th thoracic
vertebra to sacral vertebra and the L5 lumbar section of the spinal
cord was collected and frozen immediately in liquid nitrogen and
stored at −80°C until further study.
Enzyme-linked immunosorbent assay
(ELISA)
The L5 lumbar spinal cord was collected after
behavioral testing. The tissue was pooled and homogenized in
homogenization buffer (phosphate-buffered saline, pH 7.4,
containing 1% Triton-X100, 1 mM PMSF, 10 μg/ml aprotinin and 1
μg/ml leupeptin). Samples were spun at 15,000 x g for 30 min at
4°C. The supernatant was aliquoted and stored at −80°C for future
protein quantification. The concentrations of C5a (Wuhan EIAab
Science Ltd, Wuhan, China) were measured in the L5 lumbar spinal
cord using specific ELISA kits according to the manufacturer’s
instructions.
Western blot analysis
L5 lumbar spinal cord samples were homogenized in
ice-cold solubilizing solution [20 mM Tris-HCl (pH 7.0), 25 mM
β-glycerophosphate, 2 mM EGTA, 1% Triton X-100, 1 mM vanadate, 1%
aprotinin, 1 mM phenylmethylsulfonyl fluoride and 2 mM
dithiothreitol] and kept on ice for 40 min. The lysate was
centrifuged at 15,000 rpm for 15 min and the supernatants were
collected. The protein concentrations were determined using Bio-Rad
protein assay reagent (Hercules, CA, USA). Equal quantities of
protein were separated by 12% SDS-polyacrylamide gel
electrophoresis and transferred to a PVDF membrane (Millipore
Corporation, Billerica, MA, USA). The membranes were incubated with
C5aR and β-actin antibody (Santa Cruz Biotechnology, Santa Cruz,
CA, USA), washed and then incubated with peroxidase-conjugated
anti-mouse IgG (KPL, Gaithersburg, MD, USA). The immunoblot was
revealed with an ECL western blot detection kit (Amersham Pharmacia
Biotech, Buckinghamshire, UK). Densitometric analysis was performed
using ImageJ software.
Statistical analysis
All values are expressed as means ± SD. Data were
analyzed by ANOVA followed by Tukey-Kramer multiple comparisons
test using SPSS software (SPSS, Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant result.
Results
Induction of C5a and C5aR at the L5
lumbar spinal cord of morphine-tolerant rats
As an initial step in determining the association
between complement factors and morphine, we examined whether the
levels of C5 and C5aR were altered in the L5 lumbar spinal cords of
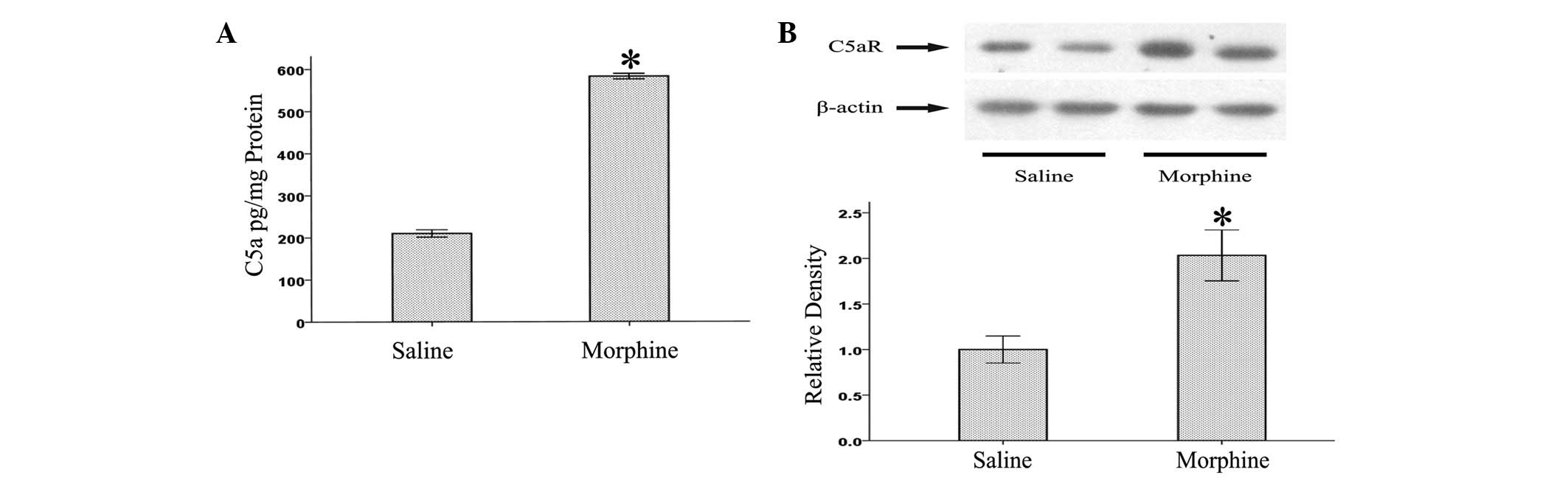
morphine-tolerant rats. As shown in Fig. 1, we found that levels of C5a and
C5aR were enhanced in the L5 lumbar spinal cords following chronic
morphine treatment. This result suggests that the complement system
might be involved in the pathogenesis of morphine tolerance.
Effects of C5a and C5aR antagonist on
morphine withdrawal-induced hyperalgesia and allodynia
In order to reveal the pathological effect of
elevated levels of C5a and C5aR in morphine-tolerant rats, we
investigated the effects of C5a and C5aR antagonist PMX53 on
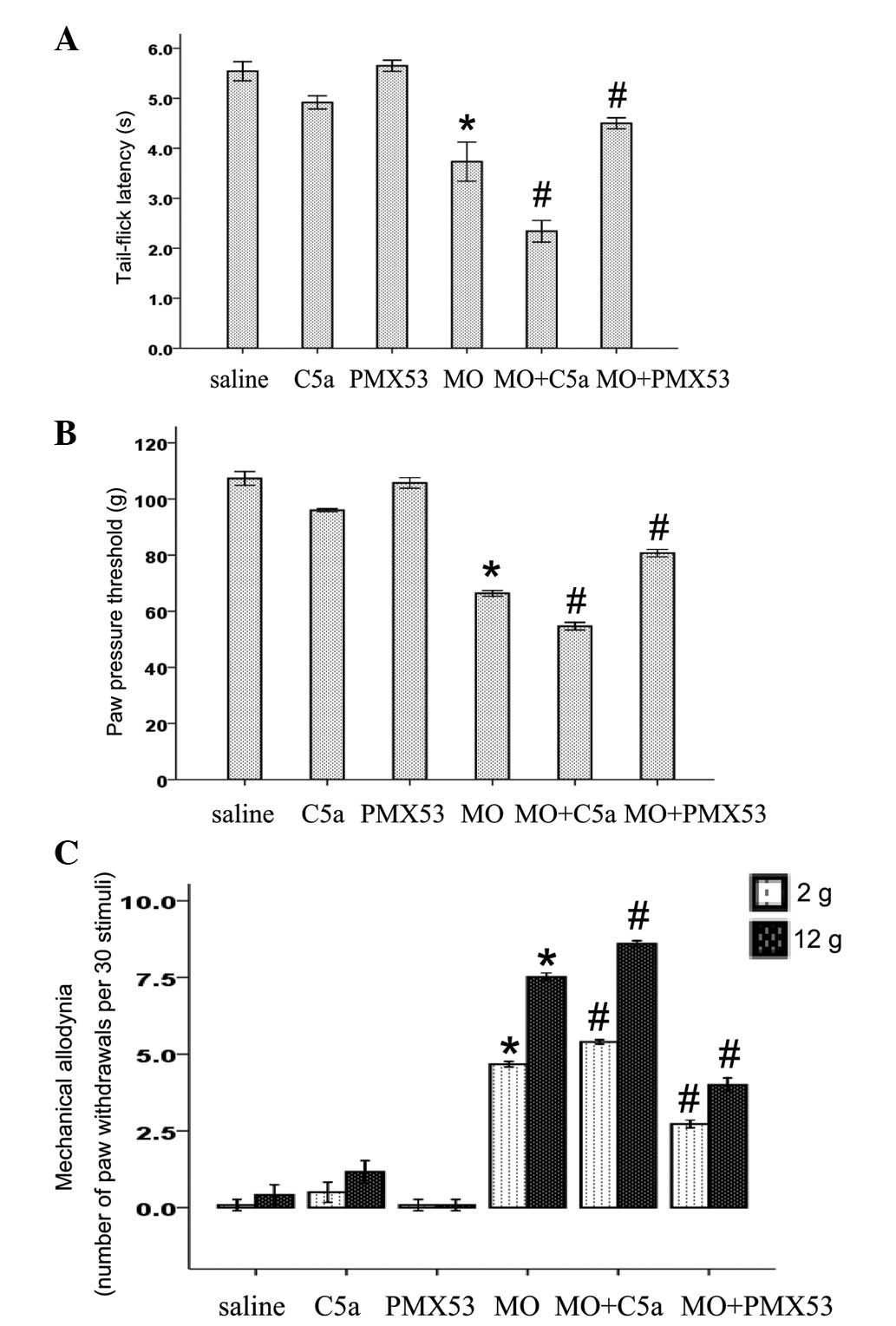
morphine withdrawal-induced hyperalgesia. As shown in Fig. 2A and B, the chronic administration
of morphine (10 mg/kg, twice daily for 5 days, s.c.) led to thermal
and mechanical hyperalgesia 16 h after the last injection.
Similarly, the chronic administration of morphine also induced
mechanical allodynia to both 2 and 12 g of mechanical stimuli
(Fig. 2C). PMX53 treatment had no
effect on nociceptive threshold in the saline-treated rats
(Fig. 2). Chronic C5a treatment
decreased nociceptive threshold in the saline-treated rats
(Fig. 2). Conversely, C5a
treatment during the induction of morphine tolerance significantly
enhanced morphine withdrawal-induced thermal and mechanical
hyperalgesia, and mechanical allodynia (Fig. 2). By contrast, the enhanced
hyperalgesia induced by morphine was significantly suppresed by
PMX53 (Fig. 2).
Effects of C5a and C5aR antagonist on the
expression of spinal antinociceptive tolerance to morphine
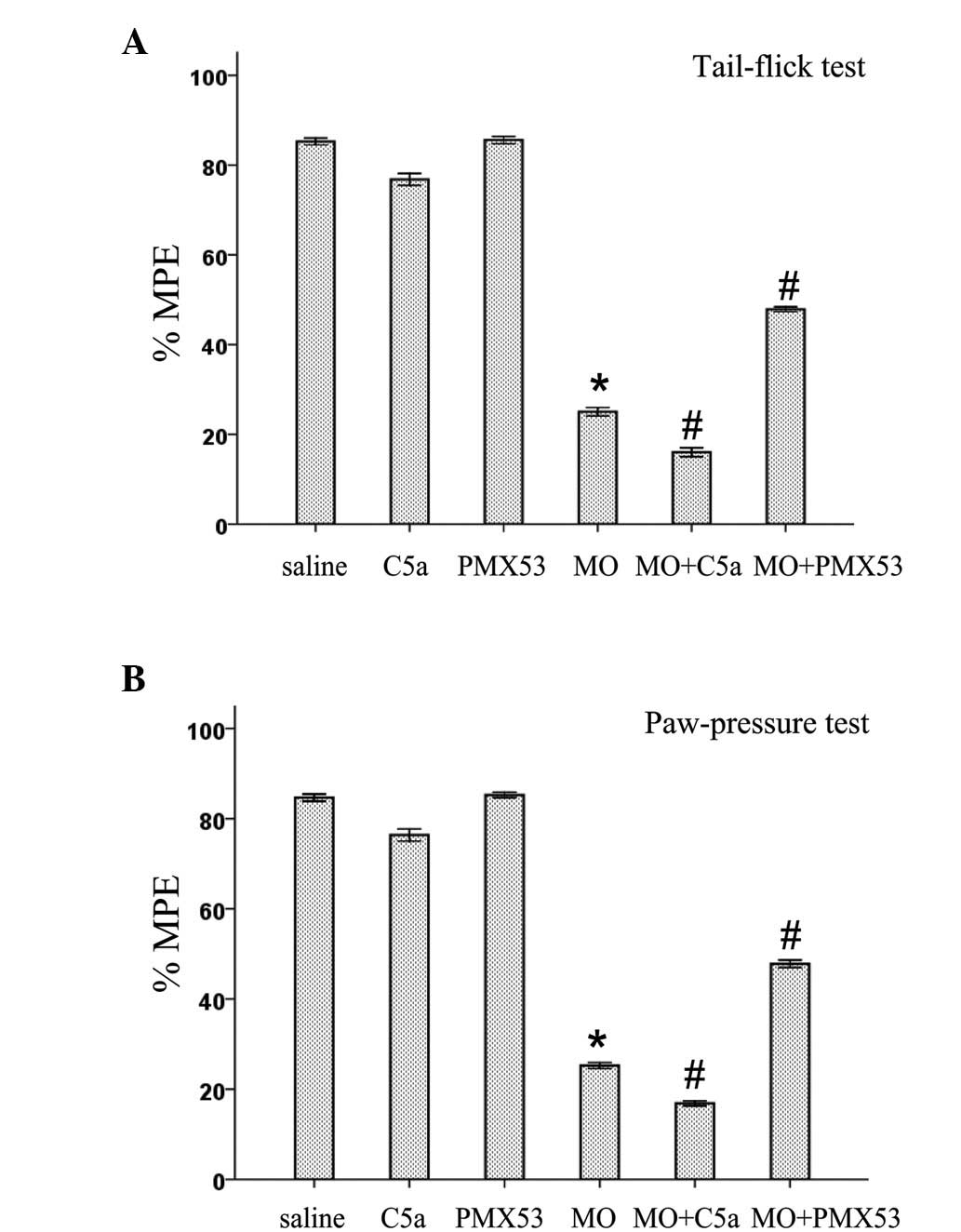
The role of C5a and C5aR in the expression of spinal
antinociceptive tolerance to morphine was examined in both
mechanical and thermal tests. As shown in Fig. 3, antinociceptive tolerance to acute
i.t. morphine (5 μg) was induced in rats injected with
saline/morphine (10 mg/kg, twice daily for 5 days, s.c.). Rats
injected with PMX53 or saline had no effect on the antinociceptive
activity of acute i.t. morphine (5 μg). Chronic C5a treatment
slightly decreased the antinociceptive activity of acute i.t.
morphine (5 μg). However, C5a treatment during the induction period
of morphine tolerance (10 mg/kg, twice daily for 5 days, s.c.)
significantly enhanced the expression of spinal antinociceptive
tolerance to i.t. morphine (5 μg) in mechanical and thermal testing
(Fig. 3). The expression of spinal
antinociceptive tolerance to i.t. morphine (5 μg) was suppressed by
PMX53.
Discussion
The repeated administration of morphine is
associated with significant problems, including antinociceptive
tolerance and hyperalgesia (22).
Accumulated evidence suggests mediators underlying morphine
tolerance and hyperalgesia. However, effective pharmacotherapy has
not been established for morphine-induced side effects. Previous
studies proposed the association between immune responses in the
spinal cord and morphine tolerance and withdrawal-induced
hyperalgesia (21,23,24).
The complement system may be a crucial regulator of morphine
tolerance and withdrawal-induced hyperalgesia, as activation of
complement cascade results in the enhancement of immune responses.
In the present study, the main finding is the identification of C5a
and C5aR as a likely promoting mediator for morphine tolerance and
withdrawal-induced hyperalgesia.
The complement system is known to be a potent
effector mechanism of the immune system. In the spinal cord,
neuron, microglia and astrocytes constitutively produce complement
components, including C5a and C5aR (25,26).
Hence, there is potential for rapid activation of the complement
cascade. Microglia and astrocytes may enhance the level of
complement components under various situations, including central
nervous system infection, brain injury and pain (6,17,27,28).
The present results demonstrate that the levels of C5a and C5aR
were increased in the L5 lumbar spinal cords of morphine-tolerant
rats. However, it is still unknown in which type(s) of these cells
that upregulated levels of C5a and C5aR occur. These results
suggest that the activation of the complement cascade is involved
in the development of morphine tolerance and withdrawal-induced
hyperalgesia.
The increased levels of C5a and C5aR in
morphine-tolerant rats suggest that the pathological role of the
upregulation of C5a and C5aR in morphine tolerance and
withdrawal-induced hyperalgesia requires elucidation. Although the
complement system plays a crucial role in host defense in times of
immunological challenge, the over-activation of the complement
cascade causes deleterious effects on the host as well. C5a/C5aR
inhibitors have shown efficacy in controlling the pathogenesis of
inflammation-associated diseases, including autoimmune diseases and
pain (25,29–31).
The present results demonstrate that C5a treatment promoted the
development of morphine tolerance and withdrawal-induced
hyperalgesia in rats. However, the C5aR antagonist PMX53 blocked
the development of these phenomena. Thus, C5a and C5aR may play
promoting roles in the development of morphine tolerance and
hyperalgesia.
Spinal inflammatory immune responses contribute to
the mechanisms of morphine tolerance. Several studies have
demonstrated that pro-inflammatory cytokines IL-1β, IL-6 and tumor
necrosis factor (TNF)-α enhances the expression of morphine
tolerance and withdrawal-induced hyperalgesia in rats (1,4,23).
The pivotal role of the activation of NF-κB in inflammation during
the induction of morphine tolerance has also been well elucidated
(32,33). C5a activates and attracts
astrocytes and microglia, and increases levels of pro-inflammatory
cytokines (34–36). It has also been reported that C5a
binds to neurons to enhance the level of intracellular calcium and
activate NF-κB (37). Therefore,
activation of C5a and C5aR may be part of a pathway to promote the
development of morphine tolerance and withdrawal-induced
hyperalgesia.
In summary, this is the first demonstration that the
complement cascade is activated in the spinal cord by repeated
injection of morphine, and that C5a and C5aR regulate morphine
tolerance and withdrawal-induced hyperalgesia. A more detailed
analysis in future studies may be useful to design an effective
therapeutic strategy for morphine tolerance and withdrawal-induced
hyperalgesia.
Acknowledgements
This study was supported by Science
and Technology Joint Special Fund of Yunnan Province
(2009CD199).
References
|
1
|
Raghavendra V, Rutkowski MD and DeLeo JA:
The role of spinal neuroimmune activation in morphine
tolerance/hyperalgesia in neuropathic and sham-operated rats. J
Neurosci. 22:9980–9989. 2002.PubMed/NCBI
|
|
2
|
Horvath RJ, Romero-Sandoval EA and De Leo
JA: Inhibition of microglial P2X4 receptors attenuates morphine
tolerance, Iba1, GFAP and mu opioid receptor protein expression
while enhancing perivascular microglial ED2. Pain. 150:401–413.
2010. View Article : Google Scholar
|
|
3
|
Ramos KM, Lewis MT, Morgan KN, et al:
Spinal upregulation of glutamate transporter GLT-1 by ceftriaxone:
therapeutic efficacy in a range of experimental nervous system
disorders. Neuroscience. 169:1888–1900. 2010. View Article : Google Scholar
|
|
4
|
Johnston IN, Milligan ED, Wieseler-Frank
J, et al: A role for proinflammatory cytokines and fractalkine in
analgesia, tolerance, and subsequent pain facilitation induced by
chronic intrathecal morphine. J Neurosci. 24:7353–7365. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hutchinson MR, Bland ST, Johnson KW, Rice
KC, Maier SF and Watkins LR: Opioid-induced glial activation:
mechanisms of activation and implications for opioid analgesia,
dependence, and reward. ScientificWorldJournal. 7:98–111. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Woodruff TM, Ager RR, Tenner AJ, Noakes PG
and Taylor SM: The role of the complement system and the activation
fragment C5a in the central nervous system. Neuromolecular Med.
12:179–192. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kemper C and Atkinson JP: T-cell
regulation: with complements from innate immunity. Nat Rev Immunol.
7:9–18. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ehrnthaller C, Ignatius A, Gebhard F and
Huber-Lang M: New insights of an old defense system: structure,
function, and clinical relevance of the complement system. Mol Med.
17:317–329. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ricklin D, Hajishengallis G, Yang K and
Lambris JD: Complement: a key system for immune surveillance and
homeostasis. Nat Immunol. 11:785–797. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huber-Lang MS, Riedeman NC, Sarma JV, et
al: Protection of innate immunity by C5aR antagonist in septic
mice. FASEB J. 16:1567–1574. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Recknagel S, Bindl R, Kurz J, et al:
C5aR-antagonist significantly reduces the deleterious effect of a
blunt chest trauma on fracture healing. J Orthop Res. 30:581–586.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hecke F, Schmidt U, Kola A, Bautsch W,
Klos A and Kohl J: Circulating complement proteins in multiple
trauma patients – correlation with injury severity, development of
sepsis, and outcome. Crit Care Med. 25:2015–2024. 1997.
|
|
13
|
Ganter MT, Brohi K, Cohen MJ, et al: Role
of the alternative pathway in the early complement activation
following major trauma. Shock. 28:29–34. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gerard C: Complement C5a in the sepsis
syndrome – too much of a good thing? N Engl J Med. 348:167–169.
2003.
|
|
15
|
Liang DY, Li X, Shi X, et al: The
complement component C5a receptor mediates pain and inflammation in
a postsurgical pain model. Pain. 153:366–372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jacob A, Hack B, Bai T, Brorson JR, Quigg
RJ and Alexander JJ: Inhibition of C5a receptor alleviates
experimental CNS lupus. J Neuroimmunol. 221:46–52. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jang JH, Liang D, Kido K, Sun Y, Clark DJ
and Brennan TJ: Increased local concentration of complement C5a
contributes to incisional pain in mice. J Neuroinflammation.
8:802011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fonseca MI, Ager RR, Chu SH, et al:
Treatment with a C5aR antagonist decreases pathology and enhances
behavioral perfor mance in murine models of Alzheimer’s disease. J
Immunol. 183:1375–1383. 2009.PubMed/NCBI
|
|
19
|
Yaksh TL and Rudy TA: Analgesia mediated
by a direct spinal action of narcotics. Science. 192:1357–1358.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stein C, Gramsch C and Herz A: Intrinsic
mechanisms of antinociception in inflammation: local opioid
receptors and beta-endorphin. J Neurosci. 10:1292–1298.
1990.PubMed/NCBI
|
|
21
|
Raghavendra V, Tanga FY and DeLeo JA:
Attenuation of morphine tolerance, withdrawal-induced hyperalgesia,
and associated spinal inflammatory immune responses by
propentofylline in rats. Neuropsychopharmacology. 29:327–334. 2004.
View Article : Google Scholar
|
|
22
|
Kamei J, Ohsawa M, Hayashi SS and
Nakanishi Y: Effect of chronic pain on morphine-induced respiratory
depression in mice. Neuroscience. 174:224–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song P and Zhao ZQ: The involvement of
glial cells in the development of morphine tolerance. Neurosci Res.
39:281–286. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hutchinson MR, Lewis SS, Coats BD, et al:
Possible involvement of toll-like receptor 4/myeloid
differentiation factor-2 activity of opioid inactive isomers causes
spinal proinflammation and related behavioral consequences.
Neuroscience. 167:880–893. 2010. View Article : Google Scholar
|
|
25
|
Rus H and Niculescu F: The complement
system in central nervous system diseases. Immunol Res. 24:79–86.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rus H, Cudrici C, David S and Niculescu F:
The complement system in central nervous system diseases.
Autoimmunity. 39:395–402. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu L, Aldskogius H and Svensson M:
Ultrastructural localization of immunoglobulin G and complement C9
in the brain stem and spinal cord following peripheral nerve
injury: an immunoelectron microscopic study. J Neurocytol.
27:737–748. 1998. View Article : Google Scholar
|
|
28
|
Davoust N, Jones J, Stahel PF, Ames RS and
Barnum SR: Receptor for the C3a anaphylatoxin is expressed by
neurons and glial cells. Glia. 26:201–211. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Twining CM, Sloane EM, Schoeniger DK, et
al: Activation of the spinal cord complement cascade might
contribute to mechanical allodynia induced by three animal models
of spinal sensitization. J Pain. 6:174–183. 2005. View Article : Google Scholar
|
|
30
|
Rioux P: TP-10 (AVANT Immunotherapeutics).
Curr Opin Investig Drugs. 2:364–371. 2001.
|
|
31
|
Shen Y and Meri S: Yin and Yang:
complement activation and regulation in Alzheimer’s disease. Prog
Neurobiol. 70:463–472. 2003.PubMed/NCBI
|
|
32
|
Sun T, Song WG, Fu ZJ, Liu ZH, Liu YM and
Yao SL: Alleviation of neuropathic pain by intrathecal injection of
antisense oligo-nucleotides to p65 subunit of NF-kappaB. Br J
Anaesth. 97:553–558. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Z, Ma W, Chabot JG and Quirion R:
Calcitonin gene-related peptide as a regulator of neuronal
CaMKII-CREB, microglial p38–NFkappaB and astroglial ERK-Stat1/3
cascades mediating the development of tolerance to morphine-induced
analgesia. Pain. 151:194–205. 2010.PubMed/NCBI
|
|
34
|
Ischenko A, Sayah S, Patte C, et al:
Expression of a functional anaphylatoxin C3a receptor by
astrocytes. J Neurochem. 71:2487–2496. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
O’Barr S and Cooper NR: The C5a complement
activation peptide increases IL-1beta and IL-6 release from
amyloid-beta primed human monocytes: implications for Alzheimer’s
disease. J Neuroimmunol. 109:87–94. 2000.PubMed/NCBI
|
|
36
|
Osaka H, McGinty A, Hoepken UE, Lu B,
Gerard C and Pasinetti GM: Expression of C5a receptor in mouse
brain: role in signal transduction and neurodegeneration.
Neuroscience. 88:1073–1082. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
O’Barr SA, Caguioa J, Gruol D, et al:
Neuronal expression of a functional receptor for the C5a complement
activation fragment. J Immunol. 166:4154–4162. 2001.PubMed/NCBI
|