Introduction
Systemic inflammatory responses,
coagulation/fibrinolytic system disorders and immune dysfunction
are among the pathophysiological features common during sepsis
(1). A previous study (1) has shown that the severity and
prognosis of sepsis are closely associated with blood coagulation
disorders or disseminated intravascular coagulation (DIC). Multiple
organ dysfunction syndrome (MODS), which is a severe consequence of
DIC, results in high mortality of septic patients. Early
manifestation of DIC is not well diagnosed. The hypercoagulable
state of early DIC has recently been named pre-DIC, characterized
by a predisposing factor for DIC and fibrinolytic coagulation
abnormalities, but with insufficient criteria for diagnosing DIC.
Successful diagnosis of pre-DIC may prevent DIC, as underlying
diseases and hematological abnormalities may be managed.
Three large-scale multi-center clinical trials were
performed to investigate the efficacy of coagulation inhibitors as
treatments for sepsis. In phase III clinical trials, recombinant
human activated protein C (APC) was found to significantly reduce
the 28-day mortality rate (2,3).
Application of APC was strongly recommended by the Society of
Critical Care Medicine (SCCM) for the treatment of severe septic
patients, who had an acute physiology and chronic health evaluation
(APACHE)-II score of >25 points (4). However, the cost of this treatment is
>6,000 US dollars for each course, which makes it extremely
expensive to apply for the majority of patients. Heparin, as an
anticoagulant, which, not only inhibits the activation of the
coagulation system, but is also an anti-inflammatory and
immunomodulatory agent, has been widely used during DIC treatment
and in the prevention and treatment of thrombotic diseases. It is
easy to obtain and inexpensive. Several non-randomized clinical
studies indicated that heparin partially inhibits sepsis by
activating the coagulation system (5). However, there is a lack of randomized
controlled prospective clinical studies. Therefore, the aim of
present study was to investigate the effects of low-dose heparin
treatment in patients with sepsis and pre-DIC to determine whether
this treatment improves the coagulation status, MODS and/or the
prognosis of patients.
Materials and methods
Reagents and patient selection
Patients were selected according to the diagnostic
criteria for sepsis of the American Thoracic Society, presented in
1992 (American College of Chest Physicians/SCCM) (6) and the pre-DIC diagnostic criteria
established at the Seventh Country Thrombosis and Bleeding Meeting
in China in 1999. Informed consent was obtained from the patients
and the study was approved by the local Ethics Review Committee of
the Hospital of Binzhou Medical University (Binzhou, China).
Exclusion criteria included
pregnancy/breast-feeding, age <16 or >70 years, requiring or
receiving anticoagulant therapy for thrombotic disease, requiring
blood or peritoneal dialysis for chronic renal failure, severe
chronic lung, kidney or liver diseases, contraindications
for the use of heparin (congenital bleeding disorders), serious
head injury, intracranial surgery or stroke <28 days prior to
enrollment, brain arteriovenous malformation, cerebral aneurysm or
a history of extensive damage of the central nervous system.
Following selection, patients were randomly divided into the
heparin and control treatment groups.
F1+2 (prothrombin fragment 1+2), TAT
(thrombin-antithrombin complex), AT-III (antithrombin-III) and
PAI-1 (plasminogen activator inhibitor-1) levels were determined
using double-antibody sandwich ELISA kits (Age Diagnostics
Laboratories Ltd., Boca Raton, FL, USA), according to the
manufacturer’s instructions.
Study design and procedure
The study was performed as a randomized,
double-blind, placebo controlled single-center clinical trial.
Following selection, patients were randomly divided into the
heparin or control treatment groups.
In the heparin group, 70 U/kg/24 h heparin was
administered by continuous infusion for 5–7 days. The input rate
and dose were adjusted according to the activated partial
thromboplastin time (APTT), which extended to 2 or 3 times. In the
placebo group, an equal amount of saline was administered. The two
groups were treated according to the sepsis cluster treatment
guidelines (4).
At 12, 18, 24, 48 and 72 h and at day 7 following
treatment, serum samples were collected and anticoagulated using
sodium citrate. Plasma F1+2, TAT, AT-III and PAI-1 levels were
determined as the primary endpoint measures using sandwich ELISA
assays. Platelet count (PLT), prothrombin time (PT), APTT,
fibrinogen (Fib) and other indicators were investigated in the
hospital laboratory. At the same time, the second endpoint
measurements, including APACHE-II-scores, were recorded and
calculated for each patient. In addition, the number of days
receiving mechanical ventilation and undergoing treatment in the
intensive care unit (ICU) were recorded, as well as the 28-day
mortality rate for the two groups. Moreover, the number of patients
suffering from MODS or DIC was established.
Statistical analysis
Data were analyzed using SPSS 11.5 (SPSS, Inc.,
Chicago, IL, USA) and are expressed as mean ± SD. Coagulation
indicators of the two groups were compared using multi-factor
repeated measurement analysis of variance. P<0.05 was considered
to indicate a statistically significant difference.
Results
Patients
In total 37 sepsis patients (21 males, 16 females;
average age, 49.3 years), treated between June 2008 and March 2009
in the ICU at Binzhou Hospital, were enrolled in the study. A list
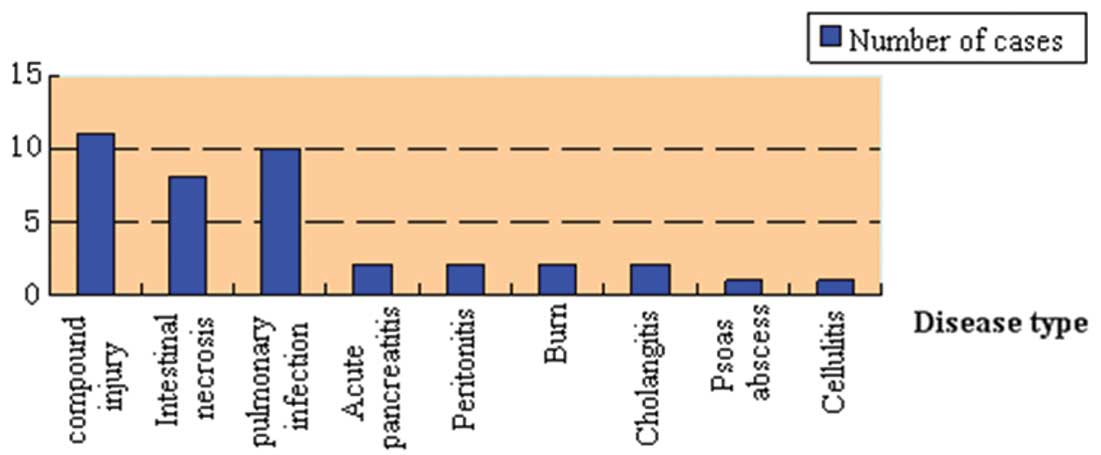
of the underlying diseases of the patients is summarized in
Fig. 1. Patients were randomly
assigned to a heparin (n=22) or control (n=15) group. There were no
clinically significant differences in the APACHE-II scores between
the two groups (Table I). However,
after seven days, the APACHE-II scores had significantly decreased
in the two groups with the decrease in the heparin group being
significantly higher (P=0.044; Table
II).
 | Table IPatient age, gender and APACHE-II
scores in the two treatment groups. |
Table I
Patient age, gender and APACHE-II
scores in the two treatment groups.
| Characteristics | Heparin (n=22) | Control (n=15) | P-value |
|---|
| Male:female | 12:10 | 9:6 | 0.254 |
| Age, years | 48.86±14.30 | 47.47±14.68 | 0.576 |
| APACHE-II score | 20.82±6.50 | 21.0±6.69 | 0.935 |
 | Table IIClinical indicators between the
low-dose heparin treatment and control groups. |
Table II
Clinical indicators between the
low-dose heparin treatment and control groups.
| Clinical
indicators | Control (n=15) | Heparin (n=22) | P-value |
|---|
| APACHE-II score |
| 0 h | 21.00±6.69 | 20.82±6.50 | 0.935 |
| 7 days | 18.26±6.12 | 15.68±6.16 | |
| 0 h-7 days | 2.73±2.49 | 5.13±4.48 | 0.044 |
| Days in ICU | 14.20±7.31 | 9.00±5.35 | 0.017 |
| Days of applying
ventilator | 11.73±8.34 | 7.00±5.74 | 0.048 |
| 28-day mortality
(%) | 6/15 (40) | 7/22 (31.8) | 0.434 |
| Complications
morbidity, n (%) |
| MODS | 11 (73.3) | 8 (36.4) | 0.030 |
| DIC | 6 (40.0) | 2 (9.1) | 0.034 |
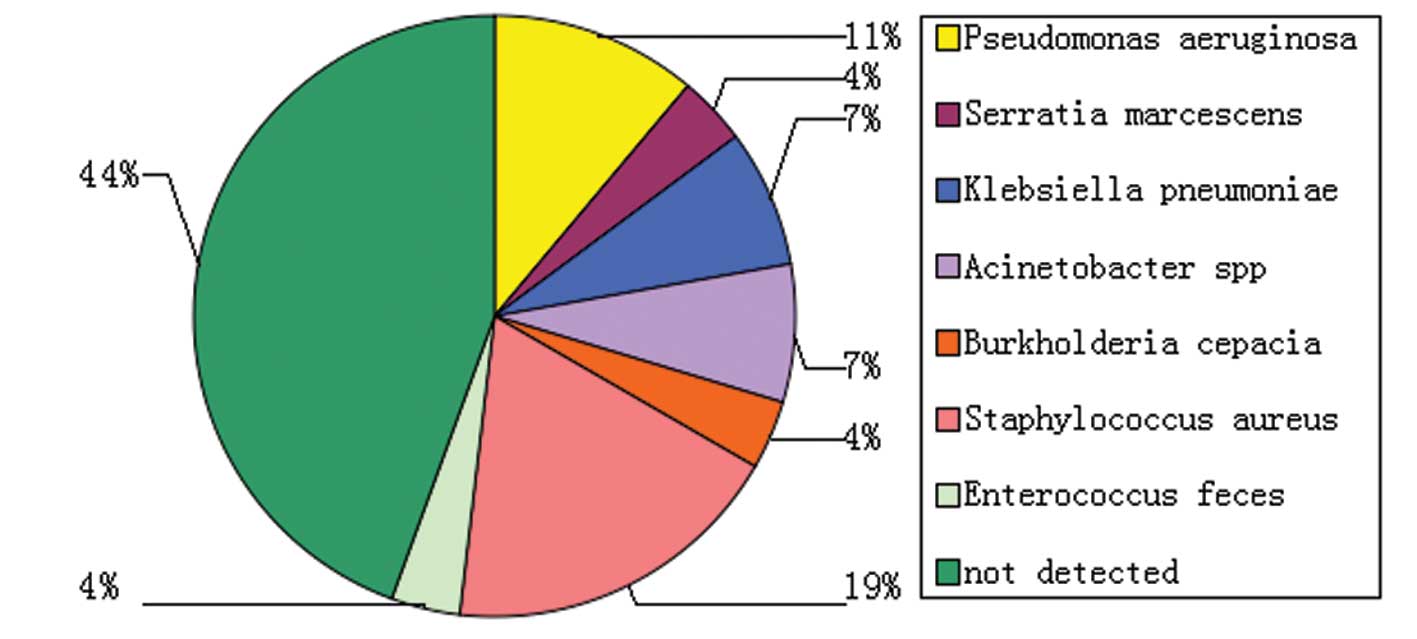
Sputum or body fluids from 15 patients of each group
were cultured and found positive for the following micro-organisms:
Pseudomonas aeruginosa, Viscous marcescens, Klebsiella
pneumoniae, Powell acinetobacter, Burkholderia cepacia,
Enterococcus feces and Staphylococcus aureus (Fig. 2).
Indicators of coagulation activation
PT, APTT, Fib concentration and PLT were assessed
prior to and following treatment. These parameters were not found
to be significantly different in the two groups. However, the F1+2
level increased significantly in the heparin group during the first
18 h, as compared to the baseline (0 h) measurement and then
decreased significantly at 72 h following the start of treatment.
TAT levels increased at 48 h and then also decreased significantly
at day 7. AT-III and PAI-1 levels in the groups did not change
significantly during treatment (P>0.05; Tables IV and V).
 | Table IVActivated coagulation parameters under
low-dose heparin treatment. |
Table IV
Activated coagulation parameters under
low-dose heparin treatment.
| F1+2, nmol/l | TAT, ng/ml |
|---|
|
|
|
|---|
| Time | Heparin | Control | Heparin | Control |
|---|
| 0 h | 1.66±0.69 | 1.68±0.81 | 4.21±2.15 | 4.38±2.19 |
| 12 h | 1.65±0.61 | 1.57±0.60 | 4.58±3.05 | 4.12±1.87 |
| 18 h | 1.82±0.77* | 1.58±0.62 | 4.82±2.89 | 4.24±1.63 |
| 24 h | 1.94±0.99 | 1.57±0.51 | 4.05±3.67 | 4.27±1.39 |
| 48 h | 1.84±1.14 | 1.59±0.70 | 4.78±4.48* | 4.34±1.43 |
| 72 h | 1.22±0.61* | 1.61±0.65 | 3.30±2.17 | 4.28±1.31 |
| 7 days | 0.88±0.48* | 1.28±0.56* | 2.44±1.51* | 3.91±1.49 |
 | Table VAnti-coagulation and fibrinolysis
parameters under low-dose heparin treatment. |
Table V
Anti-coagulation and fibrinolysis
parameters under low-dose heparin treatment.
| AT-III, IU/ml | PAI-1, ng/ml |
|---|
|
|
|
|---|
| Time | Heparin | Control | Heparin | Control |
|---|
| 0 h | 3.92±2.07 | 3.94±1.44 | 25.76±8.58 | 27.09±7.84 |
| 12 h | 3.73±1.76 | 3.86±1.28 | 26.02±11.06 | 25.46±6.80 |
| 18 h | 3.82±1.96 | 3.84±1.46 | 25.02±8.71 | 26.31±7.68 |
| 24 h | 3.82±1.45 | 3.83±1.54 | 25.35±9.08 | 23.73±6.83 |
| 48 h | 4.13±1.45 | 4.04±1.53 | 26.44±9.12 | 23.48±4.99 |
| 72 h | 4.43±1.38 | 4.13±1.47 | 22.64±10.16 | 23.77±6.46 |
| 7 days | 4.48±1.52 | 4.17±1.21 | 20.74±8.13 | 23.45±7.29 |
Mechanical ventilation time, number of
days in the ICU and 28-day mortality
Patients receiving low-dose heparin treatment
required significantly less mechanical ventilation and fewer days
in the ICU, as compared to the control group (P=0.017 and 0.048,
respectively). After 28 days, 13 patients had succumbed to the
disease, of whom 7 (31.8%) were in the heparin group and 6 (40%) in
the control group (Table II).
Occurrence of complications
Significant differences were observed for the
incidence of MODS and DIC between the two groups (P<0.05;
Table II).
Discussion
Results of an epidemiological study in the USA found
sepsis to have a high incidence of ~0.3%, which is on the increase
at a rate of 1.5% annually (7).
Financial issues are involved in the treatment of sepsis, with the
annual cost being 17.4 billion US dollars. In addition, the
mortality rate of sepsis is high, with findings of a previous study
indicating that despite intensive care, mortality remains at 30–50%
(8). More than half of the
patients with sepsis present with coagulation factor abnormalities
and the incidence of DIC is >20%. In cases of DIC incidence, the
mortality rate is almost 63% (9).
It has been confirmed that DIC leads to ischemic injury of vital
organs (10) and eventually to
MODS (11). Therefore, treating
the coagulopathy state is necessary to improve multiple organ
function, which may also be a promising approach for treating
sepsis.
Sepsis involves the production of endotoxin by
Gram-positive bacteria, a key mediator of the inflammatory response
during septic shock. In the current study, 56% of the cases
detected pathogens, 23% of which were gram-positive bacteria
(Staphylococcus aureus being the most common).
Coagulation activation is an important feature in
the pathogenesis of sepsis (9).
Thrombin generation is a key step in an activated coagulation
system. Due to the extremely short half-life of thrombin, it is
difficult to determine activated thrombin levels in blood.
Therefore, other molecular markers were used to confirm thrombin
generation. In the present study, four molecular markers were
selected to detect the early stages of coagulation. The markers are
produced at various stages and appear earlier than PT, APTT and
platelet abnormalities. In addition, the markers were selected to
help diagnose the emergence of pre-DIC. These early indicators
included F1+2, TAT, fibrinopeptide A and soluble fibrin monomer
complex. F1+2 directly reflects the amount of generated thrombin,
while the remaining three markers only partially reflect thrombin
generation. Therefore, F1+2 is considered the most sensitive
indicator of thrombin generation (12).
Heparin is a sulfated polysaccharide with a
heterogeneous structure and complex polymerization (MW, 3–57 kDa).
Heparin binds to AT-III, causing a conformational change that
increases the flexibility of the reactive site loop, activating
AT-III. The activated AT-III then inactivates thrombin and other
proteases, including factor Xa. Heparin also binds platelets to
induce platelet aggregation, resulting in a strong anticoagulant
effect (13). In the present
study, the subjects were in an early stage of DIC, during which
blood is hypercoagulable, platelets are activated and activation of
coagulation has already been initiated. In addition, during the
pre-DIC period, there is no extensive micro-thrombosis,
fibrinolysis, consumption or degradation of platelets and clotting
factors. During low-dose heparin therapy, F1+2 showed a gradual
increase, followed by a decrease over the first 24 h. At 72 h and 7
days, it was found to be present in significantly lower amounts
(P<0.05). Similarly, TAT was initially increased, but
subsequently decreased and eventually was reduced to only half the
initial level at day 7, following initiation of low-dose heparin
therapy (P<0.05). This was consistent with the observation that
therapy led to the activation of coagulation (12). Heparin is not only involved with
the coagulation factor Xa, but also collaborates with AT to inhibit
thrombin. The heparin dose used to inhibit factor Xa was much lower
than that required to inactivate thrombin, thus it is likely that
the effectiveness of low-dose heparin is dependent on this
mechanism to prevent thrombosis. Formation of thrombin is reduced
by inhibiting factor Xa and F1+2, which explains the decrease in
thrombin levels following a reduction in factor Xa. As a result,
TAT decreases, which is consistent with the observations in the
present study of decreased F1+2 and TAT levels.
AT-III is a member of the serine protease inhibitor
family, which is the most important plasma inhibitor of the
coagulation system. It is capable of inhibiting all serine
proteases at a low speed, particularly factor Xa and thrombin. In
the presence of heparin, inhibition speed increases significantly.
AT is also known as a heparin cofactor. However, activity of AT is
affected by the pH of the body, which means that loss of the
inhibitor increases during acidosis. If acidosis is enhanced, AT
activity significantly improves (14). During sepsis, elastase released
from activated neutrophils inactivates AT. Clearance of TAT
complexes, a process that occurs rapidly, results in the clearance
of AT. Heparin may significantly increase AT activity. In the
current study, following the administration of low doses of heparin
to the patients, AT levels increased gradually. However, the
patients were undergoing sepsis, which is usually associated with
disturbances in oxygen supply and demand, an acid-base imbalance
and high blood lactate (15).
AT-III is therefore in a low state of activity. Moreover, with
patients in a hypercoagulable state, when AT consumption and
degradation are increased, AT-III recovery and improvement of
oxygen metabolism are expected. This may be the reason for the
recovery of AT-III not being observed following treatment.
PAI-1 is mainly synthesized by endothelial cells and
secreted in the subendothelial space. It is the most important
factor of a variety of PAIs and functions as an inhibitor of tissue
plasminogen activator and urokinase. In the present study, PAI-1
levels of heparin-treated patients did not decrease significantly
following heparin administration. This finding indicates that the
concentration of plasma PAI-1 is important in the maintenance of
patients with sepsis and DIC (16).
The current study has demonstrated that APACHE-II
scores of the two treatment groups significantly decreased, while
low-dose heparin intervention improved the scores more
significantly. Low-dose heparin shortened the normal coagulation
time by preventing clotting. Thus, heparin treatment not only
improved blood circulation and oxygen supply, but also lowered the
necessity of mechanical ventilation significantly (P=0.048).
Altogether, this resulted in a shorter duration of stay at the
hospital. Improvements in microcirculation not only blocked the
development of DIC, but also decreased MODS.
A limitation of this study is the small number of
patients enrolled in the present study. In addition, a few patients
exited the study prematurely, not allowing time for follow-up.
However, the study was conducted as a prospective clinical trial,
which is uncommon and allows for several conclusions. No
significant differences were observed between the two treatment
groups, which confirms the results of an international clinical
study aiming to determine the effects of low-dose heparin treatment
during sepsis (17,18). The clinical results obtained
indicate that shortening the stay in the ICU and decreasing the
need for mechanical ventilation reduces the treatment costs of
sepsis patients. In addition, the application of low-dose heparin
is relatively inexpensive and safe, indicating the suitability for
the early treatment of sepsis.
Acknowledgements
The study was supported by a grant from the Nature
Science Foundation of Shandong Province, China (no. Z2007C10).
References
|
1
|
Cornet AD, Smit EG, Beishuizen A and
Groeneveld AB: The role of heparin and allied compounds in the
treatment of sepsis. Thromb Haemost. 98:579–586. 2007.PubMed/NCBI
|
|
2
|
Bernard GR, Vincent JL, Laterre PF, et al;
Recombinant human protein C Worldwide Evaluation in Severe Sepsis
(PROWESS) study group. Efficacy and safety of recombinant human
activated protein C for severe sepsis. N Engl J Med. 344:699–709.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barie PS, Hydo LJ, Shou J and Eachempati
SR: Efficacy of therapy with recombinant human activated protein C
of critically ill surgical patients with infection complicated by
septic shock and multiple organ dysfunction syndrome. Surg Infect
(Larchmt). 12:443–449. 2011. View Article : Google Scholar
|
|
4
|
Dellinger RP, Carlet JM, Masur H, et al;
Surviving Sepsis Campaign Management Guidelines Committee.
Surviving Sepsis Campaign guidelines for management of severe
sepsis and septic shock. Crit Care Med. 32:858–873. 2004.
View Article : Google Scholar
|
|
5
|
Shen JI and Winkelmayer WC: Use and safety
of unfractionated heparin for anticoagulation during maintenance
hemodialysis. Am J Kidney Dis. 60:473–486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
No authors listed. American College of
Chest Physicians/Society of Critical Care Medicine Consensus
Conference: definitions for sepsis and organ failure and guidelines
for the use of innovative therapies in sepsis. Crit Care Med.
20:864–874. 1992. View Article : Google Scholar
|
|
7
|
Angus DC, Linde-Zwirble WT, Lidicker J,
Clermont G, Carcillo J and Pinsky MR: Epidemiology of severe sepsis
in the United States: analysis of incidence, outcome, and
associated costs of care. Crit Care Med. 29:1303–1310. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gerlach H and Keh D: Sepsis in 2003: are
we still in the middle of nowhere? Curr Opin Anaesthesiol.
17:97–106. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feistritzer C and Wiedermann CJ: Effects
of anticoagulant strategies on activation of inflammation and
coagulation. Expert Opin Biol Ther. 7:855–870. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hook KM and Abrams CS: The loss of
homeostasis in hemostasis: new approaches in treating and
understanding acute disseminated intravascular coagulation in
critically ill patients. Clin Transl Sci. 5:85–92. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dempfle CE: Coagulopathy of sepsis. Thromb
Haemost. 91:213–224. 2004.
|
|
12
|
Thamas L, Yuan L, Zhu HM and Xia S:
Clinical Laboratory Diagnosis: Application and evaluation of
results. Shanghai Science and Technology Press; Shanghai: 2004
|
|
13
|
Robertson MS: Heparin: the cheap
alternative for immunomodulation in sepsis? Crit Care Resusc.
8:235–238. 2006.PubMed/NCBI
|
|
14
|
Wiedermann CJ: Clinical review: molecular
mechanisms underlying the role of antithrombin in sepsis. Crit
Care. 10:2092006. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Michaeli B, Martinez A, Revelly JP, et al:
Effects of endotoxin on lactate metabolism in humans. Crit Care.
16:R1392012. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Madoiwa S, Nunomiya S, Ono T, et al:
Plasminogen activator inhibitor 1 promotes a poor prognosis in
sepsis-induced disseminated intravascular coagulation. Int J
Hematol. 84:398–405. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jaimes F, De la Rosa G, Arango C, et al: A
randomized clinical trial of unfractioned heparin for treatment of
sepsis (the HETRASE study): design and rationale [NCT00100308].
Trials. 7:192006.PubMed/NCBI
|
|
18
|
Jaimes F, De La Rosa G, Morales C, et al:
Unfractioned heparin for treatment of sepsis: A randomized clinical
trial (The HETRASE Study). Crit Care Med. 37:1185–1196. 2009.
View Article : Google Scholar : PubMed/NCBI
|